Abstract
Lithium–sulfur batteries (LSBs) are gaining much attention because they offer a much higher theoretical energy density compared to traditional lithium-ion batteries. However, the cycling performance of LSBs with high sulfur mass loading is poor due to the shuttle effect, limiting the practical application of LSBs. In this work, a unique porous sulfur/Ti3C2Tx Mxene@selenium (S/Ti3C2Tx@Se) cathode of a LSB is synthesized by a simple hydrothermal method to address these challenges. In this composite, Ti3C2Tx forms a conductive framework and Se is tightly anchored on the framework. The Se inhibits the agglomeration of Ti3C2Tx and prevents the collapse of Ti3C2Tx. The S/Ti3C2Tx@Se composite can adsorb lithium polysulfides (LiPSs) and suppresses the shuttle effect and volume changes during cycling, improving the cycling stability of LSBs with high S loading. A high capacity of 812.2 mAh g−1 at 0.1 C with 5.0 mg cm−2 sulfur mass loading after 100 cycles is obtained. This work could inspire further research into high-performance S host materials for high-S-loading LSBs.
1. Introduction
The depletion of fossil fuels and environmental pollution have created an urgent demand for the development of renewable energy. Solar cells, lithium-ion batteries, and sodium-ion batteries are essential for sustainable energy [1,2,3,4]. Among these, lithium-ion batteries stand out for their exceptional performance, particularly in consumer electronics, flexible devices, and transportation [5,6,7,8,9,10,11,12]. However, one limitation of lithium-ion batteries is their limited energy density. Lithium–sulfur batteries (LSBs) are gaining much attention because they offer a much higher theoretical energy density (2600 Wh kg−1) compared to traditional lithium-ion batteries. The theoretical specific capacity of sulfur is 1675 mAh g−1, which also outpaces many current cathode materials [13,14,15,16,17,18]. However, there are some issues for LSBs which need to be overcome, including (i) the dissolution of intermediate products (Li2Sn, 4 ≤ n ≤ 8) which is named the “shuttle effect”; (ii) the low electrical conductivity of sulfur; and iii) the huge volume change during cycling [19,20,21,22]. These imperfections cause poor electrochemical performance, such as low specific capacity, low Coulombic efficiency, and rapid capacity fading. So far, many strategies have been developed to address LSB issues, such as developing porous host materials for sulfur [23,24,25], separator modification [26,27,28], and electrolyte engineering [29]. The most common and effective method is to design porous S host materials to enable a suppressed shuttle effect.
In recent years, a novel two-dimensional material (Ti3C2Tx Mxene) has been widely applied in energy storage owing to the controlled synthetic method, excellent conductivity and abundant functional surface groups [30,31,32,33,34]. Moreover, Ti3C2Tx Mxene exhibits significant potential to mitigate the shuttle effect and accommodate the sulfur volume expansion during lithiation owing to its layered architecture and large pore volume. In a recent study, Wang et al. [35] easily prepared S/Ti3C2Tx Mxene by a hydrothermal method. The sulfur nanoparticles are uniformly distributed within the Ti3C2Tx layers, which is crucial to the electrochemical performance of LSBs. The Ti3C2Tx ensures that sulfur, which has low intrinsic conductivity, is closely integrated with a highly electrically conductive Ti3C2Tx. This structure leads to enhanced electrochemical performance (a high initial capacity of 1277 mAh g−1 and a stable capacity of 1059 mAh g−1 at 0.5 C after 100 cycles).
Although the S/Ti3C2Tx Mxene cathode showed improved electrochemical performance, it possessed low areal sulfur loading (<3 mg cm−2) due to the Ti3C2Tx Mxene creating irreversible agglomerates via the van der Waals force, which limited its practical application. According to the commercial requirement, LSB cathodes must have a high sulfur loading (>4 mg cm−2). However, high-sulfur-loading cathodes suffer from a severe shuttle effect. Utilization of conductive coatings to prevent the severe shuttle effect has been demonstrated as an effective strategy to enhance the cycling stability of LSBs with high sulfur loading. Yu et al. reported a PDA-coated Ti3C2Tx Mxene/sulfur cathode which showed excellent cycling stability (556 mAh g−1 after 330 cycles at 0.5 C) with 4.4 mg cm−2 sulfur loading [36]. Se possesses high electrical conductivity and greatly enhances electron transport, holding great promise for a Ti3C2Tx Mxene/sulfur cathode. Thus, we have prepared a unique porous sulfur/Ti3C2Tx Mxene@selenium (S/Ti3C2Tx@Se) cathode. The Se inhibits the agglomeration of Ti3C2Tx. As a result, the S/Ti3C2Tx@Se composite shows a high capacity of 812.2 mAh g−1 after 100 cycles at 0.1 C with 5.0 mg cm−2 sulfur mass loading.
2. Experimental
2.1. Preparation of Ti3C2Tx/S@Se
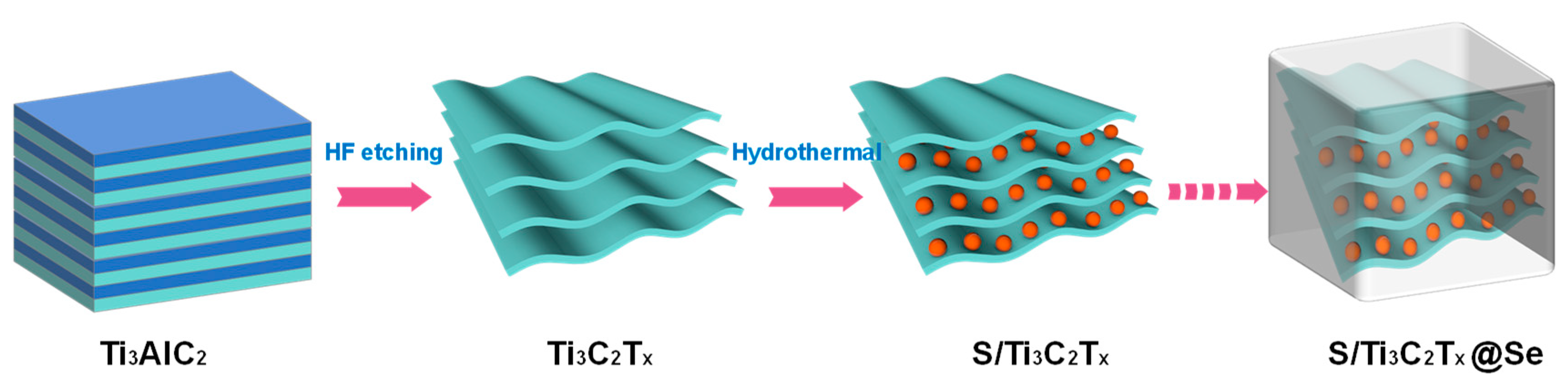
A total of 40 mL HF (Sigma-Aldrich, 48%, St. Louis, MO, USA) was added into a plastic Erlenmeyer flask, and a PTFE-coated stirring bar was used. Then, 0.5 g MAX precursor (Ti3AlC2) (Nanjing XFNANO Materials Tech Co., Ltd., Nanjing, China) was added in and the mixture was stirred for 96 h at room temperature. After etching, the sample was washed with DI water until the supernatant reached a pH above 6 and then dried for 24 h at 80 °C in an oven to obtain Ti3C2Tx Mxene. The sulfur was encapsulated into Ti3C2Tx Mxene and Se via a hydrothermal process. Typically, the Ti3C2Tx Mxene powder was added into a 7:1 (volume ratio) water/ethanol solution, followed by ultrasonic treatment for 1 h. The purpose of the ultrasonic treatment was to promote dispersion of the Ti3C2Tx Mxene in the solution, ensuring that the mixture was homogeneous. Carbon disulfide (CS2) (Sigma-Aldrich, St. Louis, MO, USA) was used as a solvent to dissolve the sulfur and selenium powders; the solution contained 600 mg of sulfur in 10 mL CS2 and 120 mg of selenium in 2 mL CS2. Subsequently, the S and Se solutions were added into the above mixture, then treated in an ultrasonic grinder for 0.1 h to disperse S and Se in the mixture. The mixture was transferred to a Teflon-lined autoclave, which was then heated to 220 °C (close to selenium’s melting point) for 12 h. In this process, the S powder was first melted and filled into the layers of Ti3C2Tx Mxene. Then, the Se was melted and coated on the surface of the S/Ti3C2Tx composite when the reaction temperature reached Se’s melting point. The mixture was transferred to a Teflon-lined autoclave, which was then heated to 220 °C (close to selenium’s melting point) for 12 h. The product was washed three times with distilled water to remove any residual products or unreacted materials. The final step involved drying the product in an oven at 80 °C for 24 h. The schematic of preparation of the S/Ti3C2Tx@Se composite is shown in Figure 1.
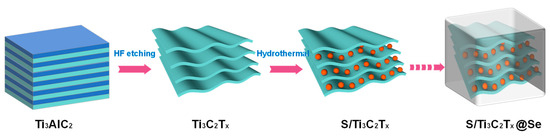
Figure 1.
Schematic of preparation of S/Ti3C2Tx@Se composite.
2.2. Characterizations
The structure of samples was characterized by X-ray diffraction (XRD) (Rigaku MiniFlexll, Rigaku Corporation, Tokyo, Japan). Scanning electron microscopy (SEM) (JEOL JSM-7800F Field Emission, Manufacturer JEOL Ltd., Tokyo, Japan) at 5 kV and corresponding energy dispersive spectroscopy (EDX) were used to analyze the samples’ morphologies. The Raman spectra (632 nm) were collected with a RE01 (Renishaw Inc., London, UK). Thermogravimetric analysis (TGA) measurements were carried out by a Mettler Toledo TGA/SDTA 851 (Zurich, Switzerland).
2.3. Electrochemical Tests
The sulfur cathode was fabricated as follows: 60 wt% active material, 30 wt% conductive carbon black, and 10 wt% polytetrafluoroethylene (PTFE) were mixed to form a paste, which was subsequently pressed onto nickel foam. The electrolyte consisted of 1 M lithium bis(trifluoromethane sulfonyl)imide dissolved in a 1:1 (volumetric ratio) 1,3-dioxolane/dimethoxyethane with 1 wt% lithium nitrate. The separator was Celgard 2400 (LLC-Headquarters & Charlotte Manufacturing Facility, North Carolina, USA). Lithium foil served as the counter electrode. The assembly of the coin cells was carried out in a glove box filled with Ar. Galvanostatic discharge and charge tests were conducted using a Land CT2001 battery tester (Wuhan Land Electronic Co. Ltd., Wuhan, Hubei, China), operating within a voltage range of 1.6 to 2.8 V versus Li/Li+. Additionally, cyclic voltammetry (CV) was performed at a scanning rate of 0.1 mV s−1, also covering the 1.6 to 2.8 V potential range, using a PGSTAT 302N electrochemical station (Wuhan Land Electronic Co., Ltd., Wuhan, Hubei, China).
3. Results and Discussion
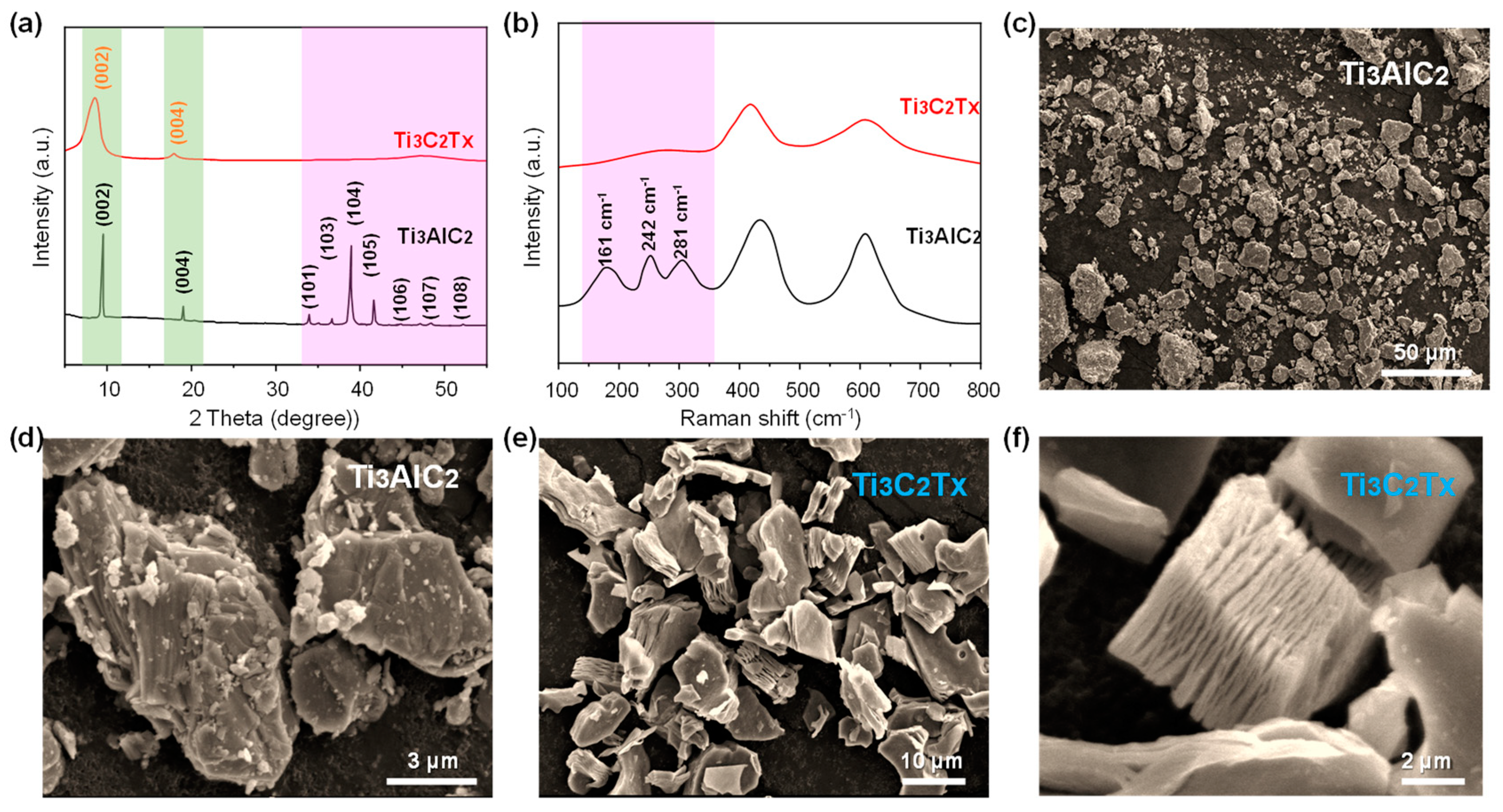
Figure 2a presents the XRD patterns of the MAX phase precursor (Ti3AlC2) and Mxene product (Ti3C2Tx). The MAX exhibits strong peaks at 2θ values of 9.6°, 19.1°, 34.1°, 36.9°, 38.9°, 42.0°, 45.0°, 48.5°, and 52.5°, corresponding to the (002), (004), (101), (103), (104), (105), (106), (107), and (108) planes of Ti3AlC2 (JCPDS No. 52-0875) [37], respectively. And the diffraction peaks are sharp, indicating high crystallinity. After HF etching, however, the XRD pattern shows a reduction in peak intensity, signifying a decrease in crystallinity. In addition, the diffraction peak corresponding to the (002) plane shows an obvious shift to a lower angle, and the peaks at the 2θ values of 36.9°, 38.9°, and 42.0° are no longer present. These changes suggest the successful removal of Al atomic layers through HF etching, resulting in an increased d-spacing within the Ti3C2Tx layers. The Raman spectra (Figure 2b) prove these observations, with peaks located at 161 cm−1, 242 cm−1, 281 cm−1, 442 cm−1, and 621 cm−1 [35]. After etching, the peaks at 161 cm−1, 242 cm−1, and 281 cm−1 are nearly absent, indicating significant structural changes during the etching process. Figure 2c–f are SEM images of Ti3AlC2 and Ti3C2Tx. Figure 2c displays the dense structure of Ti3AlC2, while Figure 2e–f illustrate the multilayered structure of Ti3C2Tx after etching. This layered morphology facilitates the uniform distribution and anchoring of nanoparticles between the layers.
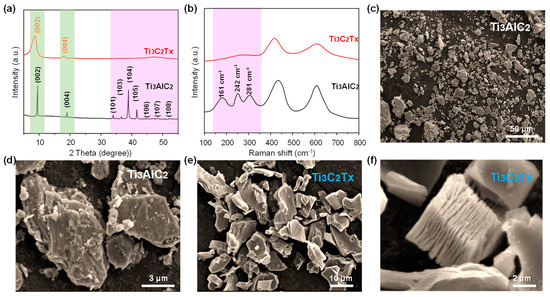
Figure 2.
(a) XRD patterns of MAX and Mxene. (b) Raman patterns of MAX and Mxene. (c,d) SEM images of MAX. (e,f) SEM images of Mxene.
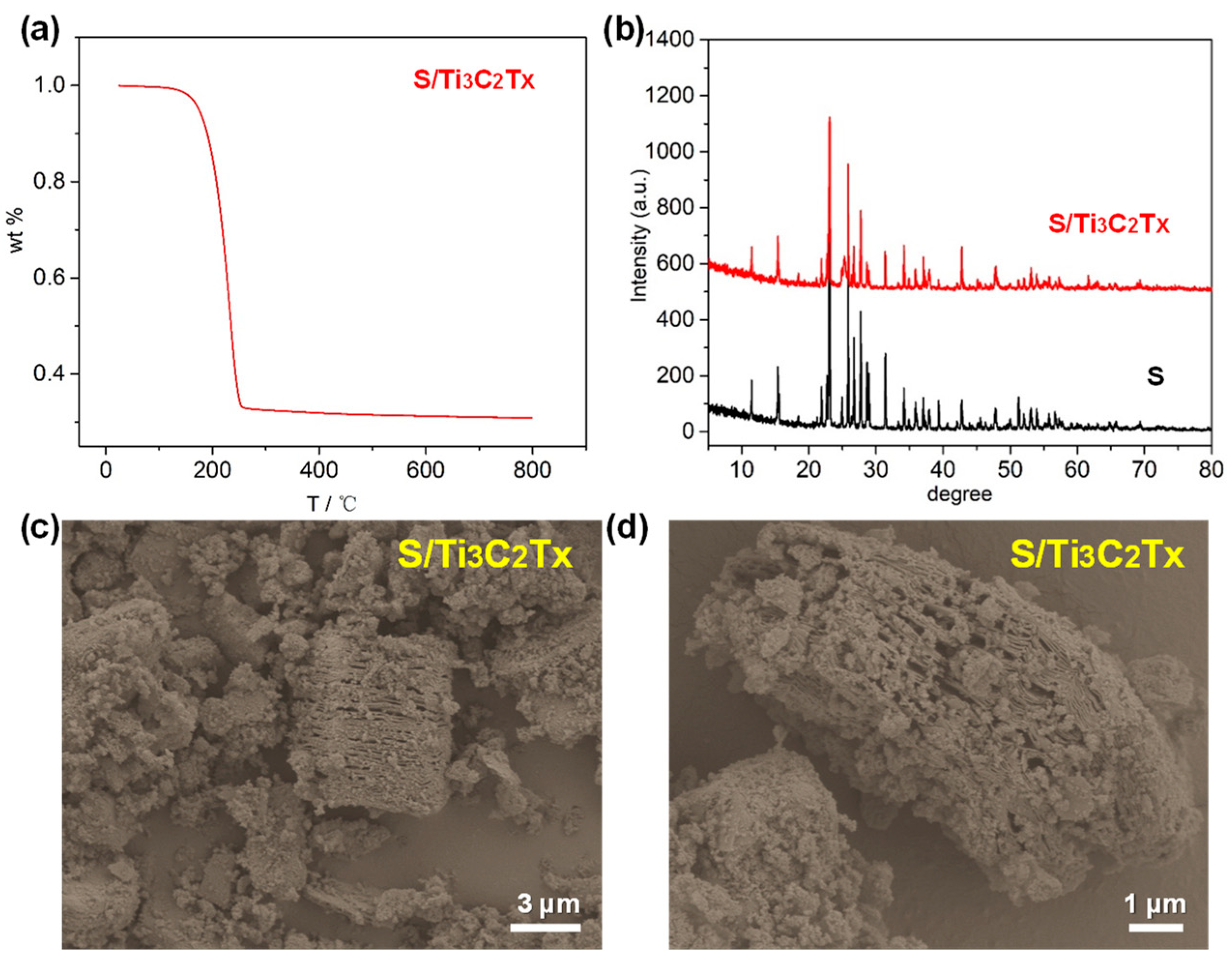
The S/Ti3C2Tx composite was synthesized using a hydrothermal reaction. To assess the sulfur content, TGA was conducted under a nitrogen atmosphere. The TGA results are illustrated in Figure 3a. There are two weight-loss steps. In the initial step, from room temperature to 110 °C, the weight decreases by 0.62%, resulting from the evaporation of surface-bound water and residual HF. Between 110 °C and 300 °C, a major weight reduction of 67.5% occurs, corresponding to the sulfur loss from the S/Ti3C2Tx composite. Thus, the sulfur content was quantified to be 67.5 wt%. Figure 3b displays XRD patterns of sulfur and the S/Ti3C2Tx composite. There is a strong resemblance between sulfur and the S/Ti3C2Tx composite due to the high content of sulfur in Ti3C2Tx. Figure 3c,d present SEM images of the S/Ti3C2Tx composite, highlighting that sulfur nanoparticles are uniformly dispersed between the Ti3C2Tx layers. The hydrothermal reaction is a simple synthesis technique performed under relatively moderate conditions. By this method, we were able to regulate the interaction between Ti3C2Tx and sulfur in solution, ensuring uniform sulfur nanoparticle distribution both within the layers and on the surface of the Ti3C2Tx.
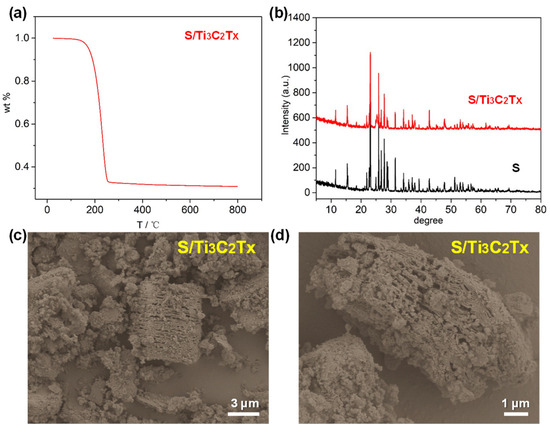
Figure 3.
(a) TGA of S/Ti3C2Tx composite. (b) XRD patterns of S and S/Ti3C2Tx composite. (c,d) SEM images of S/Ti3C2Tx composite.
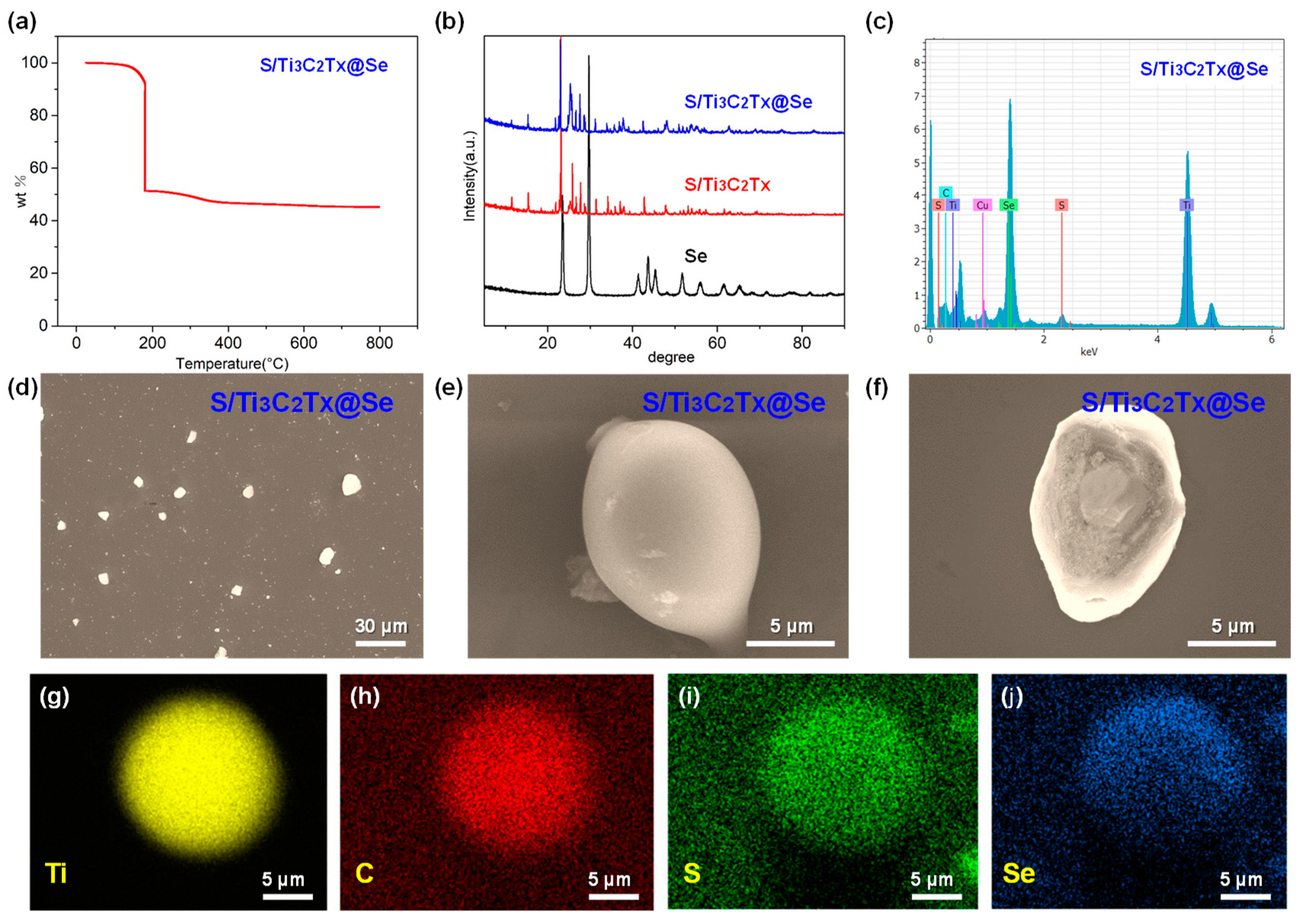
Figure 4 illustrates the morphology and structure of the S/Ti3C2Tx@Se composite. Figure 4a presents the TGA curve of the S/Ti3C2Tx@Se composite in a nitrogen atmosphere and shows a three-step weight loss. In the first step, from room temperature to 110 °C, the observed weight loss is due to the evaporation of surface-bound water. The second step, occurring between 110 °C and 200 °C, shows a major weight reduction caused by the sulfur loss from the composite. In the third step, above 200 °C, the weight loss is attributed to the evaporation of Se. Based on these results, the sulfur content was measured at 47.7 wt%, while the selenium content was found to be 6.1 wt%. Due to the relatively low content of Se, the characteristic diffraction peak for Se cannot be observed in the XRD pattern of the S/Ti3C2Tx@Se composite (Figure 4b). Figure 4d–f are SEM images of the S/Ti3C2Tx@Se composite. Figure 4d shows the S/Ti3C2Tx@Se composite’s ball-like structure. From Figure 4e, we can observe that the S/Ti3C2Tx@Se composite is wrapped by a thin layer of Se. Figure 4f is a cross-sectional SEM image of the S/Ti3C2Tx@Se composite. We can observe that the S nanoparticles are fixed in Ti3C2Tx layers, and a thin layer of Se is wrapped over the S/Ti3C2Tx composite. Figure 4g–j are SEM–EDX mapping images of the S/Ti3C2Tx@Se composite. The yellow, red, green, and blue parts represent the Ti, C, S, and Se. We can observe that Se uniformly wraps over the S/Ti3C2Tx composite.
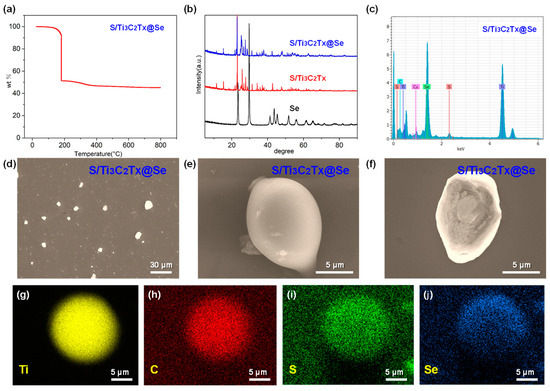
Figure 4.
(a) TGA of S/Ti3C2Tx@Se composite. (b) XRD patterns of S, S/Ti3C2Tx composite and S/Ti3C2Tx@Se composite. (c) SEM–EDX pattern of S/Ti3C2Tx@Se composite. (d–f) SEM images of S/Ti3C2Tx@Se composite. (g–j) SEM–EDX mapping images of S/Ti3C2Tx@Se composite.
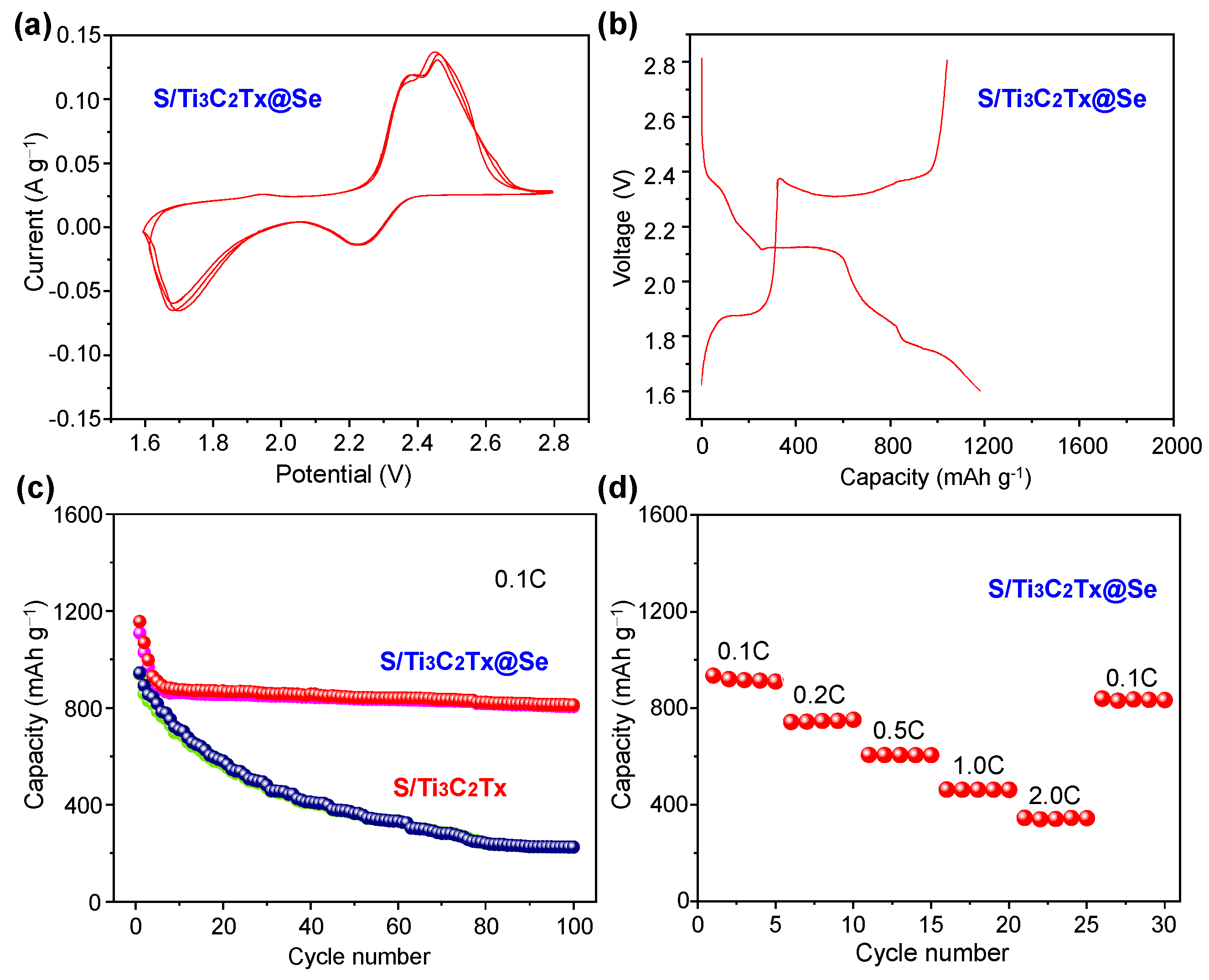
Figure 5 presents the S/Ti3C2Tx@Se composite’s electrochemical performance. Figure 5a shows the CV curves of the S/Ti3C2Tx@Se composite in which two reduction peaks at 1.72 V and 2.18 V are associated with the lithiation process, while three oxidation peaks at 1.83 V, 2.32 V, and 2.51 V correspond to the delithiation process. It is notable that the S/Ti3C2Tx@Se composite has a higher oxidation peak current and reduction peak current compared to the other S/Ti3C2Tx composite [35], which indicates that the Se can improve the conductivity of the S cathode material. The galvanostatic charge–discharge curves (Figure 5b) exhibit two distinct plateaus at 1.70 V and 2.13 V during discharge and three plateaus at 1.80 V, 2.28 V, and 2.50 V during charge. There is a differentiation of the redox peak and plateau potentials which is due to the complex side reactions in the initial cycles. Figure 5c illustrates the cycling performance of both the S/Ti3C2Tx composite and the S/Ti3C2Tx@Se composite at 0.1 C. For the S/Ti3C2Tx composite, the initial reversible capacity is 892.9 mAh g−1, which declines rapidly to 225.3 mAh g−1 after 100 cycles. In contrast, the S/Ti3C2Tx@Se composite starts with an initial reversible capacity of 1069.7 mAh g−1 and maintains a stable reversible capacity of 812.2 mAh g−1 after 100 cycles. The capacity retention of the S/Ti3C2Tx@Se composite is as high as 75.9%, which is much higher than that of S/Ti3C2Tx (25.2%). The cycling performance of the S/Ti3C2Tx@Se cathode is better than that of the previously reported S-based cathode (Table 1) [35,38,39,40,41,42]. With a multilayered 2D structure, Ti3C2Tx as a host material can buffer the volume expansion. However, the shuttle effect of LiPSs cannot be avoided by Ti3C2Tx at high S mass loading. Thus, the S/Ti3C2Tx cathode shows a low capacity retention. In the S/Ti3C2Tx@Se composite, Ti3C2Tx forms a conductive framework and Se is tightly anchored on the framework. The Se inhibits the agglomeration of Ti3C2Tx and prevents the collapse of Ti3C2Tx. The S/Ti3C2Tx@Se composite can adsorb lithium polysulfides (LiPSs) and suppresses the shuttle effect and volume changes during cycling, improving the cycling stability of LSBs. And Ti3C2Tx and Se possess high electrical conductivity and greatly enhance the electron transport [43,44,45,46,47,48,49], enabling the S/Ti3C2Tx@Se composite to exhibit an ideal rate performance. It exhibits high average capacities at different current densities, with average discharge specific capacities at current densities of 0.1–2.0 C of 881.2, 717.9, 603.1, 441.1, and 386.5, and 213.8 mAh g−1, respectively. When the current density was returned to 0.1 C, the discharge specific capacity also recovered to 821.2 mAh g−1.
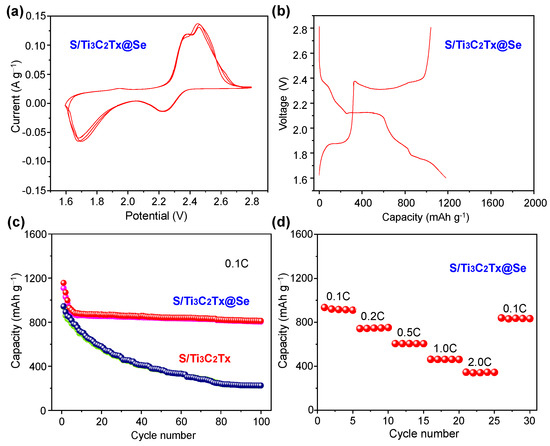
Figure 5.
(a) CV of S/Ti3C2Tx@Se composite at the scan rate of 0.1 mV s−1. (b) Galvanostatic charge/discharge curves of S/Ti3C2Tx@Se composite at 0.1 C at 1.6–2.8V. (c) Cycling performance of S/Ti3C2Tx@Se composite and S/Ti3C2Tx@Se composite at 0.1 C. (d) Rate performance of S/Ti3C2Tx@Se composite.

Table 1.
Comparison of the cycling performance of the S/Ti3C2Tx@Se composite and previously reported S-based cathode.
4. Conclusions
In this work, we easily prepared a unique porous sulfur/Ti3C2Tx Mxene@selenium (S/Ti3C2Tx@Se) cathode of a LSB by a hydrothermal method. SEM images showed that S nanoparticles are homogeneously embedded in the Ti3C2Tx sheets and that film-like Se wraps the S/Ti3C2Tx. Because of Mxene’s highly electrically conductive multilayered 2D structure with Se coatings, the S/Ti3C2Tx@Se composite showed improved reversible specific capacity and cycling performance at high sulfur mass loading. Its initial reversible capacity is 1069.7 mAh g−1 and it retains an 812.2 mAh g−1 capacity after 100 cycles at 0.1 C with a high capacity retention of 75.9% at a sulfur mass loading of 5.0 mg cm−2. These results suggest that a novel cathode material with good electrochemical performance can be used in LSBs.
Author Contributions
Conceptualization, X.D.; Methodology, L.K.; Validation, F.Z.; Formal analysis, J.X. and Z.T.; Investigation, J.X.; Writing—original draft, Y.S.; Writing—review & editing, H.D.; Supervision, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Jianbin Xu was employed by the company Tianjin Sanyuan Electric Power Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- An, T.; Zhang, Y.; Wen, J.; Dong, Z.; Du, Q.; Liu, L.; Wang, Y.; Xing, G.; Zhao, X. Multi-Level Pyramidal Microstructure-Based Pressure Sensors with High Sensitivity and Wide Linear Range for Healthcare Monitoring. ACS Sens. 2024, 9, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, Q.; Kang, H.; Gu, X.; Shan, C.; Zeng, J.; Dai, T.; Yang, Q.; Sun, X.; Li, G.; et al. Uniform Molecular Adsorption Energy-Driven Homogeneous Crystallization and Dual-Interface Modification for High Efficiency and Thermal Stability in Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2024, 34, 2408512. [Google Scholar] [CrossRef]
- Xie, Z.; Li, Y.; Li, X.; Fang, Y.; Chang, J.; Yang, Q.; Sun, X.; Miao, C.; Lu, G.; Chen, Z.; et al. High Performance Inverted Planar Perovskite Solar Cells Enhanced by Heteroatomic Functionalized Hole Transport Materials. Mater. Chem. Front. 2024, 8, 2764–2774. [Google Scholar] [CrossRef]
- Sun, X.; Fan, H.; Xu, X.; Li, G.; Gu, X.; Luo, D.; Shan, C.; Yang, Q.; Dong, S.; Miao, C.; et al. A Fluorination Strategy and Low-Acidity Anchoring Group in Self-Assembled Molecules for Efficient and Stable Inverted Perovskite Solar Cells. Chem.-A Eur. J. 2024, 30, e202400629. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.; Peng, D.; Dong, L.; Zhu, D. Soft devices empowered by mechanoluminescent materials. Soft Sci. 2023, 3, 39. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Xu, Z.; Guo, J.; Tang, S.Y.; Ma, X. Recent advancements in liquid metal enabled flexible and wearable biosensors. Soft Sci. 2023, 3, 37. [Google Scholar] [CrossRef]
- Tang, G.; Mei, D.; Zhao, X.; Zhao, C.; Li, L.; Yanjie, W. A comprehensive survey of ionic polymer metal composite transducers: Preparation, performance optimization and applications. Soft Sci. 2023, 3, 9. [Google Scholar]
- Zhang, Z.; Zhao, D.; Xu, Y.; Liu, S.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Wang, Z.; Wu, Y.; et al. A review on electrode materials of fast-charging lithium-ion batteries. Chem. Rec. 2022, 22, e202200127. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, J.; Zhang, Y. Recent advances in the design, fabrication, actuation mechanisms and applications of liquid crystal elastomers. Soft Sci. 2023, 3, 11. [Google Scholar] [CrossRef]
- Wang, X.; Qi, L.; Yang, H.; Rao, Y.; Chen, H. Stretchable synaptic transistors based on the field effect for flexible neuromorphic electronics. Soft Sci. 2023, 3, 15. [Google Scholar] [CrossRef]
- Wei, X.; Liang, X.; Meng, C.; Cao, S.; Shi, Q.; Wu, J. Multimodal electronic textiles for intelligent human-machine interfaces. Soft Sci. 2023, 3, 17. [Google Scholar] [CrossRef]
- Guess, M.; Soltis, I.; Rigo, B.; Zavanelli, N.; Kapasi, S.; Kim, H.; Yeo, W.-H. Wireless batteryless soft sensors for ambulatory cardiovascular health monitoring. Soft Sci. 2023, 3, 23. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Zhu, Z.; Cheng, X.B.; Zhu, J.; Lu, B.; Wu, Y. Heterostructures Regulating Lithium Polysulfides for Advanced Lithium-Sulfur Batteries. Adv. Mater. 2023, 35, 2303520. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.D.; Tang, X.; Feng, J.A.; Zhang, C.Y.; Liu, H.; Shi, C.; Zhao, X.X.; Song, J.J. Recent advances in vacancy engineering for reliable lithium-sulfur batteries. Rare Met. 2024, 43, 455–477. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, H.Y.; Liu, X.L.; Chen, Y.X.; Zhao, Y.; Zhang, Y.G.; Han, Q.Q.; Qin, C.L.; Bakenov, Z.; Wang, Y.C.; et al. Single Zn atoms anchored on hollow carbon nanofiber network for dendrite-free lithium metal anode of flexible Li-S full cell. Rare Met. 2023, 42, 3705–3717. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, S.; Yu, S.; Ren, J.; Zhang, Z.; Liu, S.; Liu, X.; Wang, Z.; Wu, Y.; Zhang, Y. Lychee seed-derived microporous carbon for high-performance sodium-sulfur batteries. Carbon 2023, 201, 864–870. [Google Scholar] [CrossRef]
- Rao, X.Y.; Xiang, S.F.; Zhou, J.; Zhang, Z.; Xu, X.Y.; Xu, Y.Y.; Zhou, X.C.; Pan, Z.D.; Tan, S.C.; Dong, S.X.; et al. Recent progress and strategies of cathodes toward polysulfides shuttle restriction for lithium-sulfur batteries. Rare Met. 2024, 43, 4132–4161. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, S.; Zhou, X.; Rao, X.; Xu, X.; Zhang, Z.; Pan, Z.; Wang, Q.C.; Wang, Z.; Wu, Y.; et al. Defect engineering enables an advanced separator modification for high-performance lithium-sulfur batteries. Chem. Eng. J. 2024, 487, 150574. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Hong, J.; Wang, X.; Huang, X. Boosting bidirectional conversion of polysulfide driven by the built-in electric field of MoS2/MoP Mott-Schottky heterostructures in lithium-sulfur batteries. J. Adv. Ceram. 2023, 12, 1872–1888. [Google Scholar] [CrossRef]
- Yang, W.H.; Ni, Z.C.; You, D.; Hou, J.Y.; Deng, B.N.; Huang, R.W.; Sun, S.G.; Zhao, J.B.; Li, X.; Zhang, Y.Y.; et al. Multifunctional sulfur-immobilizing GO/MXene aerogels for highly-stable and long-cycle-life lithium-sulfur batteries. Rare Met. 2023, 42, 2577–2591. [Google Scholar] [CrossRef]
- Wen, Q.; Qu, F.; Yu, Z.; Graczyk-Zajac, M.; Xiong, X.; Riedel, R. Si-based polymer-derived ceramics for energy conversion and storage. J. Adv. Ceram. 2022, 11, 197–246. [Google Scholar] [CrossRef]
- Liu, R.Q.; Jin, F.; Gu, M.; Zhang, D.W.; He, L.L.; Liu, W.X.; Zhu, W.F.; Xie, K.; Wu, J.Y.; Liu, Y.R.; et al. Titanium nitride nanorod array/carbon cloth as flexible integrated host for highly stable lithium–sulfur batteries. Rare Met. 2023, 42, 4115–4127. [Google Scholar] [CrossRef]
- Liu, J.; Wei, A.; Pan, G.; Xiong, Q.; Chen, F.; Shen, S.; Xia, X. Atomic layer deposition-assisted construction of binder-free Ni@ N-doped carbon nanospheres films as advanced host for sulfur cathode. Nano-Micro Lett. 2019, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiu, Y.; Liu, Y.; Xu, K.; Zhao, N.; Lao, C.; Shen, J.; Chen, Z. Novel 3D grid porous Li4Ti5O12 thick electrodes fabricated by 3D printing for high performance lithium-ion batteries. J. Adv. Ceram. 2022, 11, 295–307. [Google Scholar] [CrossRef]
- Gao, F.; Yue, X.A.; Xu, X.Y.; Xu, P.; Zhang, F.; Fan, H.S.; Wang, Z.L.; Wu, Y.T.; Liu, X.; Zhang, Y. A N/Co co-doped three-dimensional porous carbon as cathode host for advanced lithium–selenium batteries. Rare Met. 2023, 42, 2670–2678. [Google Scholar] [CrossRef]
- Yin, C.; Li, Z.; Zhao, D.; Yang, J.; Zhang, Y.; Du, Y.; Wang, Y. Azo-branched covalent organic framework thin films as active separators for superior sodium-sulfur batteries. ACS Nano 2022, 16, 14178–14187. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Cui, X.; Guan, S.; Tu, L.; Tang, H.; Li, Z.; Li, J. Understanding the advantageous features of bacterial cellulose-based separator in Li-S battery. Adv. Mater. Interfaces 2023, 10, 2201730. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhu, J.; Zhao, R.; Zhang, Y.; Ma, Y.; Li, C.; Zhang, H.; Chen, Y. A lithium sulfonylimide COF-modified separator for high-performance Li-S batteries. Nano Res. 2023, 16, 12601–12607. [Google Scholar] [CrossRef]
- Yan, W.; Gao, X.; Jin, X.; Liang, S.; Xiong, X.; Liu, Z.; Wang, Z.; Chen, Y.; Fu, L.; Zhang, Y.; et al. Nonporous gel electrolytes enable long cycling at high current density for lithium-metal anodes. ACS Appl. Mater. Interfaces 2021, 13, 14258–14266. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, L.; Xiang, S.; Yu, S.; Johnson, H.M.; Wang, S.; Yin, J.; Zhao, J.; Luo, Y.; Chu, P.K. Unleashing the potential of Mxene-based flexible materials for high-performance energy storage devices. Adv. Sci. 2024, 11, 2304874. [Google Scholar] [CrossRef]
- Pan, Z.; Yu, S.; Wang, L.; Li, C.; Meng, F.; Wang, N.; Zhou, S.; Xiong, Y.; Wang, Z.; Wu, Y.; et al. Recent advances in porous carbon materials as electrodes for supercapacitors. Nanomaterials 2023, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Jin, S.; Xia, Q.; Chang, Y.; Wang, L.; Zhou, A. Synthesis of Mo2C MXene with high electrochemical performance by alkali hydrothermal etching. J. Adv. Ceram. 2023, 12, 1889–1901. [Google Scholar] [CrossRef]
- Mou, J.; Li, S.; Zhang, W.; Xu, W.; Fan, S.; Bei, G. Deintercalation of Al from MoAlB by molten salt etching to achieve a Mo2AlB2 compound and 2D MoB nanosheets. J. Adv. Ceram. 2023, 12, 943–953. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Y.; Liu, L.; Liu, X.; Wang, Z.; Zhang, Y. Sulfur nanoparticles/Ti3C2Tx MXene with an optimum sulfur content as a cathode for highly stable lithium-sulfur batteries. Dalton Trans. 2021, 50, 5574–5581. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, W.; Chen, M.; Zhong, X.; Wu, X.; Zhang, H.; Yu, Y. Boosting the electrochemical performance of Li-S batteries with a dual polysulfides confinement strategy. Small 2018, 14, 1802516. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Zhang, Y.; Zhang, Y.; Wu, Y.; Tian, M.; Chen, P.; Trout, R.; Ma, Y.; Wu, T.H.; et al. A safe and fast-charging lithium-ion battery anode using MXene supported Li3VO4. J. Mater. Chem. 2019, 7, 11250–11256. [Google Scholar] [CrossRef]
- Fang, B.; Tian, X.; Wang, T.; Wang, T.; Qu, L.; Li, M. Restraining Polysulfide with High-Entropy Metal Nitride towards Long Cycle Life and High Capacity Li-S Batteries. ChemElectroChem 2020, 7, 4737–4744. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Rui, K.; Wu, X.; Wu, M.; Zhang, J. Enhanced performance of lithium sulfur battery with polypyrrole warped mesoporous carbon/sulfur composite. J. Power Sources 2014, 254, 353–359. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, W.; Zhao, W.; Li, G.; Hou, Y.; Liu, M.; Zhou, L.; Ye, F.; Li, H.; Wei, Z.; et al. High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, J.; Dong, Z.; Liu, Y.; Wu, Y.; Xu, C.; Du, G. Biomass derived activated carbon with 3D connected architecture for rechargeable lithium−sulfur batteries. Electrochim. Acta 2014, 116, 146–151. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Jiang, X.; Xia, Y.; Wang, H.; Zhang, R.; Li, H.; Fan, B.; Zhou, Y. Construction of hydrangea-like core–shell SiO2@Ti3C2Tx@CoNi microspheres for tunable electromagnetic wave absorbers. J. Adv. Ceram. 2023, 12, 711–723. [Google Scholar] [CrossRef]
- Cai, Z.; Ma, Y.F.; Wang, M.; Qian, A.-N.; Tong, Z.; Xiao, L.; Jia, S.; Chen, X. Engineering of electrolyte ion channels in MXene/holey graphene electrodes for superior supercapacitive performances. Rare Met. 2022, 41, 2084–2093. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Deng, S.L.; Sun, X.Z.; Li, B.; Chen, J. Progress of symmetrical solid oxide fuel cell. Adv. Ceram. 2023, 44, 129–141. [Google Scholar]
- Xu, H.; Zheng, R.; Du, D.; Ren, L.; Wen, X.; Wang, X.; Tian, G.; Shu, C. Adjusting the 3d Orbital Occupation of Ti in Ti3C2 MXene via Nitrogen Doping to Boost Oxygen Electrode Reactions in Li-O2 Battery. Small 2023, 19, 2206611. [Google Scholar] [CrossRef]
- Liu, W.; Cao, J.; Song, F.; Zhang, D.D.; Okhawilai, M.; Yi, J.; Qin, J.Q.; Zhang, X.Y. A double transition metal Ti2NbC2Tx MXene for enhanced lithium-ion storage. Rare Met. 2023, 42, 100–110. [Google Scholar] [CrossRef]
- Jiang, T. Fabrication technology, research and development status, development trend of the ternary laminated structure MAX phase ceramics materials. Adv. Ceram. 2023, 44, 1–23. [Google Scholar]
- Sun, Z.; Hu, Y.; Zhang, J.; Zhou, N.; Li, M.; Liu, H.; Huo, B.; Chao, M.; Zeng, K. Interfacial oxygen bridge bonding with Mo-O-Ti units in MoOx@ Ti3C2 MXene harness efficient Li-O2 Battery at high rate. Appl. Catal. Environ. Energy 2024, 351, 123984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).