Safety Aspects of Sodium-Ion Batteries: Prospective Analysis from First Generation Towards More Advanced Systems
Abstract
1. Introduction
| Materials | Potential Range | Practical Capacity (mAh g−1) | Sources |
|---|---|---|---|
| Cathode: | |||
| Li materials | vs. Li/Li+ | ||
| Doped LiCoO2 | 3.0–4.5 V | 190 | [91] |
| LiNiO2 | 3.0–4.3 V | 231 | [92] |
| LiMnO2 | 3.0–4.3 V | 210 | [93] |
| LiNi0.33Mn0.33Co0.33O2 | 3.0–4.3 V | 164 | [94] |
| LiMn2O4 | 3.8–4.3 V | 140 | [95] |
| LiFePO4 | ≈3.45 V | 150 | [96] |
| Na materials | vs. Na/Na+ | ||
| Na3V2(PO4)2F3 | 2.0–4.2 V | 120 | [58] |
| NaFeO2 | ≈3.3 V | 103 | [97] |
| Na2FeP2O7 | 2.0–4.0 V | 90 | [98] |
| Na3.32Fe2.11Ca0.23(P2O7)2 | 2.2–4.0 V | 100 | [99] |
| Na0.69CoO2 | Cutoff voltages: 4.3 V (Na0.12CoO2), 4.1 V (Na0.24CoO2), 3.5 V (Na0.52CoO2) | 147 (Na0.12CoO2), 116 (Na0.24CoO2), 49 (Na0.52CoO2) | [100] |
| Na0.44MnO2 | 2.0–4.0 V | 108 | [101] |
| NaNi0.33Fe0.33Mn0.33O2 | 2.0–4.3 V | 165 | [102] |
| Na0.9[Cu0.22Fe0.30Mn0.48]O2 | 1.0–4.0 V | 100 | [103] |
| Na[Ni0.6Co0.2Mn0.2]O2 | 2.0–4.1 V | 153 | [104] |
| NaxMnyFe(CN)6·nH2O | 3.3–3.8 V | 67 | [70] |
| Anode: | |||
| Li materials | vs. Li+/Li | ||
| Graphite | <0.4 V | 348 | [105] |
| Li4Ti5O12 | ≈1.5 V | 160 | [106] |
| Na materials | vs. Na/Na+ | ||
| Hard carbon | 0–1.5 V | 361 | [107] |
| NaxMnyMn(CN)6·nH2O | 1.7–2.5 V | 68 | [70] |
| Company | Active Materials | Salient Features |
|---|---|---|
| Contemporary Amperex Technology Co., Ltd. (CATL), Ningde, China | Prussian white analogues||HC | 160 Wh/kg, charge in 15 min to 80% SOC at RT, capacity retention > 90% at −20 °C, thermal stability fulfills national safety requirement for traction batteries [114]. |
| Faradion Limited, Sheffield, UK | Layered oxide Materials (NaaNi(1-x-y-z)MnxMgyTizO2)||HC | 160 Wh/kg in 32 Ah pouch cells, cycle life between 2300–3000 cycles at 78% DOD [115], possibility of zero volt discharge (negative voltages) [116], no flame or ignition detected during nail penetration tests [117]. |
| HiNa Battery Technology Co., Ltd., Liyang, China | Layered oxide (NaCu1-y-zFeyMnzO2)||Soft carbon | 140–155 Wh/kg, 4500 cycles at 83% DOD (2C/2D), fulfills Chinese national standard GB/T31845-2015 [118], covering thermal, mechanical, and electrical abuse [119,120]. |
| Natron Energy, Santa Clara, CA, USA | PBA (NaxMnyFe(CN)6.nH2O)||PBA (NaxMnyMn(CN)6.nH2O) | Zero strain during charge/discharge from −20 to 50 °C, over 50,000 cycles (23 Wh/kg at cell level and 10.3 Wh/kg at module level), no fire or explosion detected during mechanical and electrical mishandling [70,121,122]. |
| TIAMAT, Amiens, France | Polyanionic material (Na3V2(PO4)2F3)||HC | 100–120 Wh/kg, 10,000 cycles at 2C/5D, no thermal runaway observed during overheating, overcharging, nail penetration test, and short circuits [123]. |
| Novasis Energies, Vancouver, WA, USA | PBA (NaxMnFe(CN)6.nH2O)||HC | 100–130 Wh/kg, nail penetration in fully charged cell causes temperature increase to 100 °C but no ignition was detected. On overcharging, the cells swell and temperature increases to 90 °C with no serious safety concerns [124]. |
| Altris AB, Uppsala, Sweden | Prussian white analogues||HC | 160 Wh/kg, NaBOB salt-based electrolyte patented by Altris which is supposedly fire-resistant and complements PW cathode to improve electrochemical performance and safety [125,126]. |
2. Lessons to Be Learnt from LiBs to Develop Thermally Resilient SEI Layer in SiBs
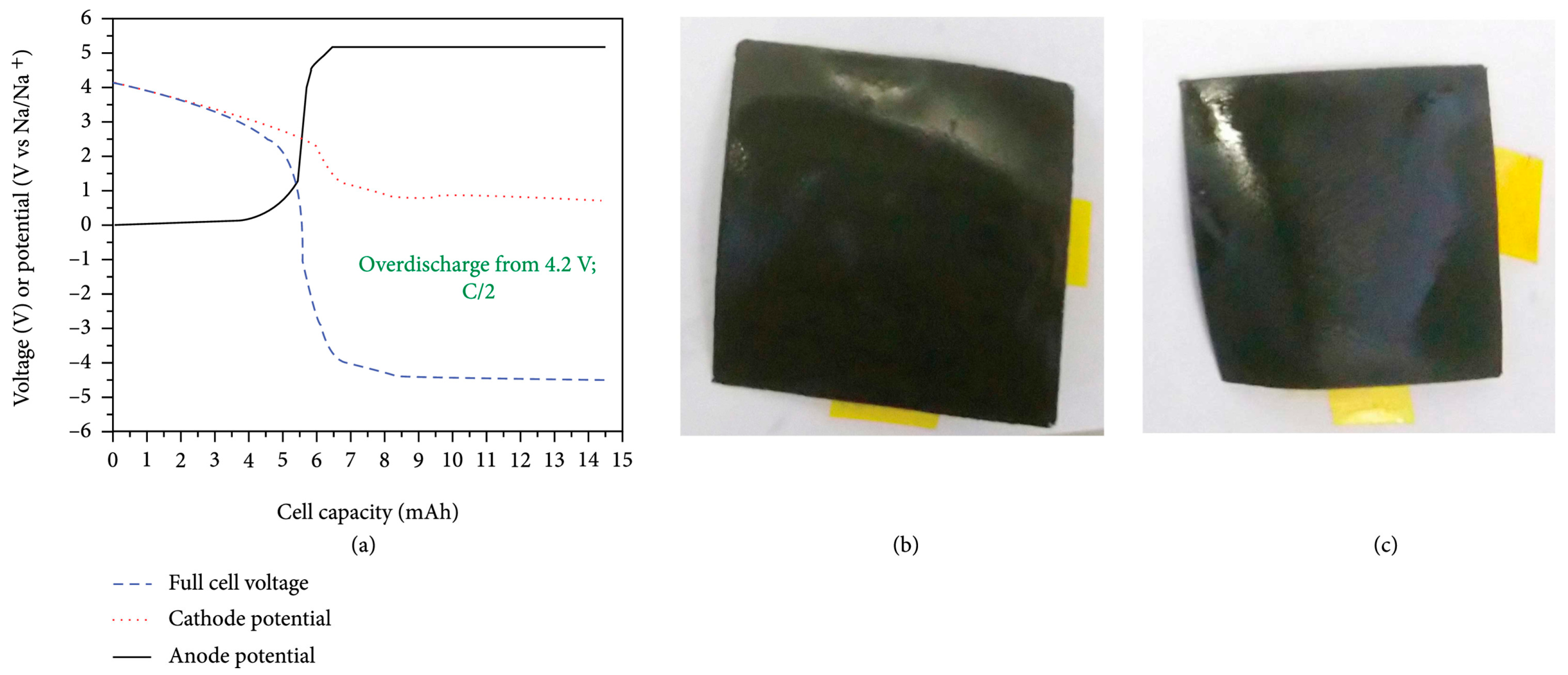
3. Is Zero-Volt Storage Possible for SiBs and What Are the Added Safety Gains?
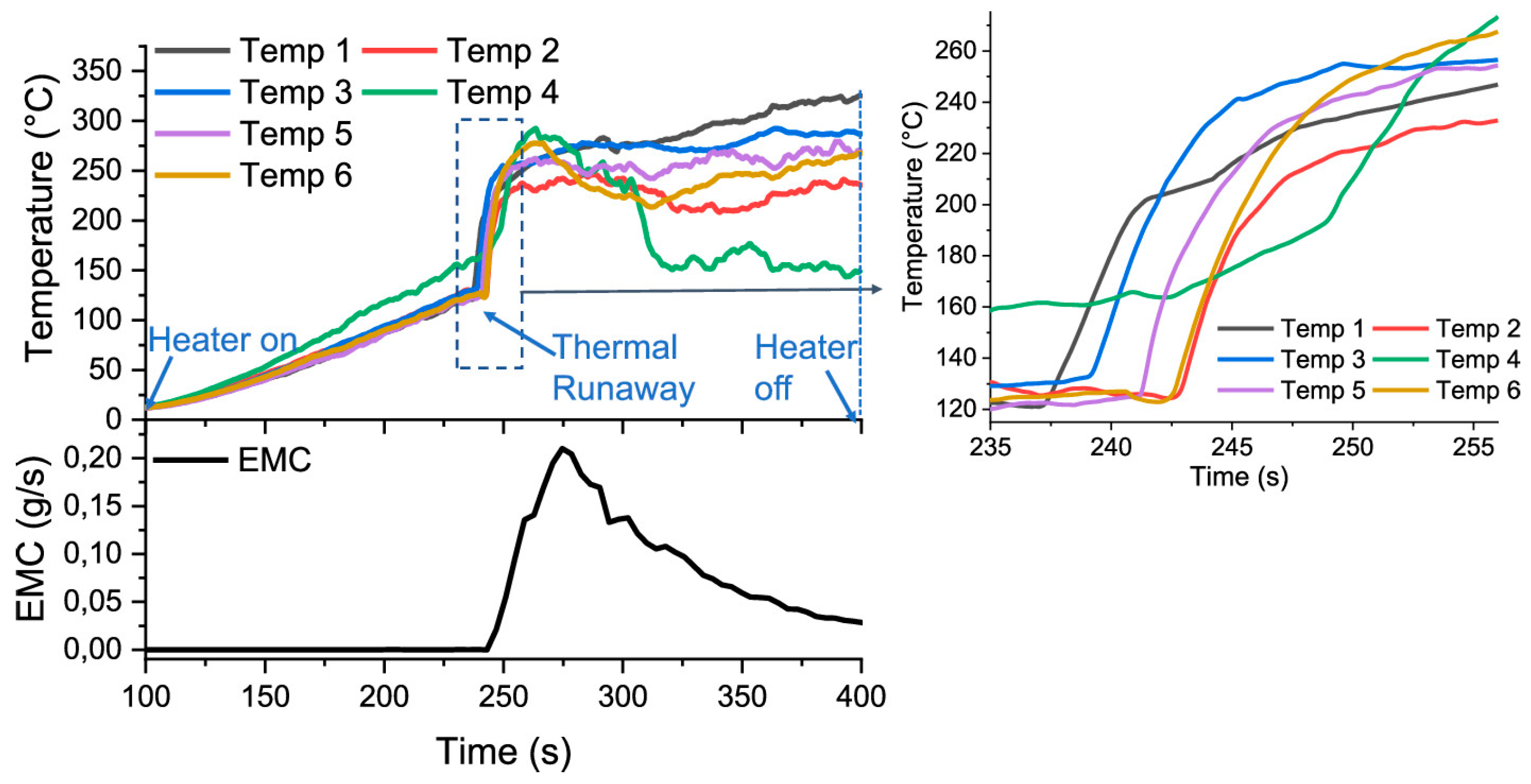
4. Comparative Thermal Studies of SiBs Versus LiBs
4.1. Component Level
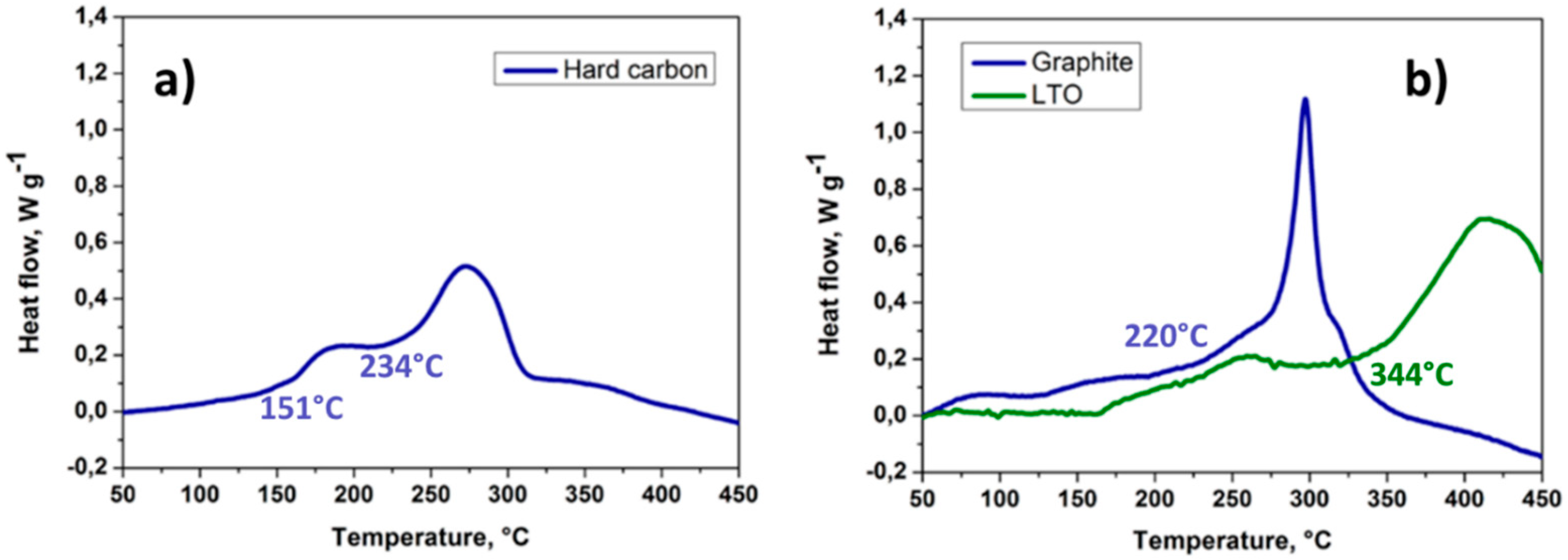
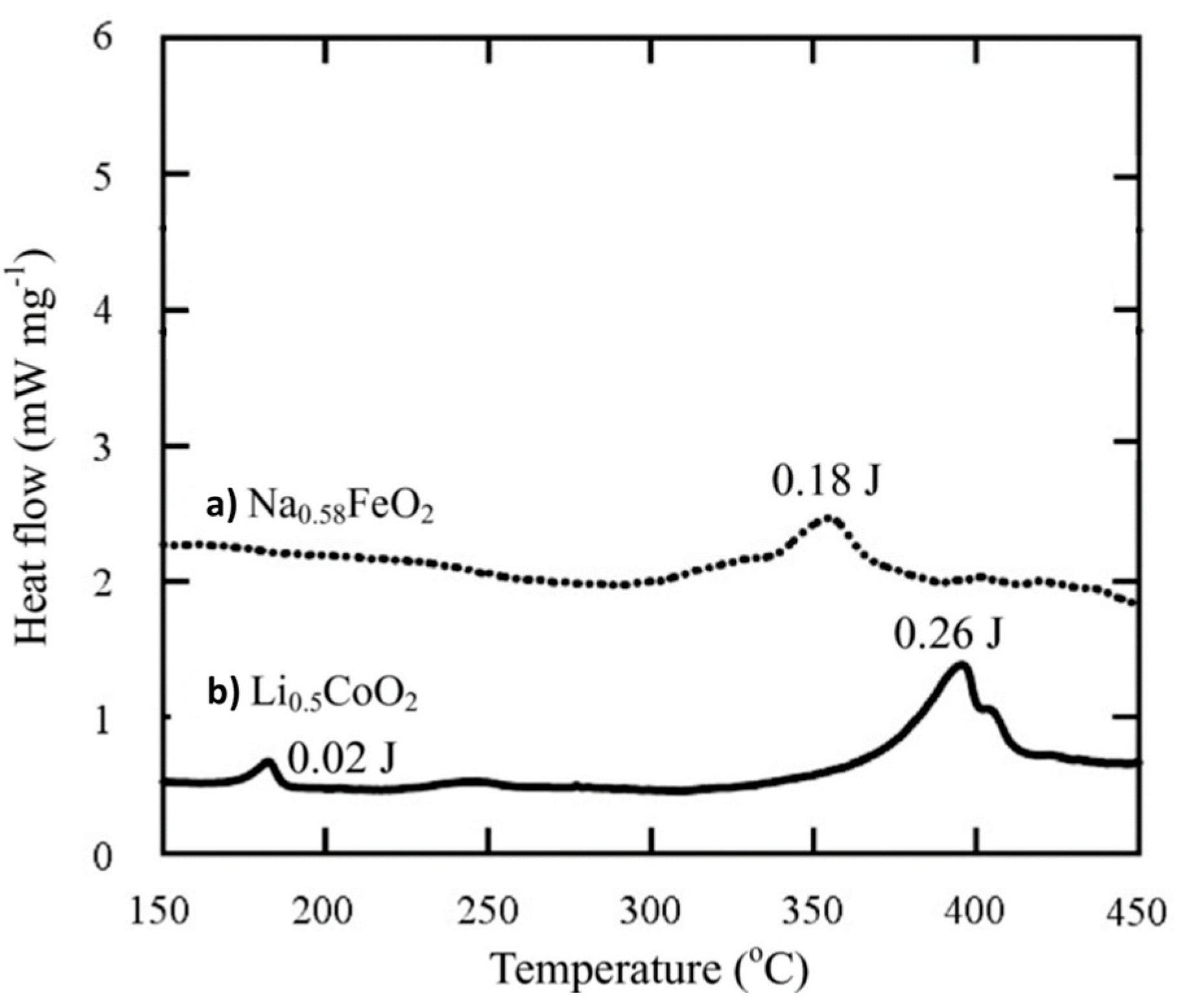
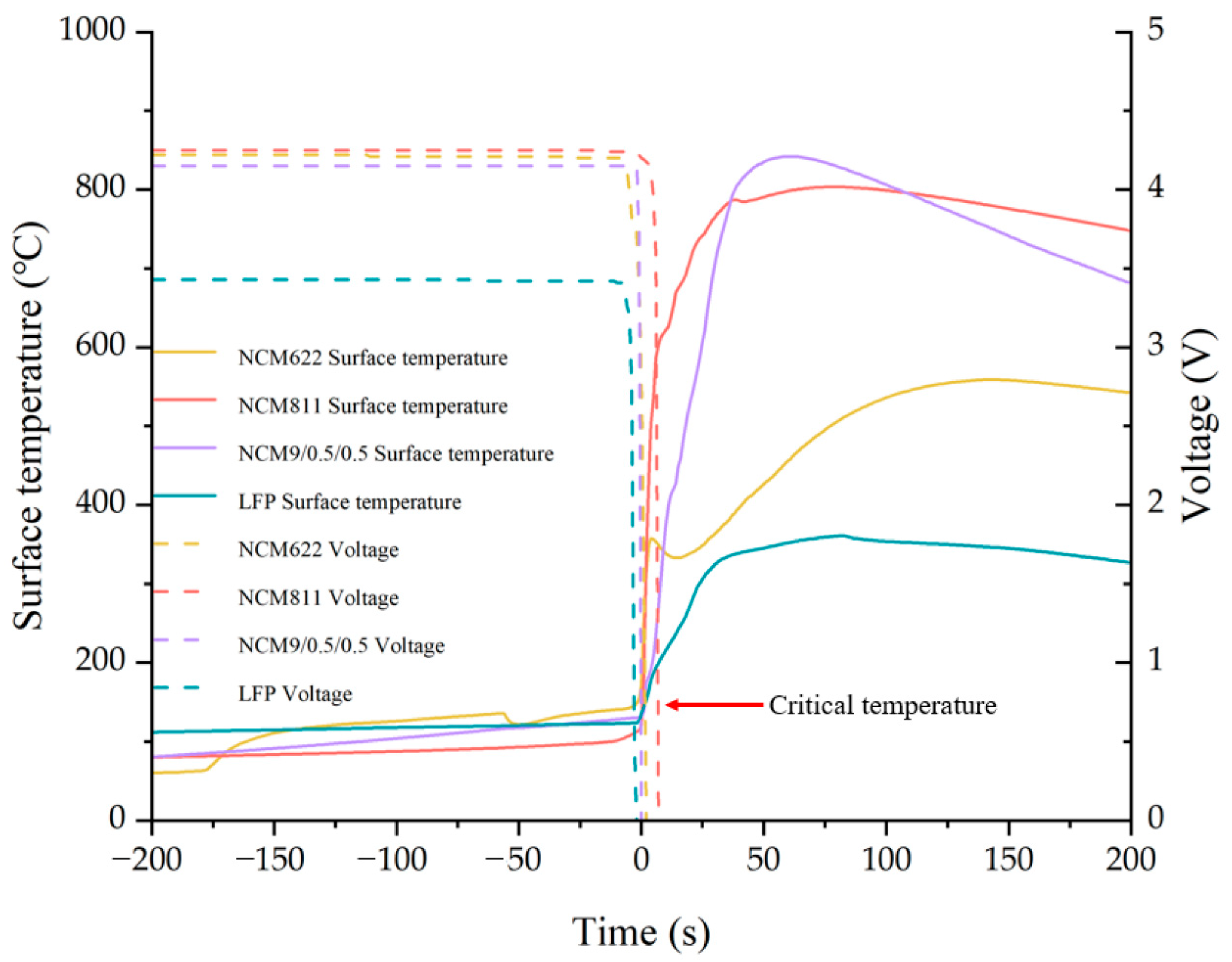
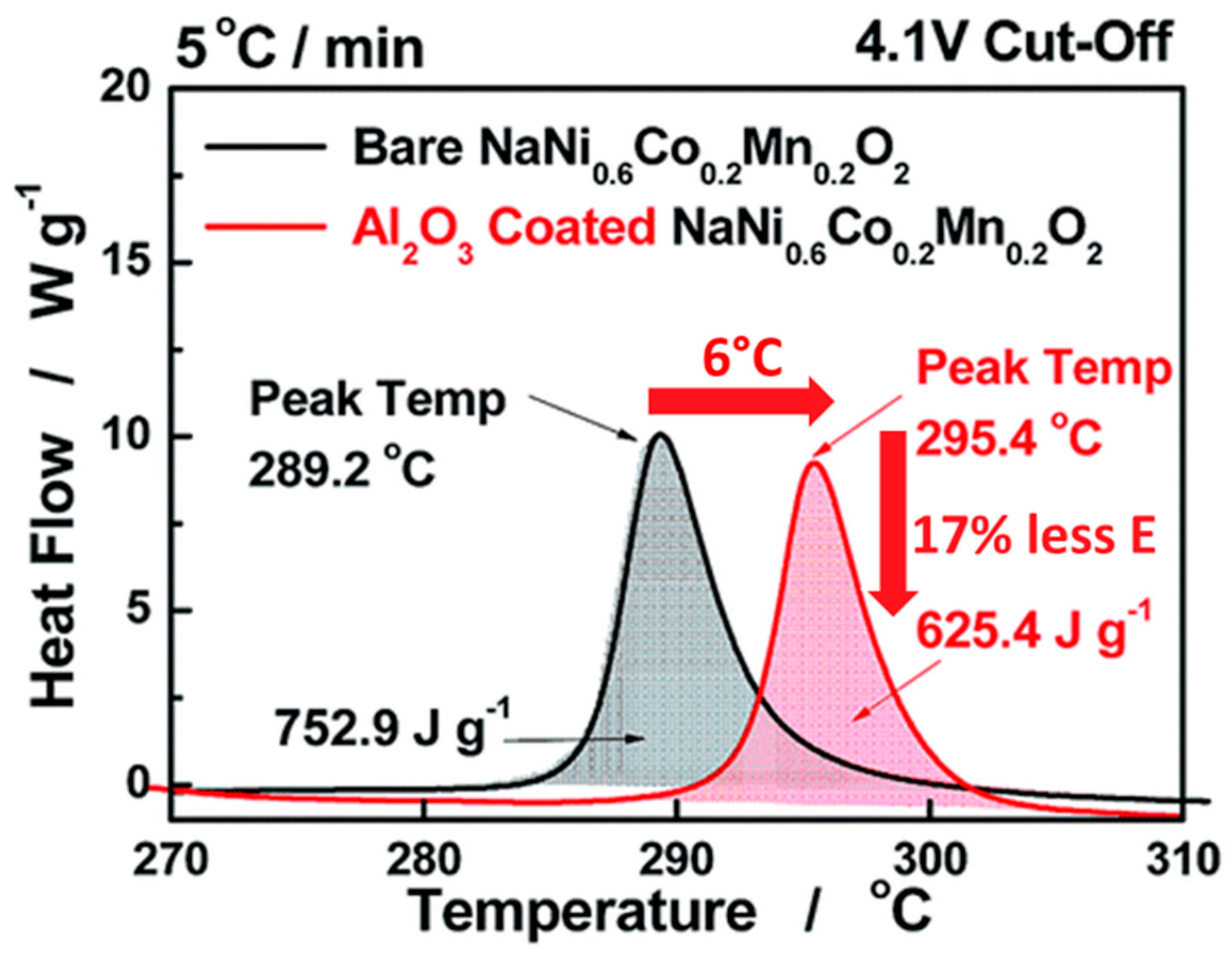
4.1.1. Cathode Material
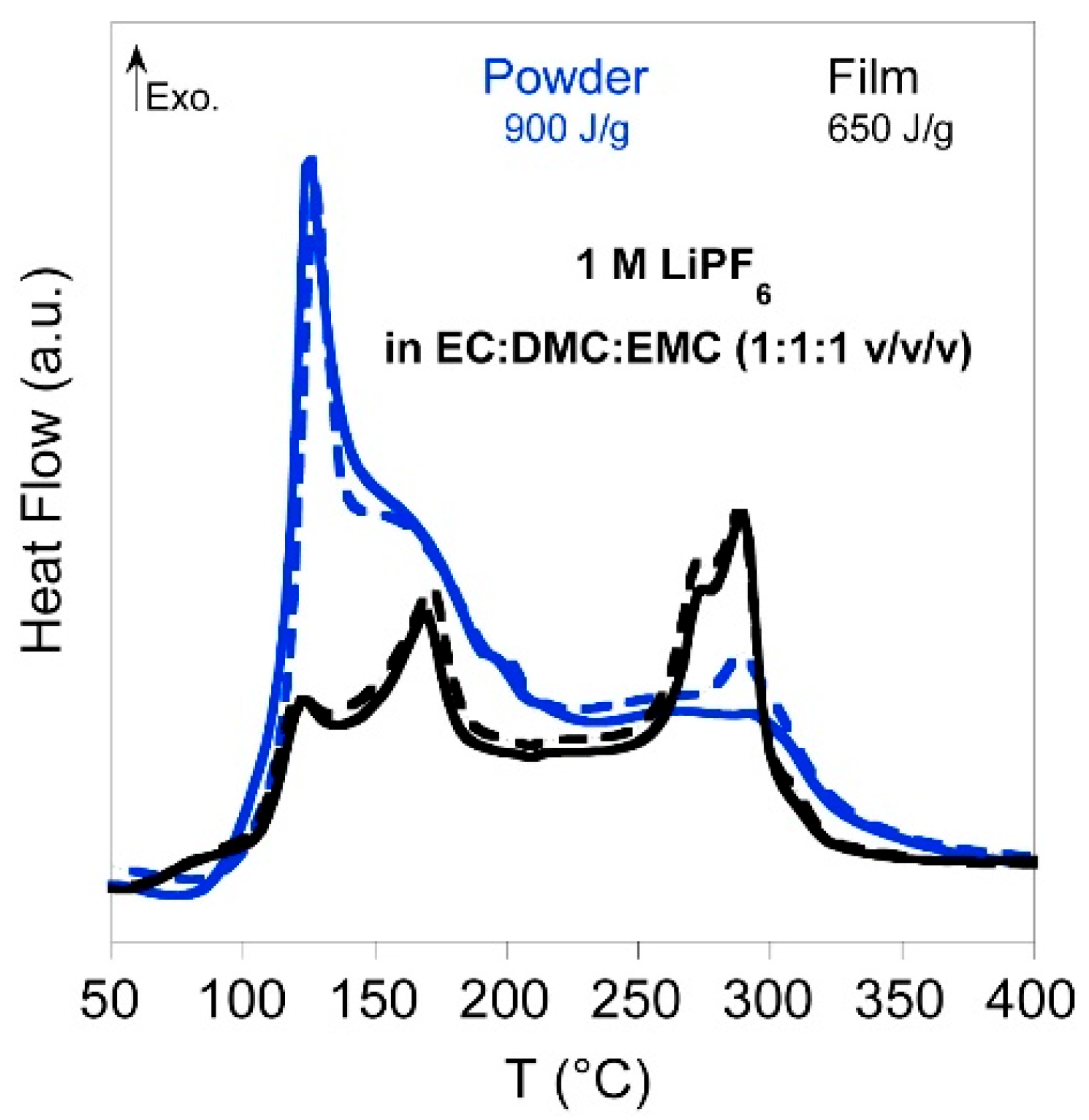
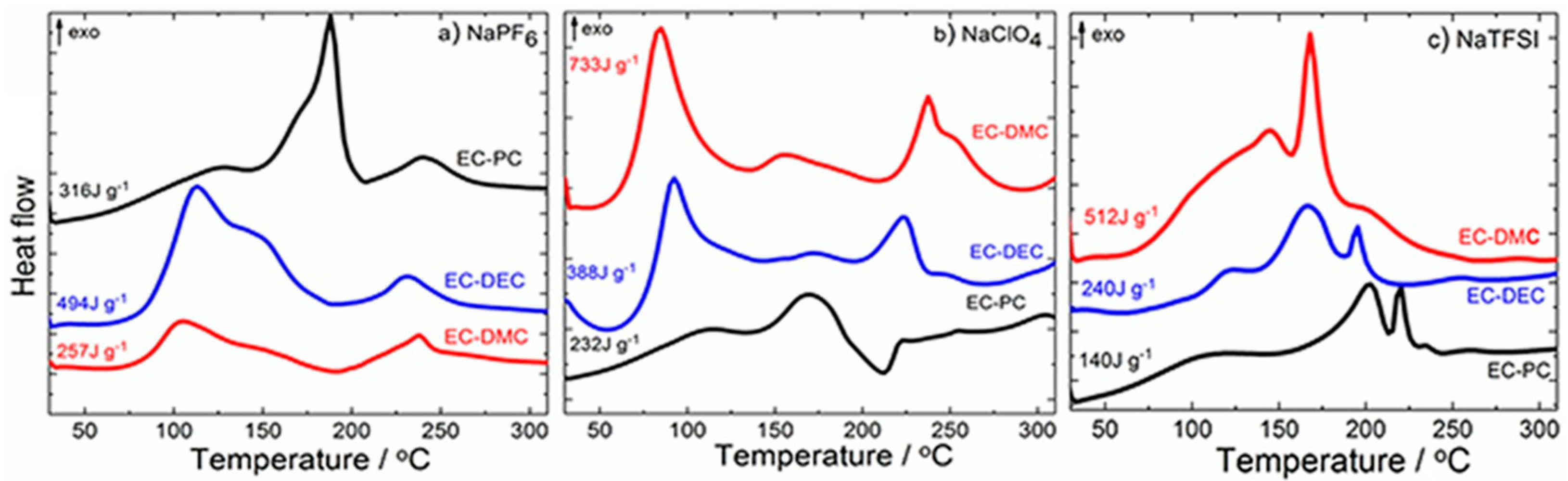
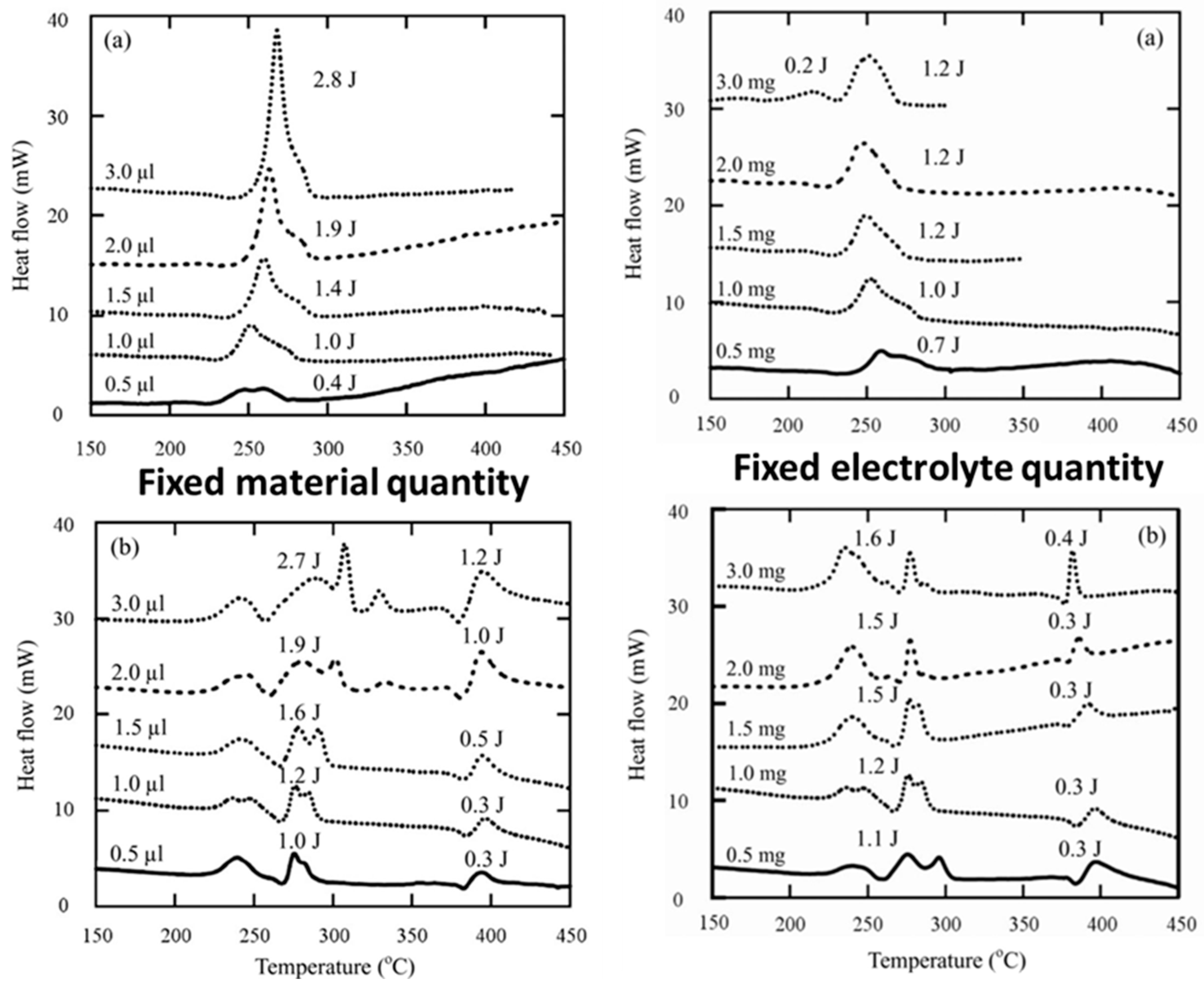
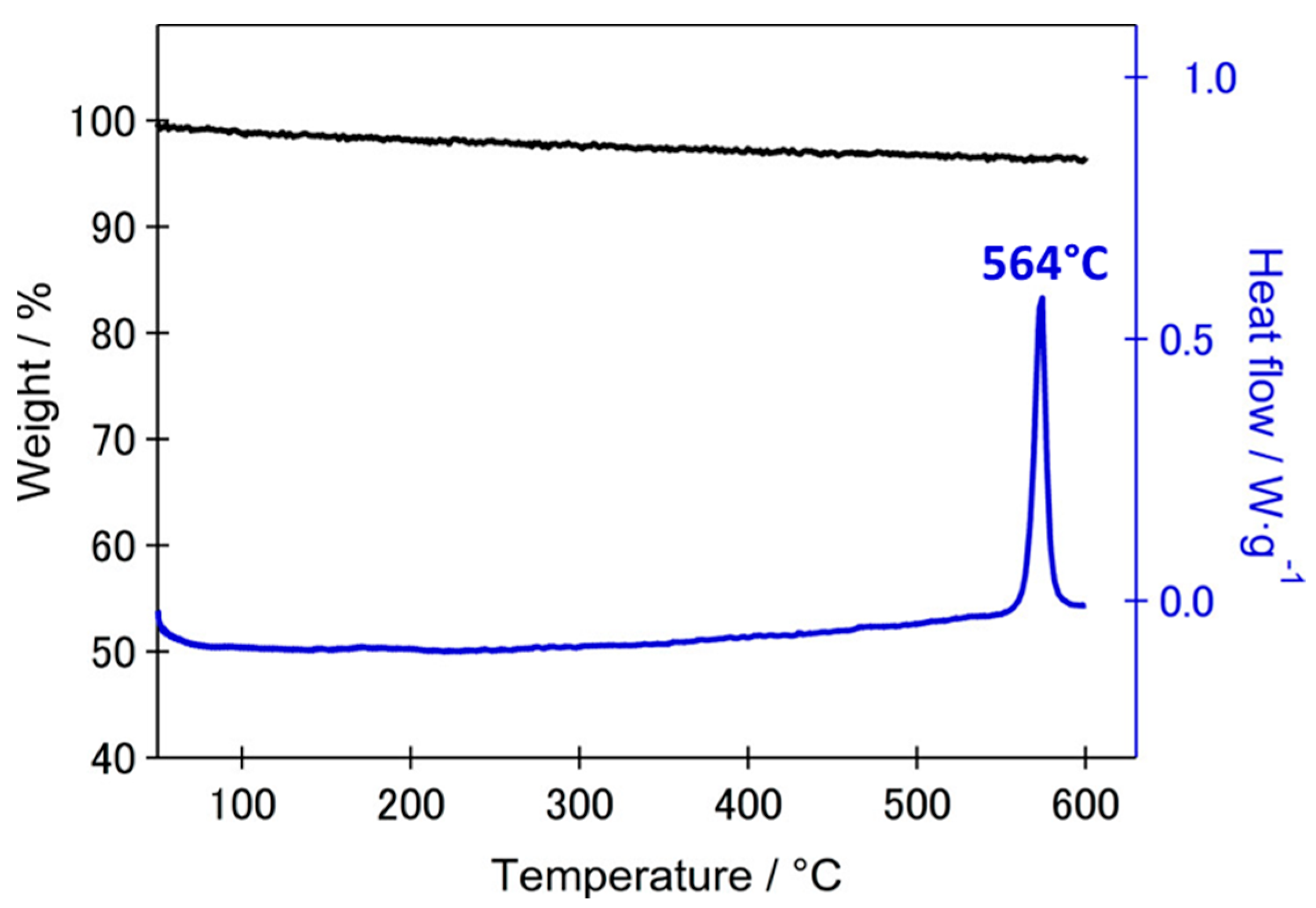
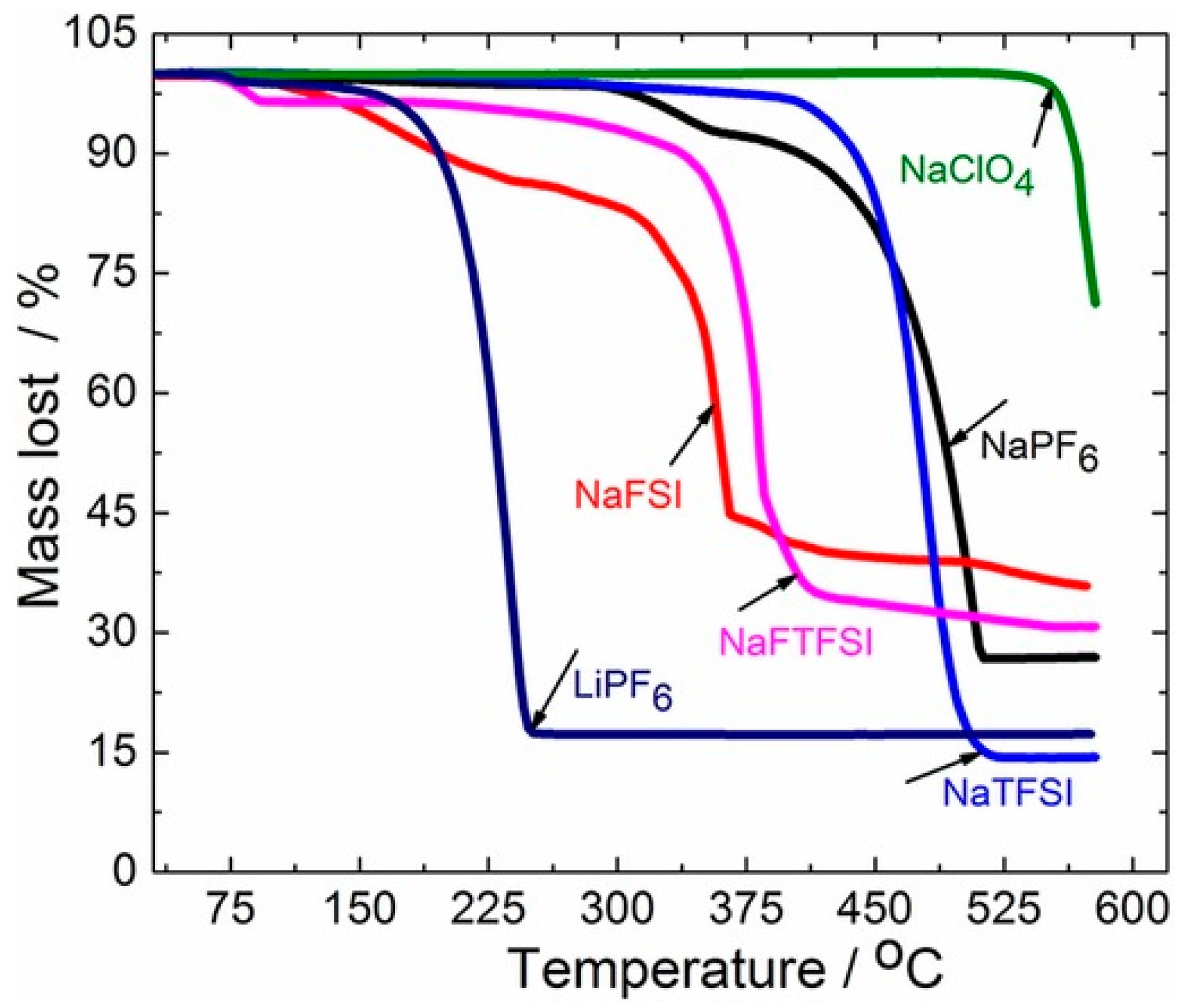
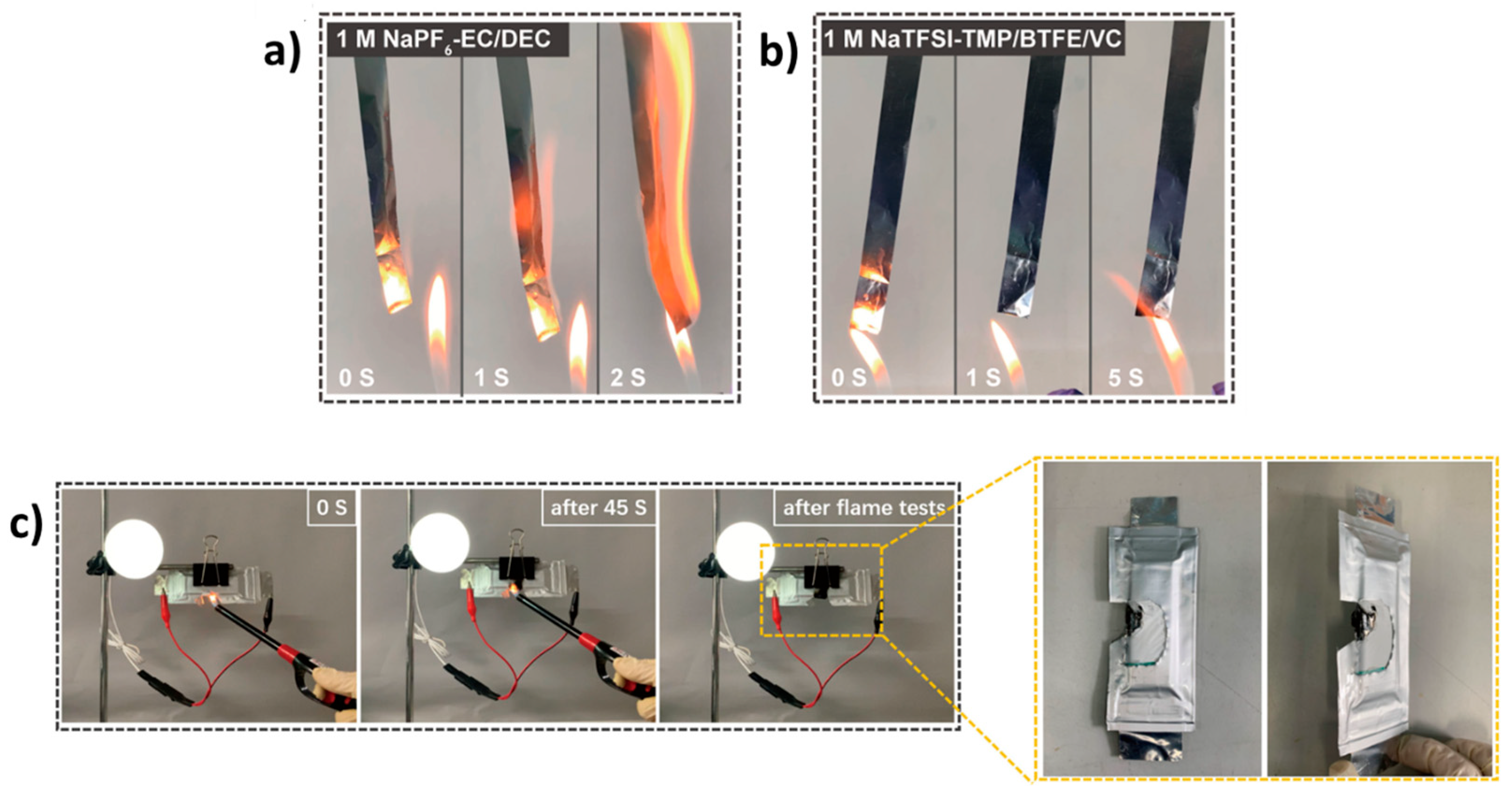
4.1.2. Electrolytes
4.2. Full Cell Level
5. Role of the Separator in Battery Safety
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of Current Development in Electrical Energy Storage Technologies and the Application Potential in Power System Operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Kurzweil, P. Gaston Planté and His Invention of the Lead–Acid Battery—The Genesis of the First Practical Rechargeable Battery. J. Power Sources 2010, 195, 4424–4434. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Sun, X.; Yang, D.; Gao, L.; Liu, J.; Kumar, R.V.; Yang, J. Preparation of Basic Lead Oxide from Spent Lead Acid Battery Paste via Chemical Conversion. Hydrometallurgy 2012, 117–118, 24–31. [Google Scholar] [CrossRef]
- Fire Hazard Assessment of Lead-Acid Batteries. Available online: https://www.nfpa.org/education-and-research/research/fire-protection-research-foundation/projects-and-reports/fire-hazard-assessment-of-lead-acid-batteries (accessed on 3 October 2024).
- Zhang, J.; Chen, C.; Zhang, X.; Liu, S. Study on the Environmental Risk Assessment of Lead-Acid Batteries. Procedia Environ. Sci. 2016, 31, 873–879. [Google Scholar] [CrossRef]
- World Health Organization. Recycling Used Lead-Acid Batteries: Health Considerations. World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151285-5. Available online: https://iris.who.int/handle/10665/259447 (accessed on 3 October 2024).
- Bernard, P. Secondary Batteries—Nickel Systems|Nickel–Cadmium: Sealed. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 459–481. ISBN 978-0-444-52745-5. Available online: https://linkinghub.elsevier.com/retrieve/pii/b9780444527455001544 (accessed on 3 October 2024).
- EU Bans Nickel-Cadmium Batteries in Portable Devices by 2025: Impact on Emergency Lighting. Available online: https://www.etaplighting.com/en/blog/end-of-cadmium-batteries-portable-applications (accessed on 16 April 2024).
- Maxianova, K.; Rusche, T.M. Restriction of Hazardous Substances: On the Need for and the Limits of Comitology. Rev. Eur. Community Int. Environ. Law 2006, 15, 202–210. [Google Scholar] [CrossRef]
- Bernard, P.; Lippert, M. Nickel–Cadmium and Nickel–Metal Hydride Battery Energy Storage. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 223–251. ISBN 978-0-444-62616-5. Available online: https://linkinghub.elsevier.com/retrieve/pii/b9780444626165000140 (accessed on 3 October 2024).
- Ikoma, M.; Yuasa, S.; Yuasa, K.; Kaida, S.; Matsumoto, I.; Iwakura, C. Charge Characteristics of Sealed-Type Nickel/Metal-Hydride Battery. J. Alloys Compd. 1998, 267, 252–256. [Google Scholar] [CrossRef]
- Winn, D.A.; Shemilt, J.M.; Steele, B.C.H. Titanium Disulphide: A Solid Solution Electrode for Sodium and Lithium. Mater. Res. Bull. 1976, 11, 559–566. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Cairns, E.J.; Shimotake, H. High-Temperature Batteries: Research in High-Temperature Electrochemistry Reveals Compact, Powerful Energy-Storage Cells. Science 1969, 164, 1347–1355. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium Ion Secondary Batteries; Past 10 Years and the Future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Forestier, C.; Grugeon, S.; Davoisne, C.; Lecocq, A.; Marlair, G.; Armand, M.; Sannier, L.; Laruelle, S. Graphite Electrode Thermal Behavior and Solid Electrolyte Interphase Investigations: Role of State-of-the-Art Binders, Carbonate Additives and Lithium Bis(Fluorosulfonyl)Imide Salt. J. Power Sources 2016, 330, 186–194. [Google Scholar] [CrossRef]
- Kim, G.-H.; Pesaran, A.; Spotnitz, R. A Three-Dimensional Thermal Abuse Model for Lithium-Ion Cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse Behavior of High-Power, Lithium-Ion Cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Nie, B.; Dong, Y.; Chang, L. The Evolution of Thermal Runaway Parameters of Lithium-Ion Batteries under Different Abuse Conditions: A Review. J. Energy Storage 2024, 96, 112624. [Google Scholar] [CrossRef]
- Tobishima, S.; Yamaki, J. A Consideration of Lithium Cell Safety. J. Power Sources 1999, 81–82, 882–886. [Google Scholar] [CrossRef]
- Tobishima, S.; Takei, K.; Sakurai, Y.; Yamaki, J. Lithium Ion Cell Safety. J. Power Sources 2000, 90, 188–195. [Google Scholar] [CrossRef]
- Kitoh, K.; Nemoto, H. 100 Wh Large Size Li-Ion Batteries and Safety Tests. J. Power Sources 1999, 81–82, 887–890. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J.; et al. An Overview on the Advances of LiCoO2 Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.J.; Park, B. Novel LiCoO 2 Cathode Material with Al2O3 Coating for a Li Ion Cell. Chem. Mater. 2000, 12, 3788–3791. [Google Scholar] [CrossRef]
- Jo, M.; Hong, Y.-S.; Choo, J.; Cho, J. Effect of LiCoO2 Cathode Nanoparticle Size on High Rate Performance for Li-Ion Batteries. J. Electrochem. Soc. 2009, 156, A430. [Google Scholar] [CrossRef]
- Ahangari, M.; Szalai, B.; Lujan, J.; Zhou, M.; Luo, H. Advancements and Challenges in High-Capacity Ni-Rich Cathode Materials for Lithium-Ion Batteries. Materials 2024, 17, 801. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing Principle for Ni-Rich Cathode Materials with High Energy Density for Practical Applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and Their Origins of Ni-Rich Layered Oxide Cathode Materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kunnikuruvan, S.; Kumar, S.; Markovsky, B.; Aurbach, D.; Dixit, M.; Major, D.T. Layered Cathode Materials for Lithium-Ion Batteries: Review of Computational Studies on LiNi1–x–yCoxMnyO2 and LiNi1–x–yCoxAl yO 2. Chem. Mater. 2020, 32, 915–952. [Google Scholar] [CrossRef]
- Vitins, G.; West, K. Lithium Intercalation into Layered LiMnO2. J. Electrochem. Soc. 1997, 144, 2587–2592. [Google Scholar] [CrossRef]
- Berg, H.; Göransson, K.; Noläng, B.; Thomas, J.O. Electronic Structure and Stability of the LixMn2O4 (0 < x < 2) System. J. Mater. Chem. 1999, 9, 2813–2820. [Google Scholar] [CrossRef]
- Cheruku, R.; Kruthika, G.; Govindaraj, G.; Vijayan, L. Electrical Relaxation Studies of Olivine Type Nanocrystalline LiMPO4 (M=Ni, Mn and Co) Materials. J. Phys. Chem. Solids 2015, 86, 27–35. [Google Scholar] [CrossRef]
- Manthiram, A. A Reflection on Lithium-Ion Battery Cathode Chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Benoit, C.; Franger, S. Chemistry and Electrochemistry of Lithium Iron Phosphate. J. Solid. State Electrochem. 2008, 12, 987–993. [Google Scholar] [CrossRef]
- Dahn, J.; Fuller, E.; Obrovac, M.; Vonsacken, U. Thermal Stability of LixCoO2, LixNiO2 and λ-MnO2 and Consequences for the Safety of Li-Ion Cells. Solid. State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Rumble, C.; Conry, T.E.; Doeff, M.; Cairns, E.J.; Penner-Hahn, J.E.; Deb, A. Structural and Electrochemical Investigation of Li(Ni0.4Co0.15Al0.05Mn0.4)O2 Cathode Material. J. Electrochem. Soc. 2010, 157, A1317. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, R.; Xu, X.-M.; Zeng, C.-S.; Shen, X.-B.; Gu, Y.-J. The Different Roles of Ni2+/Ni3+, Ni3+/Ni4+, and Mn3+/Mn4+ in Li-Rich Layered Nanostructured LiNi Mn1-O2·Li2MnO3 with High Capacity. Int. J. Electrochem. Sci. 2021, 16, 210425. [Google Scholar] [CrossRef]
- Schafzahl, L.; Mahne, N.; Schafzahl, B.; Wilkening, M.; Slugovc, C.; Borisov, S.M.; Freunberger, S.A. Singlet Oxygen during Cycling of the Aprotic Sodium-O2 Battery. Angew. Chem. Int. Ed. Engl. 2017, 56, 15728–15732. [Google Scholar] [CrossRef]
- Kalyani, P.; Kalaiselvi, N. Various Aspects of LiNiO2 Chemistry: A Review. Sci. Technol. Adv. Mater. 2005, 6, 689–703. [Google Scholar] [CrossRef]
- Salomez, B.; Grugeon, S.; Armand, M.; Tran-Van, P.; Laruelle, S. Review—Gassing Mechanisms in Lithium-Ion Battery. J. Electrochem. Soc. 2023, 170, 050537. [Google Scholar] [CrossRef]
- Kebede, M.A.; Ezema, F.I. (Eds.) Electrochemical Devices for Energy Storage Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-367-85511-6. Available online: https://www.taylorfrancis.com/books/9781000763799 (accessed on 3 October 2024).
- Mao, N.; Gadkari, S.; Wang, Z.; Zhang, T.; Bai, J.; Cai, Q. A Comparative Analysis of Lithium-Ion Batteries with Different Cathodes under Overheating and Nail Penetration Conditions. Energy 2023, 278, 128027. [Google Scholar] [CrossRef]
- Brand, M.; Gläser, S.; Geder, J.; Menacher, S.; Obpacher, S.; Jossen, A.; Quinger, D. Electrical Safety of Commercial Li-Ion Cells Based on NMC and NCA Technology Compared to LFP Technology. World Electr. Veh. J. 2013, 6, 572–580. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Resendiz, E.G.; Nwophoke, S.M.; Khanna, S.; James, C.; Brown, S.F. Review of Gas Emissions from Lithium-Ion Battery Thermal Runaway Failure—Considering Toxic and Flammable Compounds. J. Energy Storage 2024, 87, 111288. [Google Scholar] [CrossRef]
- Azuma, H.; Imoto, H.; Yamada, S.; Sekai, K. Advanced Carbon Anode Materials for Lithium Ion Cells. J. Power Sources 1999, 81–82, 1–7. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material—Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-Based Materials for Fast Charging Lithium-Ion Batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Yoon, T.; Milien, M.S.; Parimalam, B.S.; Lucht, B.L. Thermal Decomposition of the Solid Electrolyte Interphase (SEI) on Silicon Electrodes for Lithium Ion Batteries. Chem. Mater. 2017, 29, 3237–3245. [Google Scholar] [CrossRef]
- Sick, N.; Krätzig, O.; Eshetu, G.G.; Figgemeier, E. A Review of the Publication and Patent Landscape of Anode Materials for Lithium Ion Batteries. J. Energy Storage 2021, 43, 103231. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Qilu; Duo, X.; Sheng, X. A Review of Spinel Lithium Titanate (Li4Ti5O12) as Electrode Material for Advanced Energy Storage Devices. Ceram. Int. 2021, 47, 5870–5895. [Google Scholar] [CrossRef]
- Belharouak, I.; Koenig, G.M.; Amine, K. Electrochemistry and Safety of Li4Ti5O12 and Graphite Anodes Paired with LiMn2O4 for Hybrid Electric Vehicle Li-Ion Battery Applications. J. Power Sources 2011, 196, 10344–10350. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- PV magazine International Sodium-Ion Batteries—A Viable Alternative to Lithium? Available online: https://www.pv-magazine.com/2024/03/22/sodium-ion-batteries-a-viable-alternative-to-lithium/ (accessed on 23 September 2024).
- Lithium ion Battery Manufacturer and Supplier in China-DNK Power Will Sodium Batteries Replace Lithium Batteries? Available online: https://www.dnkpower.com/will-sodium-batteries-replace-lithium-batteries/ (accessed on 23 September 2024).
- Desai, P.; Forero-Saboya, J.; Meunier, V.; Rousse, G.; Deschamps, M.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. Mastering the Synergy between Na3V2(PO4)2F3 Electrode and Electrolyte: A Must for Na-Ion Cells. Energy Storage Mater. 2023, 57, 102–117. [Google Scholar] [CrossRef]
- Tournevis Sans Fil DEXTER Sodium 3.6 V 0.7 Ah|Leroy Merlin. Available online: https://www.leroymerlin.fr/produits/outillage/outillage-electroportatif/visseuse-et-tournevis-electrique/visseuse/tournevis-sans-fil-dexter-sodium-3-6-v-0-7-ah-86650845.html (accessed on 8 July 2024).
- Barker, J.; Heap, R.J.; Roche, N.; Tan, C.; Sayers, R.; Whitley, J.; Liu, Y. High Energy Density Na-Ion Battery Technology. Meet. Abstr. 2016, MA2016-03, 796. [Google Scholar] [CrossRef]
- Faradion Transport Applications. Available online: https://faradion.co.uk/applications/transport-applications/ (accessed on 14 June 2023).
- Gupta, Y.; Siwatch, P.; Karwasra, R.; Sharma, K.; Tripathi, S.K. Recent Progress of Layered Structured P2- and O3- Type Transition Metal Oxides as Cathode Material for Sodium-Ion Batteries. Renew. Sustain. Energy Rev. 2024, 192, 114167. [Google Scholar] [CrossRef]
- Wang, P.-F.; You, Y.; Yin, Y.-X.; Guo, Y.-G. Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance. Adv. Energy Mater. 2018, 8, 1701912. [Google Scholar] [CrossRef]
- Grépin, E.; Jacquet, Q.; Moiseev, I.A.; Iadecola, A.; Rousse, G.; Avdeev, M.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. Mastering the Synthesis of High Na-Content, Moisture-Stable Layered Oxide Cathode for Na-Ion Batteries. J. Power Sources 2024, 613, 234962. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, W.; Li, K.; Zhu, X. Na-Deficient P2-Type Layered Oxide Cathodes for Practical Sodium-Ion Batteries. Microstructures 2024, 4, 2024027. [Google Scholar] [CrossRef]
- Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Polyanion-Type Electrode Materials for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600275. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Yang, C.; Hu, Y.-S. Polyanionic Cathode Materials for Practical Na-Ion Batteries toward High Energy Density and Long Cycle Life. ACS Cent. Sci. 2023, 9, 1721–1736. [Google Scholar] [CrossRef]
- Li, H.; Xu, M.; Zhang, Z.; Lai, Y.; Ma, J. Engineering of Polyanion Type Cathode Materials for Sodium-Ion Batteries: Toward Higher Energy/Power Density. Adv. Funct. Mater. 2020, 30, 2000473. [Google Scholar] [CrossRef]
- Lv, Z.; Ling, M.; Yue, M.; Li, X.; Song, M.; Zheng, Q.; Zhang, H. Vanadium-Based Polyanionic Compounds as Cathode Materials for Sodium-Ion Batteries: Toward High-Energy and High-Power Applications. J. Energy Chem. 2021, 55, 361–390. [Google Scholar] [CrossRef]
- He, M.; Davis, R.; Chartouni, D.; Johnson, M.; Abplanalp, M.; Troendle, P.; Suetterlin, R.-P. Assessment of the First Commercial Prussian Blue Based Sodium-Ion Battery. J. Power Sources 2022, 548, 232036. [Google Scholar] [CrossRef]
- Sodium-Ion Batteries & Sustainable Energy|Natron Energy. Available online: https://natron.energy/ (accessed on 8 July 2024).
- Bie, X.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. Synthesis and Electrochemical Properties of Na-Rich Prussian Blue Analogues Containing Mn, Fe, Co, and Fe for Na-Ion Batteries. J. Power Sources 2018, 378, 322–330. [Google Scholar] [CrossRef]
- Han, J.; Lin, Y.; Yang, Y.; Zuo, D.; Wang, C.; Liu, X. Dominant Role of M Element on the Stability and Properties of Prussian Blue Analogues NaxMFe(CN)6 (M = 3d Transition Metal) as Cathode Material for the Sodium-Ion Batteries. J. Alloys Compd. 2021, 870, 159533. [Google Scholar] [CrossRef]
- Ojwang, D.O.; Häggström, L.; Ericsson, T.; Ångström, J.; Brant, W.R. Influence of Sodium Content on the Thermal Behavior of Low Vacancy Prussian White Cathode Material. Dalton Trans. 2020, 49, 3570–3579. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, J.; Zhao, H.; Li, J.; Zhang, G.; Zhang, D.; Guo, X.; Gao, H.; Wang, Y.; Chen, J.; et al. Prussian Blue Analogues for Sodium-Ion Battery Cathodes: A Review of Mechanistic Insights, Current Challenges, and Future Pathways. Small 2024, 2401957. [Google Scholar] [CrossRef]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in Anode Materials for Sodium Ion Batteries—A Review. Renew. Sustain. Energy Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Fu, C.; Ding, Y.; Liu, G.; Cao, Y.; Chen, Z. Recent Advances in Conversion-Type Electrode Materials for Post Lithium-Ion Batteries. ACS Mater. Lett. 2021, 3, 956–977. [Google Scholar] [CrossRef]
- Lübke, M.; Howard, D.; Armer, C.F.; Gardecka, A.J.; Lowe, A.; Reddy, M.V.; Liu, Z.; Darr, J.A. High Energy Lithium Ion Battery Electrode Materials; Enhanced Charge Storage via Both Alloying and Insertion Processes. Electrochim. Acta 2017, 231, 247–254. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.J.; Ikuhara, Y. Why Is Sodium-Intercalated Graphite Unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard Carbons for Sodium-Ion Batteries: Structure, Analysis, Sustainability, and Electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Liu, X.; Yang, Z.; He, X.; Li, L.; Qiao, Y.; Chen, W.; Zeng, R.; Wang, Y.; et al. Organic Cathode Materials for Sodium-Ion Batteries: From Fundamental Research to Potential Commercial Application. Adv. Funct. Mater. 2022, 32, 2107718. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Lei, Y. Organic Materials for Rechargeable Sodium-Ion Batteries. Mater. Today 2018, 21, 60–78. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, Y.; Gong, Y.-T.; Qiu, Z.-X.; Qian, J.; Bai, Y.; Wang, Z.-L.; Zhang, R.-P.; Wu, C. Constructing Three-Dimensional Architectures to Design Advanced Anodes Materials for Sodium-Ion Batteries: From Nanoscale to Microscale. Energy Mater. 2024, 4, 400002. [Google Scholar] [CrossRef]
- Song, B.; Li, W.; Oh, S.-M.; Manthiram, A. Long-Life Nickel-Rich Layered Oxide Cathodes with a Uniform Li2 ZrO3 Surface Coating for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 9718–9725. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Shen, X.; Li, W.; Gao, Y.; Banis, M.N.; Li, M.; Chen, K.; Zhu, L.; Yu, R.; et al. Surface Doping to Enhance Structural Integrity and Performance of Li-Rich Layered Oxide. Adv. Energy Mater. 2018, 8, 1802105. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Grugeon, S.; Gachot, G.; Mathiron, D.; Armand, M.; Laruelle, S. LiFSI vs. LiPF6 Electrolytes in Contact with Lithiated Graphite: Comparing Thermal Stabilities and Identification of Specific SEI-Reinforcing Additives. Electrochim. Acta 2013, 102, 133–141. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Diemant, T.; Hekmatfar, M.; Grugeon, S.; Behm, R.J.; Laruelle, S.; Armand, M.; Passerini, S. Impact of the Electrolyte Salt Anion on the Solid Electrolyte Interphase Formation in Sodium Ion Batteries. Nano Energy 2019, 55, 327–340. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the Capacity Limit of Lithium Cobalt Oxide in Lithium Ion Batteries via Lanthanum and Aluminium Doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Tynjälä, P.; Lassi, U. Precipitation and Calcination of High-Capacity LiNiO2 Cathode Material for Lithium-Ion Batteries. Appl. Sci. 2020, 10, 8988. [Google Scholar] [CrossRef]
- Myung, S.-T.; Komaba, S.; Kumagai, N. Orthorhombic LiMnO2 as a High Capacity Cathode for Lithium-Ion Battery Synthesized by Hydrothermal Route at 170 °C. Chem. Lett. 2001, 30, 80–81. [Google Scholar] [CrossRef]
- Wagner, A.C.; Bohn, N.; Geßwein, H.; Neumann, M.; Osenberg, M.; Hilger, A.; Manke, I.; Schmidt, V.; Binder, J.R. Hierarchical Structuring of NMC111-Cathode Materials in Lithium-Ion Batteries: An In-Depth Study on the Influence of Primary and Secondary Particle Sizes on Electrochemical Performance. ACS Appl. Energy Mater. 2020, 3, 12565–12574. [Google Scholar] [CrossRef]
- Taddesse, P.; Gebrekiros, H.; Semu, G.; Duressa, M.; Chemeda, Y.C.; Murali, N.; Babu, K.V. Investigation of Structural, Vibrational Spectroscopic and Properties Study of LiMn2O4 and LiMn1.9Cu0.05Fe0.05O4 Cathode Materials. Results Mater. 2021, 12, 100224. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Wu, D.; Wu, J.; Zhu, Y.; Hu, X. Ultrahigh-Capacity and Fire-Resistant LiFePO4-Based Composite Cathodes for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1802930. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Dimov, N.; Okada, S.; Nishida, T. Electrochemical and Thermal Properties of α-NaFeO2 Cathode for Na-Ion Batteries. J. Electrochem. Soc. 2013, 160, A3077–A3081. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2 FeP2O7: A Safe Cathode for Rechargeable Sodium-Ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Indris, S.; Hua, W.; Casati, N.P.M.; Tayal, A.; Darma, M.S.D.; Wang, G.; Liu, Y.; Wu, C.; et al. The Structural Origin of Enhanced Stability of Na3.32Fe2.11Ca0.23(P2O7)2 Cathode for Na-Ion Batteries. Nano Energy 2021, 79, 105417. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, Y.; Jo, E.; Chung, K.Y.; Choi, W.; Kim, S.M.; Chang, W. Investigation of Thermal Stability of P2–NaxCoO2 Cathode Materials for Sodium Ion Batteries Using Real-Time Electron Microscopy. ACS Appl. Mater. Interfaces 2017, 9, 18883–18888. [Google Scholar] [CrossRef]
- Feng, J.; An, Y.; Ci, L.; Xiong, S. Nonflammable Electrolyte for Safer Non-Aqueous Sodium Batteries. J. Mater. Chem. A 2015, 3, 14539–14544. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, G.-L.; Che, H.; Wang, H.; Yang, K.; Yang, X.; Guo, F.; Ren, Y.; Chen, Z.; Amine, K.; et al. Probing Thermal and Chemical Stability of NaxNi1/3Fe1/3Mn1/3O2 Cathode Material toward Safe Sodium-Ion Batteries. Chem. Mater. 2018, 30, 4909–4918. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Qi, X.; Rong, X.; Li, H.; Huang, X.; Chen, L. Advanced Sodium-Ion Batteries Using Superior Low Cost Pyrolyzed Anthracite Anode: Towards Practical Applications. Energy Storage Mater. 2016, 5, 191–197. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Choi, J.U.; Yoon, C.S.; Yashiro, H.; Sun, Y.-K. Resolving the Degradation Pathways of the O3-Type Layered Oxide Cathode Surface through the Nano-Scale Aluminum Oxide Coating for High-Energy Density Sodium-Ion Batteries. J. Mater. Chem. A 2017, 5, 23671–23680. [Google Scholar] [CrossRef]
- Endo, K.; Zhang, H.P.; Fu, L.J.; Lee, K.J.; Sekine, K.; Takamura, T.; Jeong, Y.U.; Wu, Y.P.; Holze, R.; Wu, H.Q. Electrochemical Performance of a Novel Surface Modified Spherical Graphite as Anode Material for Lithium Ion Batteries. J. Appl. Electrochem. 2006, 36, 1307–1310. [Google Scholar] [CrossRef]
- Shu, J. Electrochemical Behavior and Stability of Li4Ti5O12 in a Broad Voltage Window. J. Solid. State Electrochem. 2009, 13, 1535–1539. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Yan, C.; Yuan, H.; Park, H.S.; Huang, J.-Q. Perspective on the Critical Role of Interface for Advanced Batteries. J. Energy Chem. 2020, 47, 217–220. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase—The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Z. Für Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, F.; Liu, X.; Yang, S.; Ma, J. Formation and Modification of Cathode Electrolyte Interphase: A Mini Review. Electrochem. Commun. 2021, 122, 106870. [Google Scholar] [CrossRef]
- Ma, L.A.; Naylor, A.J.; Nyholm, L.; Younesi, R. Strategies for Mitigating Dissolution of Solid Electrolyte Interphases in Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 4855–4863. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Grugeon, S.; Kim, H.; Jeong, S.; Wu, L.; Gachot, G.; Laruelle, S.; Armand, M.; Passerini, S. Comprehensive Insights into the Reactivity of Electrolytes Based on Sodium Ions. ChemSusChem 2016, 9, 462–471. [Google Scholar] [CrossRef]
- CATL Unveils Its Latest Breakthrough Technology by Releasing Its First Generation of Sodium-Ion Batteries. Available online: https://www.catl.com/en/news/665.html (accessed on 23 September 2024).
- Faradion Strong Performance. Available online: https://faradion.co.uk/technology-benefits/strong-performance/ (accessed on 3 October 2024).
- Rudola, A.; Wright, C.J.; Barker, J. Reviewing the Safe Shipping of Lithium-Ion and Sodium-Ion Cells: A Materials Chemistry Perspective. Energy Mater. Adv. 2021, 2021, 9798460. [Google Scholar] [CrossRef]
- Faradion Superior Safety. Available online: https://faradion.co.uk/technology-benefits/superior-safety/ (accessed on 23 September 2024).
- GB/T 31845-2015; Mechanical Structures for Electrotechnical and Electronic equipment—Thermal Design Specification (English Version). Code of China: Beijing, China, 2015.
- HiNa Achievements—Research & Development—HiNa Battery Technology Co., Ltd. Available online: https://www.hinabattery.com/en/index.php?catid=15 (accessed on 23 September 2024).
- CNEVPOST Hina Battery Becomes 1st Battery Maker to Put Sodium-Ion Batteries in EVs in China. Available online: https://cnevpost.com/2023/02/23/hina-battery-puts-sodium-ion-batteries-in-sehol-e10x/ (accessed on 23 September 2024).
- Natron Energy Battery Safety. Available online: https://natron.energy/our-technology/safety (accessed on 23 September 2024).
- Natron Energy Battery Performance. Available online: https://natron.energy/our-technology/performance (accessed on 23 September 2024).
- He, M.; El Mejdoubi, A.; Chartouni, D.; Morcrette, M.; Troendle, P.; Castiglioni, R. High Power NVPF/HC-Based Sodium-Ion Batteries. J. Power Sources 2023, 588, 233741. [Google Scholar] [CrossRef]
- Bauer, A.; Song, J.; Vail, S.; Pan, W.; Barker, J.; Lu, Y. The Scale-up and Commercialization of Nonaqueous Na-Ion Battery Technologies. Adv. Energy Mater. 2018, 8, 1702869. [Google Scholar] [CrossRef]
- Altris Altris Reaches New Milestone with 160 Wh/Kg Battery Cell. Available online: https://www.altris.se/news/altris-reaches-new-milestone-with-160-wh-kg-battery-cell (accessed on 23 September 2024).
- Sodium Battery Hub Altris Sodium-Ion Batteries: Performance, Safety, and Sustainability. Available online: https://sodiumbatteryhub.com/2024/08/02/altris-sodium-ion-batteries-performance-safety-and-sustainability/ (accessed on 23 September 2024).
- Liaw, H.-J.; Liou, Y.-R.; Liu, P.-H.; Chen, H.-Y.; Shu, C.-M. Increased Flammability Hazard When Ionic Liquid [C6mim][Cl] Is Exposed to High Temperatures. J. Hazard. Mater. 2019, 367, 407–417. [Google Scholar] [CrossRef]
- 01—Home|EV Fire Safe. Available online: https://www.evfiresafe.com/ (accessed on 22 April 2024).
- EPRI BESS Failure Incident Database. Available online: https://storagewiki.epri.com/index.php/BESS_Failure_Incident_Database (accessed on 29 July 2024).
- Guy, M.; Amandine, L.; Arnaud, B.; Paul, C. Truchot Benjamin Key Learnings From Recent Lithium-Ion Battery Incidents That Have Impacted e-Mobility and Energy Storage Fast Growing Markets. Chem. Eng. Trans. 2022, 90, 643–648. [Google Scholar] [CrossRef]
- CALCE and the University of Maryland Safety. Available online: https://web.calce.umd.edu/batteries/safety.html (accessed on 20 September 2023).
- Park Taejon Renault-Samsung’s Electric Vehicle Catches Fire Due to Ignition from Bonnet. Available online: https://english.etnews.com/20160127200001 (accessed on 20 September 2023).
- CTIF International Association of Fire and Rescue Services Large Explosion and Fire at French Lithium Battery Warehouse. Available online: https://ctif.org/news/large-explosion-and-fire-french-lithium-battery-warehouse (accessed on 20 September 2023).
- South Korea: Exploding Lithium Batteries Spark Deadly Factory Fire. Available online: https://www.bbc.com/news/articles/crgggmeyjj7o (accessed on 9 July 2024).
- DESTINY PhD Programme Marie Skłodowska-Curie Actions COFUND. Available online: https://www.destiny-phd.eu/topic-22---2 (accessed on 22 April 2024).
- Regulation—2023/1542—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2023/1542/oj (accessed on 22 April 2024).
- Patil, A.; Patil, V.; Wook Shin, D.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and Challenges Facing Rechargeable Thin Film Lithium Batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon. 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Hausbrand, R. Electronic Energy Levels at Li-Ion Cathode–Liquid Electrolyte Interfaces: Concepts, Experimental Insights, and Perspectives. J. Chem. Phys. 2020, 152, 180902. [Google Scholar] [CrossRef]
- Abe, K.; Yoshitake, H.; Kitakura, T.; Hattori, T.; Wang, H.; Yoshio, M. Additives-Containing Functional Electrolytes for Suppressing Electrolyte Decomposition in Lithium-Ion Batteries. Electrochim. Acta 2004, 49, 4613–4622. [Google Scholar] [CrossRef]
- Cometto, C.; Yan, G.; Mariyappan, S.; Tarascon, J.-M. Means of Using Cyclic Voltammetry to Rapidly Design a Stable DMC-Based Electrolyte for Na-Ion Batteries. J. Electrochem. Soc. 2019, 166, A3723–A3730. [Google Scholar] [CrossRef]
- Zhang, L.; Tsolakidou, C.; Mariyappan, S.; Tarascon, J.-M.; Trabesinger, S. Unraveling Gas Evolution in Sodium Batteries by Online Electrochemical Mass Spectrometry. Energy Storage Mater. 2021, 42, 12–21. [Google Scholar] [CrossRef]
- Yan, G.; Reeves, K.; Foix, D.; Li, Z.; Cometto, C.; Mariyappan, S.; Salanne, M.; Tarascon, J. A New Electrolyte Formulation for Securing High Temperature Cycling and Storage Performances of Na-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901431. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Samigullin, R.R.; Drozhzhin, O.A.; Antipov, E.V. Comparative Study of the Thermal Stability of Electrode Materials for Li-Ion and Na-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 14–19. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries. Micromachines 2024, 15, 310. [Google Scholar] [CrossRef]
- Shakourian-Fard, M.; Kamath, G.; Smith, K.; Xiong, H.; Sankaranarayanan, S.K.R.S. Trends in Na-Ion Solvation with Alkyl-Carbonate Electrolytes for Sodium-Ion Batteries: Insights from First-Principles Calculations. J. Phys. Chem. C 2015, 119, 22747–22759. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. State-of-Electrode (SOE) Analytics of Lithium-Ion Cells under Overdischarge Extremes. Energy Storage Mater. 2023, 54, 60–74. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Börner, M.; Streipert, B.; Meister, P.; Wagner, R.; Cekic Laskovic, I.; Winter, M. Lithium Ion Battery Cells under Abusive Discharge Conditions: Electrode Potential Development and Interactions between Positive and Negative Electrode. J. Power Sources 2017, 362, 278–282. [Google Scholar] [CrossRef]
- Flügel, M.; Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Detection of Copper Deposition on Anodes of Over-Discharged Lithium Ion Cells by GD-OES Depth Profiling. ChemPhysChem 2020, 21, 2047–2050. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of High Energy Density Sodium-Ion Batteries: Faradion’s Journey and Outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Huo, H.; Xing, Y.; Pecht, M.; Züger, B.J.; Khare, N.; Vezzini, A. Safety Requirements for Transportation of Lithium Batteries. Energies 2017, 10, 793. [Google Scholar] [CrossRef]
- DSV Class 9A Lithium Batteries Dangerous Goods. Available online: https://www.dsv.com/en-gb/our-solutions/modes-of-transport/value-added-services/transporting-dangerous-goods/9-classes-of-dangerous-goods/class-9a-lithium-batteries (accessed on 19 September 2023).
- He, X.; Hu, Z.; Restuccia, F.; Fang, J.; Rein, G. Experimental Study of the Effect of the State of Charge on Self-Heating Ignition of Large Ensembles of Lithium-Ion Batteries in Storage. Appl. Therm. Eng. 2022, 212, 118621. [Google Scholar] [CrossRef]
- ICAO. International Civil Aviation Organization Techincal Instructions for the Safe Transport of Dangerous Goods by Air (Doc 9284) 2015–2016 Edition. Available online: https://www.icao.int/safety/dangerousgoods/addendumcorrigendum%20to%20the%20technical%20instructions/doc%209284-2015-2016.add-3.pdf (accessed on 3 October 2024).
- IATA. 2024 Lithium Battery Guidance Document Transport of Lithium Metal and Lithium Ion Batteries. 2024. Available online: https://www.iata.org/contentassets/05e6d8742b0047259bf3a700bc9d42b9/lithium-battery-guidance-document.pdf (accessed on 3 October 2024).
- Brennan, D. Reduced charge for vehicles powered by lithium ion, lithium metal or sodium ion batteries. In Proceedings of the Dangerous Goods Panel (DGP) Twenty-Ninth Meeting, Montréal, QC, Canada, 13 November 2023; Available online: https://www.icao.int/safety/DangerousGoods/DGP29/DGP.29.WP.026.4.en.pdf (accessed on 3 October 2024).
- United Nations. Recommendations on the Transport of Dangerous Goods Model Regulations Volume I; United Nations: New York, NY, USA; Geneva, Switzerland, 2023. [Google Scholar]
- UN/SCETDG/55/INF.38. Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals. In Proceedings of the Sub-Committee of Experts on the Transport of Dangerous Goods Fifty-Fifth Session, Geneva, Switzerland, 27 June 2019. [Google Scholar]
- Desai, P.; Huang, J.; Foix, D.; Tarascon, J.-M.; Mariyappan, S. Zero Volt Storage of Na-Ion Batteries: Performance Dependence on Cell Chemistry! J. Power Sources 2022, 551, 232177. [Google Scholar] [CrossRef]
- Baba, Y. Thermal Stability of LixCoO2 Cathode for Lithium Ion Battery. Solid. State Ion. 2002, 148, 311–316. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. The Reaction of Charged Cathodes with Nonaqueous Solvents and Electrolytes: I. Li0.5CoO2. J. Electrochem. Soc. 2001, 148, A1205. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. The Reactions of Li[Sub 0.5]CoO[Sub 2] with Nonaqueous Solvents at Elevated Temperatures. J. Electrochem. Soc. 2002, 149, A912. [Google Scholar] [CrossRef]
- Hossain, M.S.; Furusawa, T.; Sato, M. Hydrothermal Synthesis, Characterization and Thermal Stability Studies of α-Fe2O3 Hollow Microspheres. Adv. Powder Technol. 2022, 33, 103797. [Google Scholar] [CrossRef]
- Darezereshki, E.; Bakhtiari, F.; Alizadeh, M.; Behrad Vakylabad, A.; Ranjbar, M. Direct Thermal Decomposition Synthesis and Characterization of Hematite (α-Fe2O3) Nanoparticles. Mater. Sci. Semicond. Process. 2012, 15, 91–97. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Chen, M.; Yang, Y.; Zhao, J. Unveiling the Origin of Air Stability in Polyanion and Layered-Oxide Cathode Materials for Sodium-Ion Batteries and Their Practical Application Considerations. Adv. Funct. Mater. 2024, 34, 2308257. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Chihara, K.; Okada, S.; Yamaki, J.; Matsumoto, S.; Kuze, S.; Nakane, K. Electrochemical and Thermal Properties of Hard Carbon-Type Anodes for Na-Ion Batteries. J. Power Sources 2013, 244, 752–757. [Google Scholar] [CrossRef]
- SODIUM PERCHLORATE|CAMEO Chemicals|NOAA. Available online: https://cameochemicals.noaa.gov/chemical/1514 (accessed on 26 April 2024).
- Bhutia, P.T.; Grugeon, S.; Bertrand, J.-P.; Binotto, G.; Bordes, A.; El Mejdoubi, A.; Laruelle, S.; Marlair, G. Fire Hazards of Carbonate-Based Electrolytes for Sodium-Ion Batteries: What Changes from Lithium-Ion Batteries? J. Power Sources 2024, 622, 235234. [Google Scholar] [CrossRef]
- Tribouilloy Benoit; Paillery Esteban; Binotto Ghislain; Marlair Guy Flammability of Halogenated Liquids: Flash Points Limits. Chem. Eng. Trans. 2022, 90, 529–534. [CrossRef]
- Yang, Z.; He, J.; Lai, W.; Peng, J.; Liu, X.; He, X.; Guo, X.; Li, L.; Qiao, Y.; Ma, J.; et al. Fire-Retardant, Stable-Cycling and High-Safety Sodium Ion Battery. Angew. Chem. 2021, 133, 27292–27300. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Xu, X.; Lin, K.; Zhou, D.; Liu, Q.; Qin, X.; Wang, S.; He, S.; Kang, F.; Li, B.; Wang, G. Quasi-Solid-State Dual-Ion Sodium Metal Batteries for Low-Cost Energy Storage. Chem. 2020, 6, 902–918. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, S.; Lv, Z.; Xu, G.; Huang, L.; Wang, Q.; Cui, Z.; Cui, G. A Temperature-Responsive Electrolyte Endowing Superior Safety Characteristic of Lithium Metal Batteries. Adv. Energy Mater. 2020, 10, 1903441. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, N.; Mishra, K.; Shahid, R.; Arif, T.; Kanchan, D.K. Sodium Ion Conducting Flame-Retardant Gel Polymer Electrolyte for Sodium Batteries and Electric Double Layer Capacitors (EDLCs). J. Energy Storage 2022, 46, 103899. [Google Scholar] [CrossRef]
- Yang, H.; Tian, W.; Chen, X.; Li, Z.; Liu, P.; Wang, Q.; Nie, X.; Wang, Q.; Jiao, L. Flame-Retardant Polymer Electrolyte for Sodium-Ion Batteries. Batter. Supercaps 2024, e202400383. [Google Scholar] [CrossRef]
- Hyung, Y.E.; Vissers, D.R.; Amine, K. Flame-Retardant Additives for Lithium-Ion Batteries. J. Power Sources 2003, 119–121, 383–387. [Google Scholar] [CrossRef]
- Lee, C.W. A Novel Flame-Retardant Additive for Lithium Batteries. Electrochem. Solid-State Lett. 1999, 3, 63. [Google Scholar] [CrossRef]
- Belov, D.G.; Shieh, D.T. A Study of Tetrabromobisphenol A (TBBA) as a Flame Retardant Additive for Li-Ion Battery Electrolytes. J. Power Sources 2014, 247, 865–875. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Chesnaye, A.; Lore, P.-A.L.; Lecocq, A. Safety Evaluation of a Sodium-Ion Cell: Assessment of Vent Gas Emissions under Thermal Runaway. ACS Energy Lett. 2022, 7, 3386–3391. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Herreyre, S.; Papin, A.; Desprez, P.; Lecocq, A. New Insight on the Risk Profile Pertaining to Lithium-Ion Batteries under Thermal Runaway as Affected by System Modularity and Subsequent Oxidation Regime. J. Energy Storage 2022, 52, 104790. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; De Persis, S. Identification and Quantification of Gases Emitted during Abuse Tests by Overcharge of a Commercial Li-Ion Battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, J.N.; Brown, S.F. Assessment of Thermal Runaway in Commercial Lithium Iron Phosphate Cells Due to Overheating in an Oven Test. Energy Procedia 2018, 151, 74–78. [Google Scholar] [CrossRef]
- Townsend, D.I.; Tou, J.C. Thermal Hazard Evaluation by an Accelerating Rate Calorimeter. Thermochim. Acta 1980, 37, 1–30. [Google Scholar] [CrossRef]
- Jhu, C.-Y.; Wang, Y.-W.; Wen, C.-Y.; Shu, C.-M. Thermal Runaway Potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 Batteries Determined with Adiabatic Calorimetry Methodology. Appl. Energy 2012, 100, 127–131. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H. Experimental Analysis of Thermal Runaway in 18650 Cylindrical Li-Ion Cells Using an Accelerating Rate Calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef]
- Rosalind, E. Franklin Crystallite Growth in Graphitizing and Non-Graphitizing Carbons. Proc. R. Soc. Lond. A 1951, 209, 196–218. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, H.; Fang, Y.; Li, W.; Lan, S.; Wei, S.; Yin, Z.; Tang, Y.; Ren, Y.; Liu, Q. Typical Cathode Materials for Lithium-ion and Sodium-ion Batteries: From Structural Design to Performance Optimization. Carbon. Neutralization 2023, 2, 339–377. [Google Scholar] [CrossRef]
- Darga, J.; Lamb, J.; Manthiram, A. Industrialization of Layered Oxide Cathodes for Lithium-Ion and Sodium-Ion Batteries: A Comparative Perspective. Energy Tech. 2020, 8, 2000723. [Google Scholar] [CrossRef]
- Pan, Y.; Chou, S.; Liu, H.K.; Dou, S.X. Functional Membrane Separators for Next-Generation High-Energy Rechargeable Batteries. Natl. Sci. Rev. 2017, 4, 917–933. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. (John) Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Li, A.; Yuen, A.C.Y.; Wang, W.; De Cachinho Cordeiro, I.M.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A Review on Lithium-Ion Battery Separators towards Enhanced Safety Performances and Modelling Approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef]
- Orendorff, C.J. The Role of Separators in Lithium-Ion Cell Safety. Interface Mag. 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Parekh, M.; Gray, F.; Fleetwood, J.; Adams, T.; Tomar, V.; Pol, V.G.; Mukherjee, P.P. The Role of Separator Thermal Stability in Safety Characteristics of Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 090521. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Zu, C.; Li, J.; Cai, B.; Qiu, J.; Zhao, Y.; Yang, Q.; Li, H.; Yu, H. Separators with Reactive Metal Oxide Coatings for Dendrite-Free Lithium Metal Anodes. J. Power Sources 2023, 555, 232336. [Google Scholar] [CrossRef]
- Ho, V.; Nguyen, B.T.D.; Thi, H.Y.N.; Kim, J.F.; Mun, J. Poly(Dopamine) Surface-modified Polyethylene Separator with Electrolyte-philic Characteristics for Enhancing the Performance of Sodium-ion Batteries. Intl J. Energy Res. 2022, 46, 5177–5188. [Google Scholar] [CrossRef]
- Kim, J.I.; Heo, J.; Park, J.H. Tailored Metal Oxide Thin Film on Polyethylene Separators for Sodium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1965–A1969. [Google Scholar] [CrossRef]






| Layered Oxides | Polyanionic Materials | Prussian Blue Analogues | |
|---|---|---|---|
| Energy density of cell |  |  |  |
| Structural stability of material |  |  |  |
| Toxic gases emission from cell |  |  |  |
| Thermal runaway density of cell |  |  |  |
| Gases Emitted (mg/g of Electrolyte Burnt) | HF | POF3 |
|---|---|---|
| EC/DMC (1:1 wt. ratio) LiPF6 1 M | 59.1 ± 0.7 | 25.5 ± 1.1 |
| EC/DMC (1:1 wt. ratio) NaPF6 1 M | 16.5 ± 0.5 | 1.2 ± 0.1 |
| Cell Chemistry | Base Electrolyte | Additive(s) | Safety Improvement |
|---|---|---|---|
| LiNi0.8Co0.2O2||Graphite | 1 M LiPF6 in 1:1 EC:DEC | Triphenylphosphate (TPP) and Tributylphosphate (TBP) | Underwriters Laboratories (UL) test standard 94 showed, with TPP additive, decreased flame propagation rates. ARC experiments on charged graphite electrode and electrolyte with TPP or TBP additives demonstrated lower exothermic heat generation [178]. |
| LiNi0.8Co0.2O2||Li | 1 M LiPF6 in 1:1 EC:DMC | Hexamethoxycyclotri-phosphazene ([NP(OCH3)2]3) | Thermal stability of fully lithiated graphite electrolyte with additive decreased the overall heat of production. ARC results showed that the maximum self-heating profile of electrolyte without and with additives improved from 0.68 °C/min to 0.19 °C/min respectively [179]. |
| LiMn2O4: Li(Ni1/3Co1/3Mn1/3)O2 (8:2)||Li4Ti5O12 | 1 M LiPF6 in 1:2 EC:EMC with 2% VC | 3,3′,5,5′-tetrabromobisphenol A (TBBA) | Safety tests were performed on 100% SOC charged 18650 cells developed on in-house safety procedure. The cells with TBBA additives had flame-retardant (self-extinguishing) ability even at 1 wt% of TBBA content [180]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhutia, P.T.; Grugeon, S.; El Mejdoubi, A.; Laruelle, S.; Marlair, G. Safety Aspects of Sodium-Ion Batteries: Prospective Analysis from First Generation Towards More Advanced Systems. Batteries 2024, 10, 370. https://doi.org/10.3390/batteries10100370
Bhutia PT, Grugeon S, El Mejdoubi A, Laruelle S, Marlair G. Safety Aspects of Sodium-Ion Batteries: Prospective Analysis from First Generation Towards More Advanced Systems. Batteries. 2024; 10(10):370. https://doi.org/10.3390/batteries10100370
Chicago/Turabian StyleBhutia, Pempa Tshering, Sylvie Grugeon, Asmae El Mejdoubi, Stéphane Laruelle, and Guy Marlair. 2024. "Safety Aspects of Sodium-Ion Batteries: Prospective Analysis from First Generation Towards More Advanced Systems" Batteries 10, no. 10: 370. https://doi.org/10.3390/batteries10100370
APA StyleBhutia, P. T., Grugeon, S., El Mejdoubi, A., Laruelle, S., & Marlair, G. (2024). Safety Aspects of Sodium-Ion Batteries: Prospective Analysis from First Generation Towards More Advanced Systems. Batteries, 10(10), 370. https://doi.org/10.3390/batteries10100370






