Inkjet-Printed Silver Lithiophilic Sites on Copper Current Collectors: Tuning the Interfacial Electrochemistry for Anode-Free Lithium Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Ag Printing
2.2. Electrochemical Measurements
2.3. Structural and Morphological Characterization
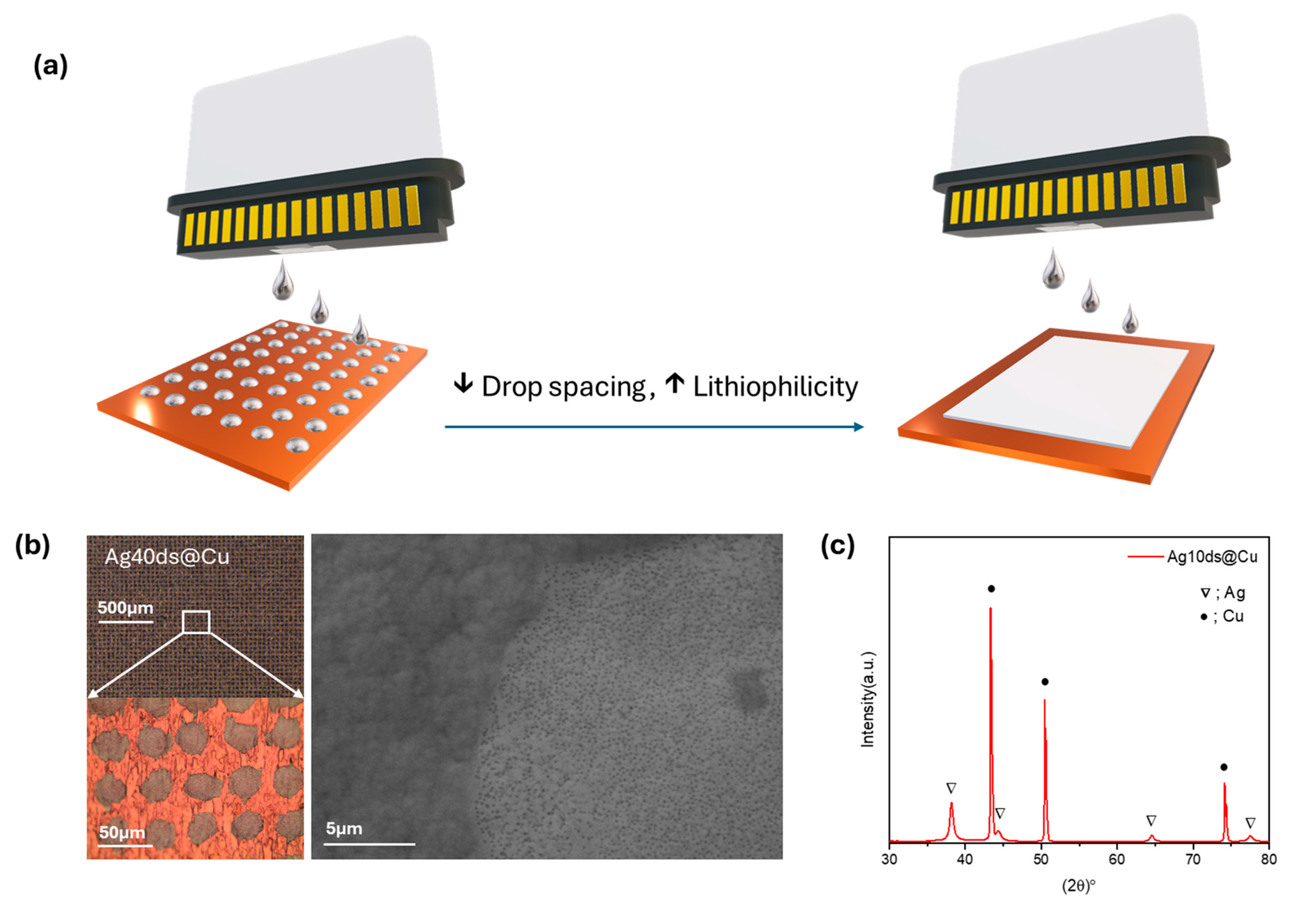
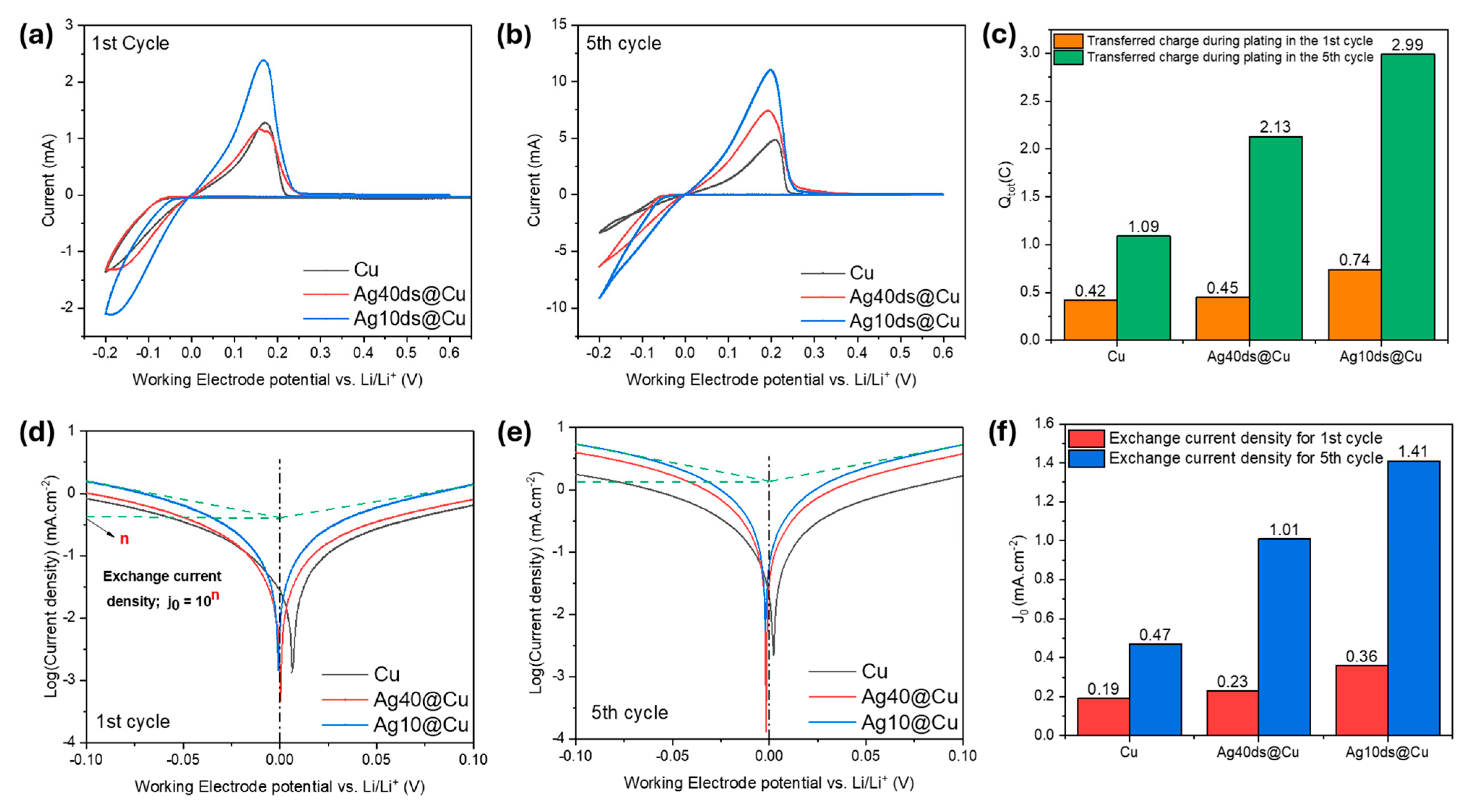
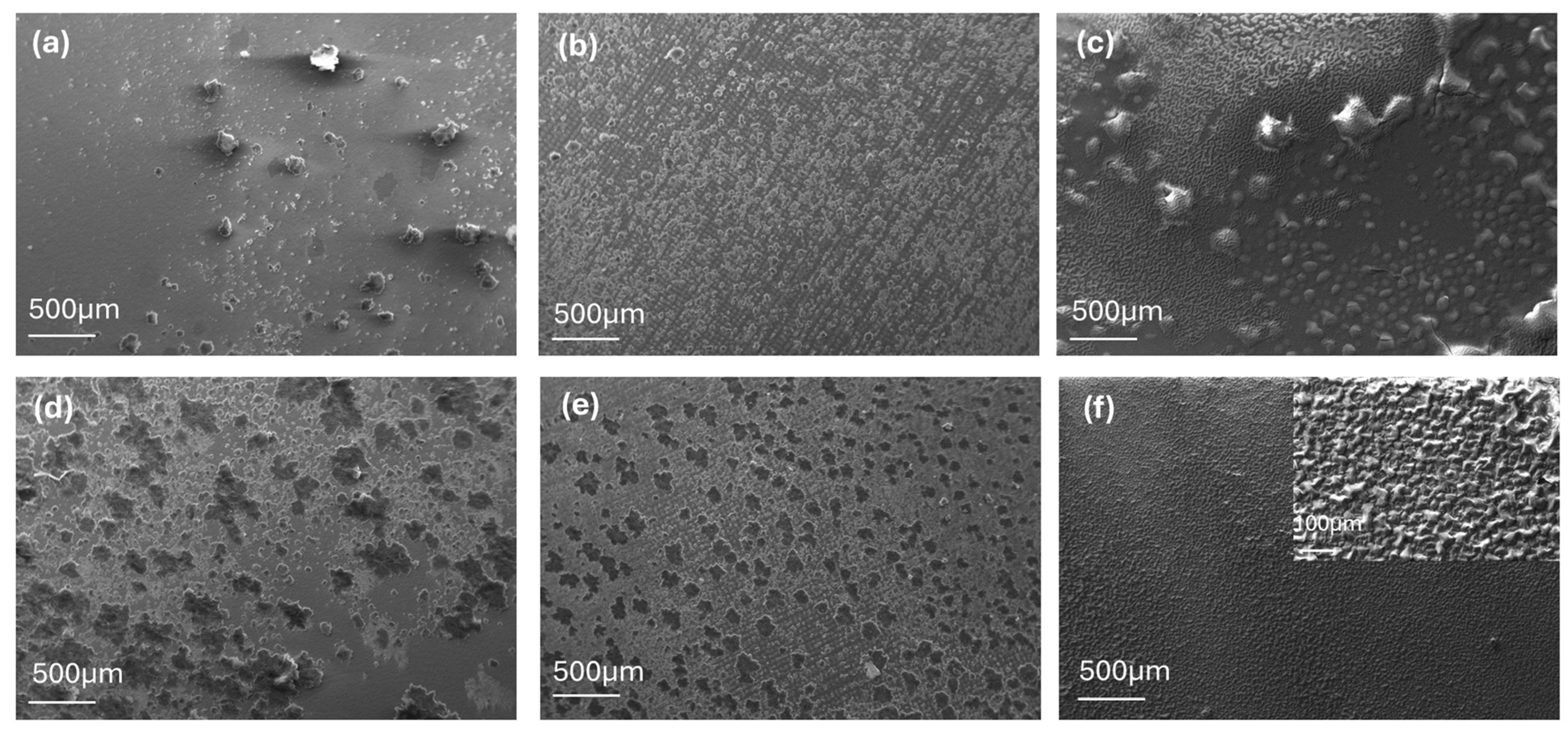
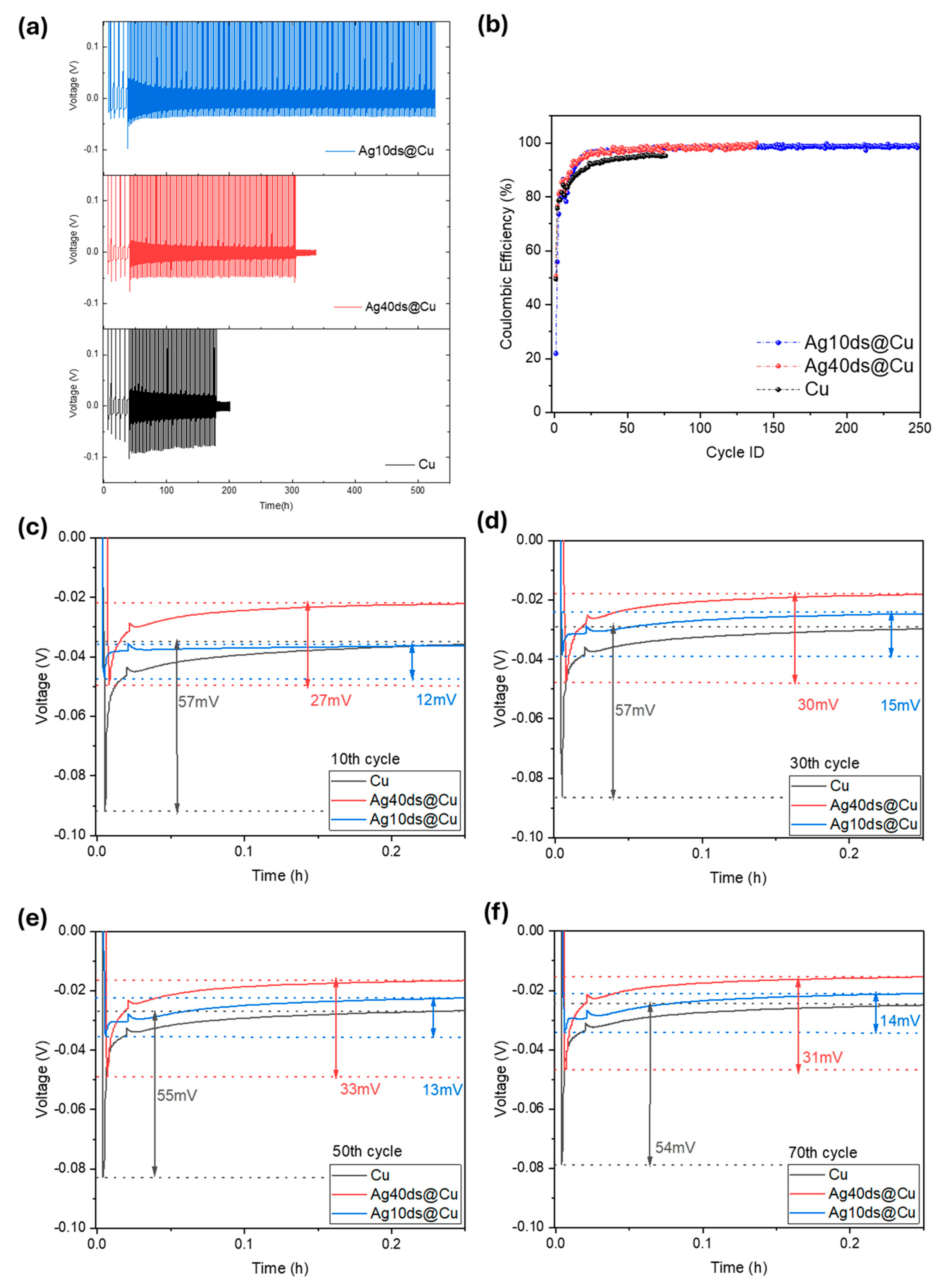
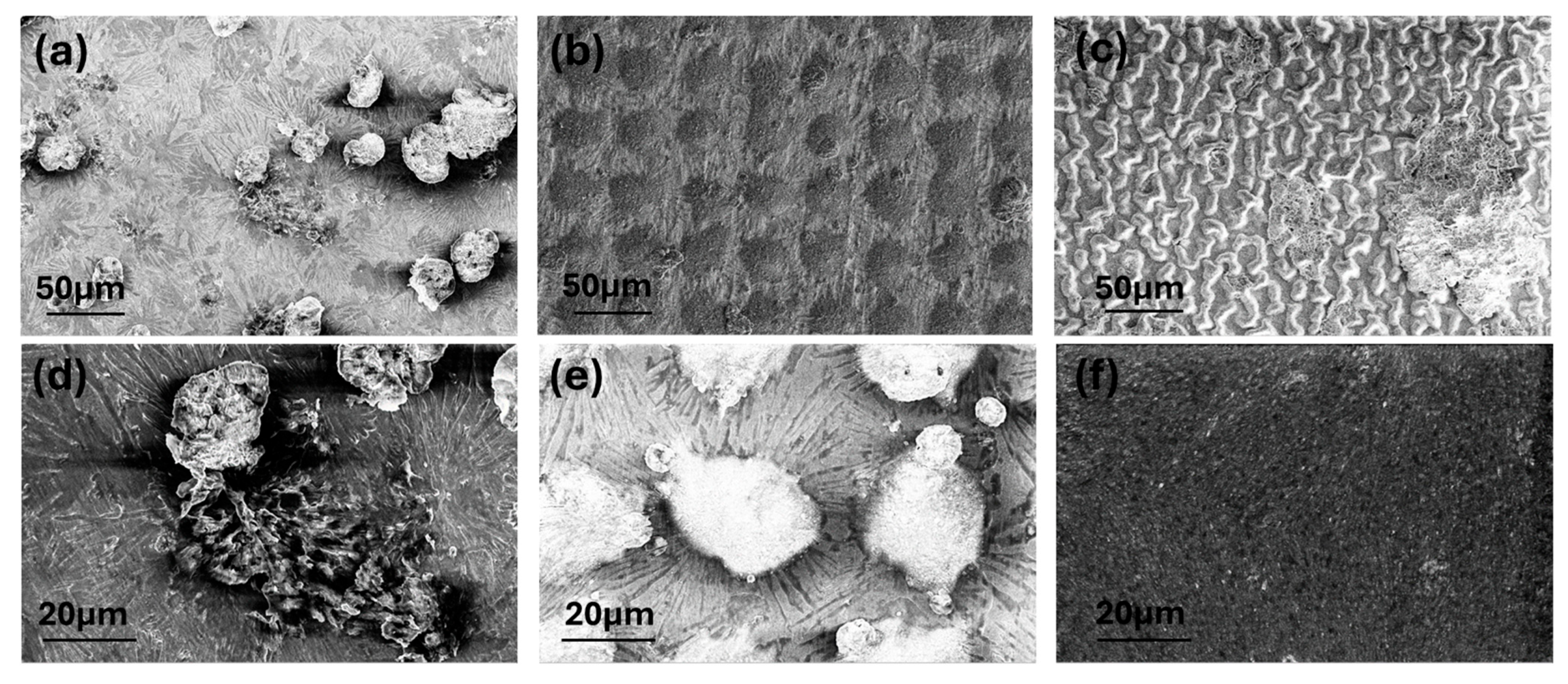
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yue, X.; Wang, J.; Abudula, A.; Guan, G. Anode-free rechargeable lithium metal batteries: Progress and prospects. Energy Storage Mater. 2020, 32, 386–401. [Google Scholar] [CrossRef]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; et al. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, B.; Shen, Y.; Wu, J.; Zhao, Z.; Zhong, C.; Hu, W. Confronting the Challenges in Lithium Anodes for Lithium Metal Batteries. Adv. Sci. 2021, 8, 2101111. [Google Scholar] [CrossRef]
- Fang, C.; Wang, X.; Meng, Y.S. Key Issues Hindering a Practical Lithium-Metal Anode. Trends Chem. 2019, 1, 152–158. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Heubner, C.; Maletti, S.; Auer, H.; Hüttl, J.; Voigt, K.; Lohrberg, O.; Nikolowski, K.; Partsch, M.; Michaelis, A. From Lithium-Metal toward Anode-Free Solid-State Batteries: Current Developments, Issues, and Challenges. Adv. Funct. Mater. 2021, 31, 2106608. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Jiang, H.; Xiong, S.; Feng, J.; Qian, Y. Recently advances and perspectives of anode-free rechargeable batteries. Nano Energy 2020, 78, 105344. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. Anode-Free Full Cells: A Pathway to High-Energy Density Lithium-Metal Batteries. Adv. Energy Mater. 2021, 11, 2000804. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.-R.; Chen, X.; Cheng, X.-B.; Zhang, X.-Q.; Yan, C.; Zhang, Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. Int. Ed. 2017, 56, 7764–7768. [Google Scholar] [CrossRef]
- Zhang, S.S.; Fan, X.; Wang, C. A tin-plated copper substrate for efficient cycling of lithium metal in an anode-free rechargeable lithium battery. Electrochim. Acta 2017, 258, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Liu, Z.; Weng, S.; Li, X.; Wang, X.; Gao, Y.; Wang, Z.; Chen, L. Phase Diagram Determined Lithium Plating/Stripping Behaviors on Lithiophilic Substrates. ACS Energy Lett. 2021, 6, 4118–4126. [Google Scholar] [CrossRef]
- Li, J.; Su, H.; Liu, Y.; Zhong, Y.; Wang, X.; Tu, J. Li Alloys in All Solid-State Lithium Batteries: A Review of Fundamentals and Applications. Electrochem. Energy Rev. 2024, 7, 18. [Google Scholar] [CrossRef]
- Chen, X.-R.; Chen, X.; Yan, C.; Zhang, X.-Q.; Zhang, Q.; Huang, J.-Q. Role of Lithiophilic Metal Sites in Lithium Metal Anodes. Energy Fuels 2021, 35, 12746–12752. [Google Scholar] [CrossRef]
- Jin, S.; Ye, Y.; Niu, Y.; Xu, Y.; Jin, H.; Wang, J.; Sun, Z.; Cao, A.; Wu, X.; Luo, Y.; et al. Solid–Solution-Based Metal Alloy Phase for Highly Reversible Lithium Metal Anode. J. Am. Chem. Soc. 2020, 142, 8818–8826. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Lai, C. Li-containing alloys beneficial for stabilizing lithium anode: A review. Eng. Rep. 2021, 3, e12339. [Google Scholar] [CrossRef]
- Wondimkun, Z.T.; Tegegne, W.A.; Shi-Kai, J.; Huang, C.-J.; Sahalie, N.A.; Weret, M.A.; Hsu, J.-Y.; Hsieh, P.-L.; Huang, Y.-S.; Wu, S.-H.; et al. Highly-lithiophilic Ag@PDA-GO film to Suppress Dendrite Formation on Cu Substrate in Anode-free Lithium Metal Batteries. Energy Storage Mater. 2021, 35, 334–344. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Wang, B.; Yang, Y.; Cao, G.; Xiong, Y.; Zhang, H. Interface structure regulation of a Ag lithiophilic layer towards uniform lithium nucleation/growth. Sustain. Energy Fuels 2019, 3, 2995–2999. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Wang, C.; Dou, H.; Zhang, X. Targeted Deposition in a Lithiophilic Silver-Modified 3D Cu Host for Lithium-Metal Anodes. Energy Environ. Mater. 2023, 6, e12412. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, P.; Yang, W.; Wei, Y.; Xiao, J.; Deng, L.; Gong, Y. Large-Scale Modification of Commercial Copper Foil with Lithiophilic Metal Layer for Li Metal Battery. Small 2020, 16, 1905620. [Google Scholar] [CrossRef]
- Xia, H.; Wang, D.; Wang, Y.; Fu, Z. Study on Stable Lithiophilic Ag Modification Layer on Copper Current Collector for High Coulombic-Efficiency Lithium Metal Anode. J. Electrochem. Soc. 2023, 170, 060546. [Google Scholar] [CrossRef]
- Hou, Z.; Yu, Y.; Wang, W.; Zhao, X.; Di, Q.; Chen, Q.; Chen, W.; Liu, Y.; Quan, Z. Lithiophilic Ag Nanoparticle Layer on Cu Current Collector toward Stable Li Metal Anode. ACS Appl. Mater. Interfaces 2019, 11, 8148–8154. [Google Scholar] [CrossRef]
- Li, Q.; Quan, B.; Li, W.; Lu, J.; Zheng, J.; Yu, X.; Li, J.; Li, H. Electro-plating and stripping behavior on lithium metal electrode with ordered three-dimensional structure. Nano Energy 2018, 45, 463–470. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Jin, D.; Phatak, C.; Cho, K.Y.; Lee, Y.-G.; Hong, S.; Ryou, M.-H.; Lee, Y.M. Size effects of micro-pattern on lithium metal surface on the electrochemical performance of lithium metal secondary batteries. J. Power Sources 2018, 408, 136–142. [Google Scholar] [CrossRef]
- Zou, P.; Wang, Y.; Chiang, S.-W.; Wang, X.; Kang, F.; Yang, C. Directing lateral growth of lithium dendrites in micro-compartmented anode arrays for safe lithium metal batteries. Nat. Commun. 2018, 9, 464. [Google Scholar] [CrossRef]
- Sztymela, K.; Rossignol, F.; Bienia, M.; Zapp, N.; Nikolowski, K.; Cerbelaud, M. Fabrication of 3D silicon anode by inkjet printing: Opportunities and challenges. J. Energy Storage 2024, 75, 109567. [Google Scholar] [CrossRef]
- Rodriguez, R.; Deiner, L.J.; Tsao, B.H.; Fellner, J.P. Aerosol Jet-Printed LFP Cathodes with Bimodal Pore Distribution Improve the Rate Capability of LIB Cells. ACS Appl. Energy Mater. 2021, 4, 9507–9512. [Google Scholar] [CrossRef]
- Deiner, L.J.; Jenkins, T.; Powell, A.; Howell, T.; Rottmayer, M. High Capacity Rate Capable Aerosol Jet Printed Li-Ion Battery Cathode. Adv. Eng. Mater. 2019, 21, 1801281. [Google Scholar] [CrossRef]
- Zhou, S.; Usman, I.; Wang, Y.; Pan, A. 3D printing for rechargeable lithium metal batteries. Energy Storage Mater. 2021, 38, 141–156. [Google Scholar] [CrossRef]
- Clement, B.; Lyu, M.; Sandeep Kulkarni, E.; Lin, T.; Hu, Y.; Lockett, V.; Greig, C.; Wang, L. Recent Advances in Printed Thin-Film Batteries. Engineering 2022, 13, 238–261. [Google Scholar] [CrossRef]
- Park, M.-S.; Hyun, S.-H.; Nam, S.-C. Mechanical and electrical properties of a LiCoO2 cathode prepared by screen-printing for a lithium-ion micro-battery. Electrochim. Acta 2007, 52, 7895–7902. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Li, W.; Cai, W.; Jiang, Z. Electrochemical properties of LiCoO2 thin film electrode prepared by ink-jet printing technique. Thin Solid Films 2008, 516, 3314–3319. [Google Scholar] [CrossRef]
- Milroy, C.A.; Jang, S.; Fujimori, T.; Dodabalapur, A.; Manthiram, A. Inkjet-Printed Lithium–Sulfur Microcathodes for All-Printed, Integrated Nanomanufacturing. Small 2017, 13, 1603786. [Google Scholar] [CrossRef]
- Lawes, S.; Sun, Q.; Lushington, A.; Xiao, B.; Liu, Y.; Sun, X. Inkjet-printed silicon as high performance anodes for Li-ion batteries. Nano Energy 2017, 36, 313–321. [Google Scholar] [CrossRef]
- Sztymela, K.; Bienia, M.; Rossignol, F.; Mailley, S.; Ziesche, S.; Varghese, J.; Cerbelaud, M. Fabrication of modern lithium ion batteries by 3D inkjet printing: Opportunities and challenges. Heliyon 2022, 8, e12623. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Q.; Liu, L.; Xu, J.; Yan, M.; Jiang, Z. A novel and facile route of ink-jet printing to thin film SnO2 anode for rechargeable lithium ion batteries. Electrochim. Acta 2006, 51, 2639–2645. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, G.; Liu, L.; Jiang, Z. High-performance thin-film Li4Ti5O12 electrodes fabricated by using ink-jet printing technique and their electrochemical properties. J. Solid State Electrochem. 2009, 13, 705–711. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhuang, L.; Xu, J.; Shi, Y.; Su, C.; Lian, P.; Tian, B. Lithiophilic Silver Coating on Lithium Metal Surface for Inhibiting Lithium Dendrites. Front. Chem. 2020, 8, 109. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Kim, Y.; Park, Y.; Kim, H.; Choi, J.W. Surface Overpotential as a Key Metric for the Discharge–Charge Reversibility of Aqueous Zinc-Ion Batteries. J. Am. Chem. Soc. 2023, 145, 15776–15787. [Google Scholar] [CrossRef]
- Xie, X.; Liang, S.; Gao, J.; Guo, S.; Guo, J.; Wang, C.; Xu, G.; Wu, X.; Chen, G.; Zhou, J. Manipulating the ion-transfer kinetics and interface stability for high-performance zinc metal anodes. Energy Environ. Sci. 2020, 13, 503–510. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Sadd, M.; Kapitanova, O.O.; Krivchenko, V.A.; Ban, J.; Wang, J.; Jiao, X.; Song, Z.; Song, J.; et al. Insight into the Critical Role of Exchange Current Density on Electrodeposition Behavior of Lithium Metal. Adv. Sci. 2021, 8, 2003301. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Liu, Z. Optimization of lithium nucleation by current density toward dendrite-free Li metal anode. J. Alloys Compd. 2022, 893, 162389. [Google Scholar] [CrossRef]
- Fan, H.; Gao, C.; Dong, Q.; Hong, B.; Fang, Z.; Hu, M.; Lai, Y. Silver sites guide spatially homogeneous plating of lithium metal in 3D host. J. Electroanal. Chem. 2018, 824, 175–180. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, S.-W.; Li, D.; Wang, X.; Chen, X.; Shi, R.; Shen, N.; Jin, M.; Zhang, X.; Lian, Q.; et al. Sandwiched Li plating between Lithiophilic-Lithiophobic gradient Silver@Fullerene interphase layer for ultrastable lithium metal anodes. Chem. Eng. J. 2022, 429, 132156. [Google Scholar] [CrossRef]
- Han, K.H.; Seok, J.Y.; Kim, I.H.; Woo, K.; Kim, J.H.; Yang, G.G.; Choi, H.J.; Kwon, S.; Jung, E.I.; Kim, S.O. A 2D Ultrathin Nanopatterned Interlayer to Suppress Lithium Dendrite Growth in High-Energy Lithium-Metal Anodes. Adv. Mater. 2022, 34, 2203992. [Google Scholar] [CrossRef]
- Tian, R.; Chen, R.; Xu, Z.; Wan, S.; Guan, L.; Duan, H.; Li, H.; Zhu, H.; Sun, D.; Liu, H. Electrodeposition behavior of lithium metal on carbon substrates with surface silvering. Carbon 2019, 152, 503–510. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Q.; Bi, Y.; Cai, M.; Dunn, B.; Glossmann, T.; Liu, J.; Osaka, T.; Sugiura, R.; Wu, B.; et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 2020, 5, 561–568. [Google Scholar] [CrossRef]
- Yang, F.; Wang, D.; Zhao, Y.; Tsui, K.-L.; Bae, S.J. A study of the relationship between coulombic efficiency and capacity degradation of commercial lithium-ion batteries. Energy 2018, 145, 486–495. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Y.; He, S.; Xie, H.; Hitz, E.; Hu, L. Ultrafine Silver Nanoparticles for Seeded Lithium Deposition toward Stable Lithium Metal Anode. Adv. Mater. 2017, 29, 1702714. [Google Scholar] [CrossRef]
- Liu, Z.; Ha, S.; Liu, Y.; Wang, F.; Tao, F.; Xu, B.; Yu, R.; Wang, G.; Ren, F.; Li, H. Application of Ag-based materials in high-performance lithium metal anode: A review. J. Mater. Sci. Technol. 2023, 133, 165–182. [Google Scholar] [CrossRef]
- Orazem, M.E.; Ulgut, B. On the Proper Use of a Warburg Impedance. J. Electrochem. Soc. 2024, 171, 040526. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirbagheri, S.; Gibertini, E.; Magagnin, L. Inkjet-Printed Silver Lithiophilic Sites on Copper Current Collectors: Tuning the Interfacial Electrochemistry for Anode-Free Lithium Batteries. Batteries 2024, 10, 369. https://doi.org/10.3390/batteries10100369
Mirbagheri S, Gibertini E, Magagnin L. Inkjet-Printed Silver Lithiophilic Sites on Copper Current Collectors: Tuning the Interfacial Electrochemistry for Anode-Free Lithium Batteries. Batteries. 2024; 10(10):369. https://doi.org/10.3390/batteries10100369
Chicago/Turabian StyleMirbagheri, Seyedalireza, Eugenio Gibertini, and Luca Magagnin. 2024. "Inkjet-Printed Silver Lithiophilic Sites on Copper Current Collectors: Tuning the Interfacial Electrochemistry for Anode-Free Lithium Batteries" Batteries 10, no. 10: 369. https://doi.org/10.3390/batteries10100369
APA StyleMirbagheri, S., Gibertini, E., & Magagnin, L. (2024). Inkjet-Printed Silver Lithiophilic Sites on Copper Current Collectors: Tuning the Interfacial Electrochemistry for Anode-Free Lithium Batteries. Batteries, 10(10), 369. https://doi.org/10.3390/batteries10100369






