Nanomaterial Endocytosis: Quantification of Adsorption and Ingestion Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials
2.3. Cell Culture
2.4. Experiment Procedure
2.5. Beads per Cell
2.6. Adsorption Isotherms
2.7. DLVO Analysis
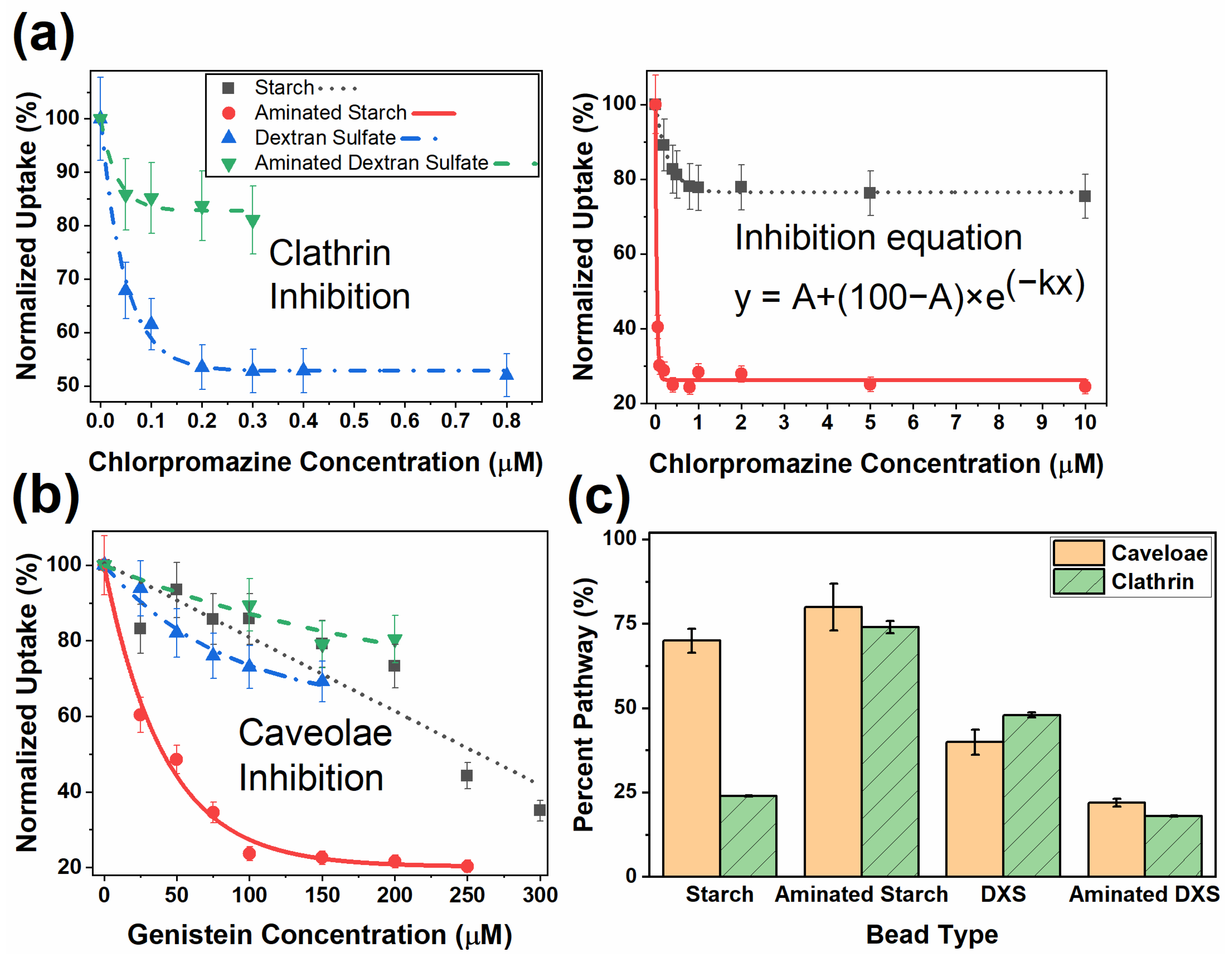
2.8. Inhibition Experiments
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, E.J.; Leon, L.; Rinaldi, C. Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier: Amsterdam, Netherlands, 2019; ISBN 0128166630. Available online: https://www.elsevier.com/books/nanoparticles-for-biomedical-applications/chung/978-0-12-816662-8 (accessed on 1 March 2020).
- Donovan, R. (Ed.) Applications of Nanotechnology in Drug Delivery; Scitus Academics LLC: Wilmington, Delaware, 2016; ISBN 9781681172385. Available online: https://books.google.com/books/about/Application_of_Nanotechnology_in_Drug_De.html?id=jzPjjwEACAAJ (accessed on 15 April 2019).
- Steinmetz, N.F.; Manchester, M. Viral Nanoparticles: Tools for Material Science and Biomedicine; Pan Stanford Publishing: Singapore, 2011; ISBN 9814267457. [Google Scholar] [CrossRef]
- Guerrini, G.; Magrì, D.; Gioria, S.; Medaglini, D.; Calzolai, L. Characterization of Nanoparticles-Based Vaccines for COVID-19. Nat. Nanotechnol. 2022, 17, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Effah, C.Y.; Wu, L.; Yu, F.; Wei, J.; Mao, G.; Xiong, Y.; He, L. Endocytosis and Intracellular RNAs Imaging of Nanomaterials-Based Fluorescence Probes. Talanta 2022, 243, 123377. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J. (Ed.) Safety of Nanoparticles: From Manufacturing to Medical Applications, 1st ed.; Springer: New York, NY, USA, 2008; ISBN 978-0-387-78608-7. [Google Scholar] [CrossRef]
- Hanot, C.C.; Choi, Y.S.; Anani, T.B.; Soundarrajan, D.; David, A.E. Effects of Iron-Oxide Nanoparticle Surface Chemistry on Uptake Kinetics and Cytotoxicity in CHO-K1 Cells. Int. J. Mol. Sci. 2015, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Leary, J.F. Fundamentals of Nanomedicine; Cambridge University Press: Cambridge, UK, 2022; ISBN 1009258311. Available online: https://www.cambridge.org/us/academic/subjects/engineering/biomedical-engineering/fundamentals-nanomedicine (accessed on 1 September 2022).
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Hill, H.L.; Yang, V.C. Polyethylene Glycol Modified, Cross-Linked Starch-Coated Iron Oxide Nanoparticles for Enhanced Magnetic Tumor Targeting. Biomaterials 2011, 32, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, F.; Lévy, M.; Wilhelm, C. Optimizing Magnetic Nanoparticle Design for Nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, L.; Papa, A.-L.; Dumont, L.; Bouyer, F.; Walker, P.; Vandroux, D.; Millot, N. Influence of Surface Charge and Polymer Coating on Internalization and Biodistribution of Polyethylene Glycol-Modified Iron Oxide Nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stenzel, M.H. Entry of Nanoparticles into Cells: The Importance of Nanoparticle Properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- David, A.E.; Cole, A.J.; Chertok, B.; Park, Y.S.; Yang, V.C. A Combined Theoretical and in Vitro Modeling Approach for Predicting the Magnetic Capture and Retention of Magnetic Nanoparticles In Vivo. J. Control. Release 2011, 152, 67–75. [Google Scholar] [CrossRef]
- Li, Y.; Kröger, M.; Liu, W.K. Endocytosis of PEGylated Nanoparticles Accompanied by Structural and Free Energy Changes of the Grafted Polyethylene Glycol. Biomaterials 2014, 35, 8467–8478. [Google Scholar] [CrossRef]
- Ohshima, H.; Makino, K. Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 0444626085. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Hou, J.; Baker, G.L.; Khan, S.A. Colloidal Interactions between Particles with Tethered Nonpolar Chains Dispersed in Polar Media: Direct Correlation between Dynamic Rheology and Interaction Parameters. Langmuir 2000, 16, 1066–1077. [Google Scholar] [CrossRef]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.-L.; Gramatke, A.M.; Joksimovic, R.; Sokolowski, M.; Gradzielski, M.; Haase, A. Size and Cell Type Dependent Uptake of Silica Nanoparticles. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Zhou, C.; Choi, Y.S.; David, A.E.; Todd, P.W.; Hanley, T.R. Nanomaterial Endocytosis: Estimation of Particles per Cell by Magnetic Measurement. IEEE Magn. Lett. 2018, 9, 7–11. [Google Scholar] [CrossRef]
- Pisciotti, M.L.M.; Lima, E., Jr.; Mansilla, M.V.; Tognoli, V.E.; Troiani, H.E.; Pasa, A.A.; Creczynski-Pasa, T.B.; Silva, A.H.; Gurman, P.; Colombo, L.; et al. In Vitro and in Vivo Experiments with Iron Oxide Nanoparticles Functionalized with DEXTRAN or Polyethylene Glycol for Medical Applications: Magnetic Targeting. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.L.; Botos, E. Endocytosis via Caveolae: Alternative Pathway with Distinct Cellular Compartments to Avoid Lysosomal Degradation? J. Cell. Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and Exocytosis of Nanoparticles in Mammalian Cells. Int. J. Nanomed. 2014, 9, 51. [Google Scholar] [CrossRef]
- Jing, Y.; Mal, N.; Williams, P.S.; Mayorga, M.; Penn, M.S.; Chalmers, J.J.; Zborowski, M. Quantitative Intracellular Magnetic Nanoparticle Uptake Measured by Live Cell Magnetophoresis. FASEB J. 2008, 22, 4239–4247. [Google Scholar] [CrossRef]
- Robert, D.; Pamme, N.; Conjeaud, H.; Gazeau, F.; Iles, A.; Wilhelm, C. Cell Sorting by Endocytotic Capacity in a Microfluidic Magnetophoresis Device. Lab Chip 2011, 11, 1902–1910. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano. Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Haddad, S.; Marshall, R.J.; Lázaro, I.A.; Boix, G.; Imaz, I.; Maspoch, D.; Forgan, R.S.; Fairen-Jimenez, D. Tuning the Endocytosis Mechanism of Zr-Based Metal–Organic Frameworks through Linker Functionalization. ACS Appl. Mater. Interfaces 2017, 9, 35516–35525. [Google Scholar] [CrossRef]
- Wilhelm, C.; Billotey, C.; Roger, J.; Pons, J.N.; Bacri, J.-C.; Gazeau, F. Intracellular Uptake of Anionic Superparamagnetic Nanoparticles as a Function of Their Surface Coating. Biomaterials 2003, 24, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Gazeau, F.; Roger, J.; Pons, J.N.; Bacri, J.-C. Interaction of Anionic Superparamagnetic Nanoparticles with Cells: Kinetic Analyses of Membrane Adsorption and Subsequent Internalization. Langmuir 2002, 18, 8148–8155. [Google Scholar] [CrossRef]
- Liang, L.; Everest-Dass, A.V.; Kostyuk, A.B.; Khabir, Z.; Zhang, R.; Trushina, D.B.; Zvyagin, A.V. The Surface Charge of Polymer-Coated Upconversion Nanoparticles Determines Protein Corona Properties and Cell Recognition in Serum Solutions. Cells 2022, 11, 3644. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Kamm, R.D.; Kah, J.C.Y. Influence of Protein Corona and Caveolae-Mediated Endocytosis on Nanoparticle Uptake and Transcytosis. Nanoscale 2018, 10, 12386–12397. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tian, X.; Wu, A.; Li, J.; Tian, J.; Chong, Y.; Chai, Z.; Zhao, Y.; Chen, C.; Ge, C. Protein Corona Influences Cellular Uptake of Gold Nanoparticles by Phagocytic and Nonphagocytic Cells in a Size-Dependent Manner. ACS Appl. Mater. Interfaces 2015, 7, 20568–20575. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Tanaka, Y.; Koide, Y.; Tanaka, M.; Hara, M. Mechanism Underlying Bioinertness of Self-Assembled Monolayers of Oligo (Ethyleneglycol)-Terminated Alkanethiols on Gold: Protein Adsorption, Platelet Adhesion, and Surface Forces. Phys. Chem. Chem. Phys. 2012, 14, 10196–10206. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Wang, S.-H.; Lee, C.-W.; Chiou, A.; Wei, P.-K. Size-Dependent Endocytosis of Gold Nanoparticles Studied by Three-Dimensional Mapping of Plasmonic Scattering Images. J. Nanobiotechnology 2010, 8, 33. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical Properties of Nanoparticles: Basics and Applications. J. Phys. D. Appl. Phys. 2013, 47, 13001. [Google Scholar] [CrossRef]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; Smedt, S.C.D.; Sanders, N.N.; Braeckmans, K. The Use of Inhibitors to Study Endocytic Pathways of Gene Carriers: Optimization and Pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef]
- Anani, T. Matrix Metalloproteinase-Responsive Superparamagnetic Iron Oxide Nanoparticles (SPIONs) to Distinguish between Aggressive and Indolent Cancer. Master’s Thesis, Auburn University, Auburn, AL, USA, 2018. Available online: http://hdl.handle.net/10415/6167 (accessed on 1 January 2019).
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric Ferrozine-Based Assay for the Quantitation of Iron in Cultured Cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Semisch, A.; Ohle, J.; Witt, B.; Hartwig, A. Cytotoxicity and Genotoxicity of Nano-and Microparticulate Copper Oxide: Role of Solubility and Intracellular Bioavailability. Part. Fibre Toxicol. 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Fuerstenau, D.W. Zeta Potentials in the Flotation of Oxide and Silicate Minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Fike, R.M.; Van Oss, C.J. Zeta-Potentials of Intact Cell Monolayers Determined by Electro-Osmosis. In Vitro 1976, 12, 428–435. [Google Scholar] [CrossRef]
- Bruinsma, R.; Behrisch, A.; Sackmann, E. Adhesive Switching of Membranes: Experiment and Theory. Phys. Rev. E 2000, 61, 4253. [Google Scholar] [CrossRef]
- Roth, C.M.; Neal, B.L.; Lenhoff, A.M. Van Der Waals Interactions Involving Proteins. Biophys. J. 1996, 70, 977–987. [Google Scholar] [CrossRef]
- Shutava, T.G.; Livanovich, K.S. Colloidal Stability of Silver Nanoparticles with Layer-by-Layer Shell of Chitosan Copolymers. J. Nanoparticle Res. 2020, 22, 154. [Google Scholar] [CrossRef]
- Dong, X.; Al-Jumaily, A.; Escobar, I.C. Investigation of the Use of a Bio-Derived Solvent for Non-Solvent-Induced Phase Separation (NIPS) Fabrication of Polysulfone Membranes. Membranes 2018, 8, 23. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The Effect of Surface Charge of Functionalized Fe3O4 Nanoparticles on Protein Adsorption and Cell Uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef]
- Ehi-Eromosele, C.O. The Effect of Polyethylene Glycol (PEG) Coating on the Magneto-Structural Properties and Colloidal Stability of Co0.8Mg0.2Fe2O4 Nanoparticles for Potential Biomedical Applications. Dig. J. Nanomater. Biostructures 2016, 11, 7–14. Available online: http://eprints.covenantuniversity.edu.ng/id/eprint/5968 (accessed on 1 March 2019).
- Hirohara, M.; Maekawa, T.; Mondarte, E.A.Q.; Nyu, T.; Mizushita, Y.; Hayashi, T. Proteomic Analysis of Biomaterial Surfaces after Contacting with Body Fluids by MALDI-ToF Mass Spectroscopy. Coatings 2020, 10, 12. [Google Scholar] [CrossRef]
- Molday, R.S.; Yen, S.P.S.; Rembaum, A. Application of Magnetic Microspheres in Labelling and Separation of Cells. Nature 1977, 268, 437. [Google Scholar] [CrossRef] [PubMed]
- Bertorelle, F.; Wilhelm, C.; Roger, J.; Gazeau, F.; Ménager, C.; Cabuil, V. Fluorescence-Modified Superparamagnetic Nanoparticles: Intracellular Uptake and Use in Cellular Imaging. Langmuir 2006, 22, 5385–5391. [Google Scholar] [CrossRef] [PubMed]
- Streibel, M.J. Electrophoretic and Microelectrode Studies of the Electrical Surface of Cultured Mammalian Cells; Penn State University: State College, PA, USA, 1972. [Google Scholar]
- Bhattacharjee, S.; Chen, J.Y.; Elimelech, M. DLVO Interaction Energy between Spheroidal Particles and a Flat Surface. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 165, 143–156. [Google Scholar] [CrossRef]
- Yang, E.-B.; Wang, D.-F.; Mack, P.; Cheng, L.-Y. Genistein, a Tyrosine Kinase Inhibitor, Reduces EGF-Induced EGF Receptor Internalization and Degradation in Human Hepatoma HepG2 Cells. Biochem. Biophys. Res. Commun. 1996, 224, 309–317. [Google Scholar] [CrossRef]
- De Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; Van Nieuwkoop, S.; Bestebroer, T.M.; Van Den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-Approved Compound Library Identifies Four Small-Molecule Inhibitors of Middle East Respiratory Syndrome Coronavirus Replication in Cell Culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. [Google Scholar] [CrossRef]
- Otręba, M.; Kośmider, L.; Rzepecka-Stojko, A. Antiviral Activity of Chlorpromazine, Fluphenazine, Perphenazine, Prochlorperazine, and Thioridazine towards RNA-Viruses. A Review. Eur. J. Pharmacol. 2020, 173553. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of Quantum Dot Nanoparticle Cellular Uptake. Toxicol. Sci. 2009, 110, 138–155. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted Nanoparticles in Mitochondrial Medicine. WIREs Nanomed. Nanobiotechnology 2015, 7, 315–329. [Google Scholar] [CrossRef]
- Müller, E.K.; Białas, N.; Epple, M.; Hilger, I. Nanoparticles Carrying NF-ΚB P65-Specific SiRNA Alleviate Colitis in Mice by Attenuating NF-ΚB-Related Protein Expression and Pro-Inflammatory Cellular Mediator Secretion. Pharmaceutics 2022, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. The Role of Specific and Non-Specific Interactions in Receptor-Mediated Endocytosis of Nanoparticles. Biomaterials 2007, 28, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shipton, M.K.; Ryan, J.; Kaufman, E.D.; Franzen, S.; Feldheim, D.L. Synthesis, Stability, and Cellular Internalization of Gold Nanoparticles Containing Mixed Peptide− Poly (Ethylene Glycol) Monolayers. Anal. Chem. 2007, 79, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
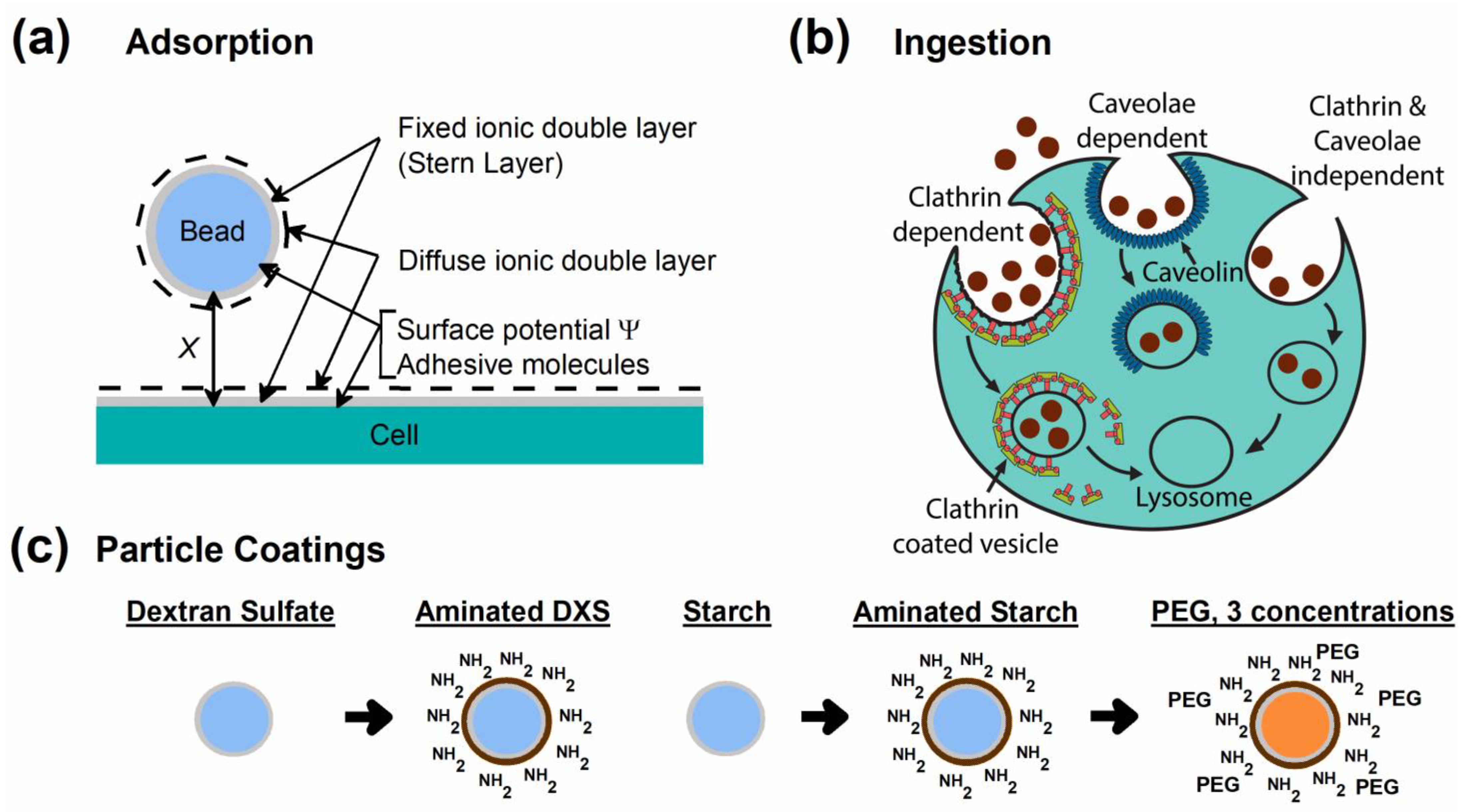
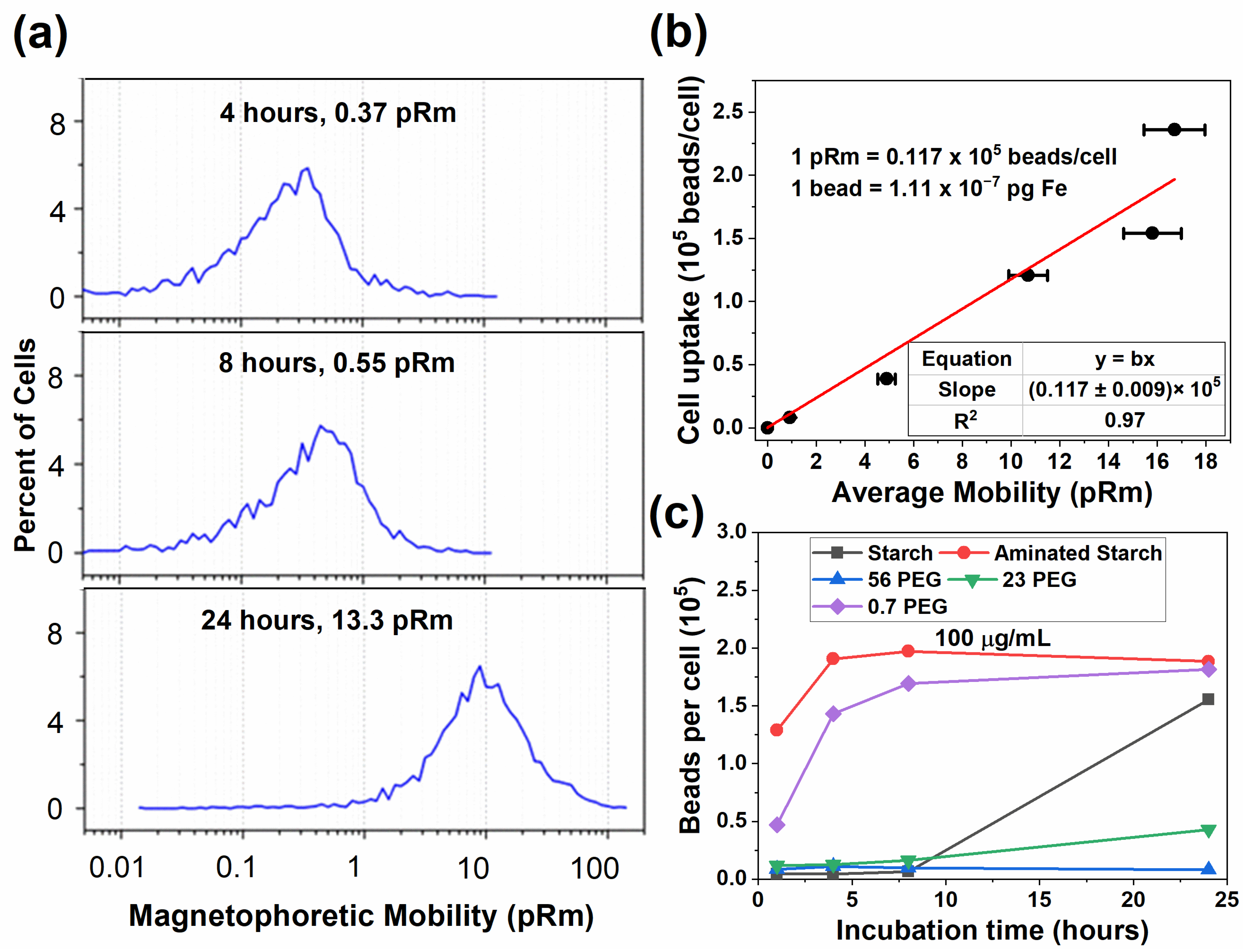
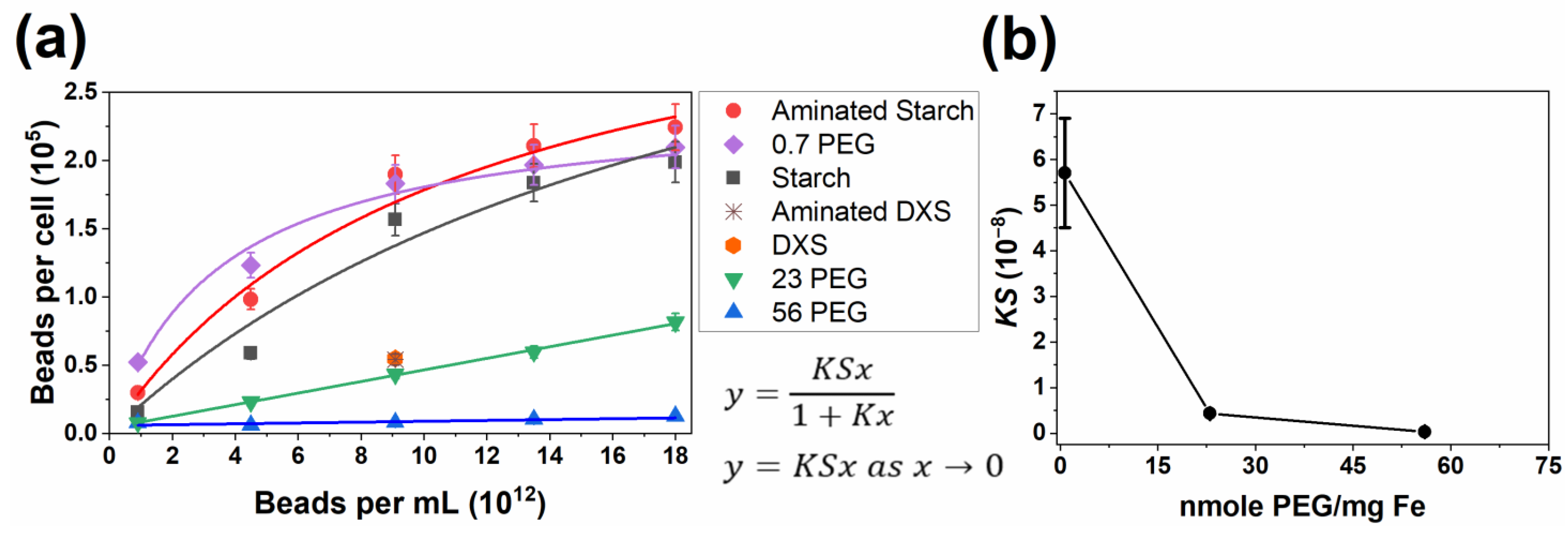
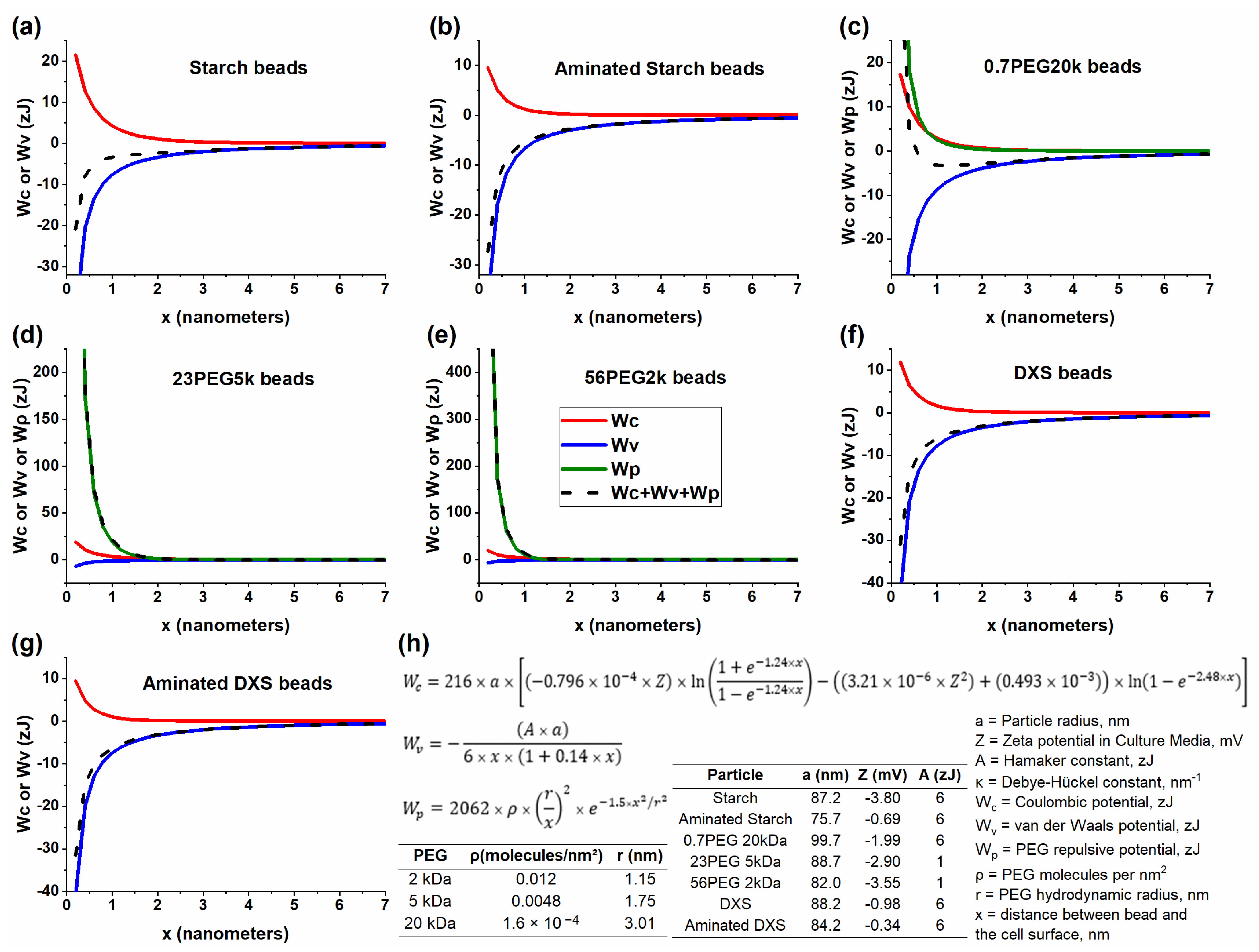
| Designation | Diameter in H2O (nm) | Diameter in CM (nm) | ZP in H2O (mV) | ZP in CM (mV) |
|---|---|---|---|---|
| Starch | 123.9 ± 22.1 | 174.4 ± 26.2 | −8.99 ± 6.38 | −3.78 ± 0.60 |
| Aminated Starch | 169.6 ± 32.6 | 151.4 ± 28.7 | +39.6 ± 5.94 | −0.69 ± 0.20 |
| * 0.7 PEG 20 kDa | 220.1 ± 45.3 | 199.5 ± 38.4 | +18.8 ± 4.1 | −1.99 ± 0.31 |
| * 23 PEG 5 kDa | 171.9 ± 29.9 | 177.5 ± 36.9 | +16.2 ± 5.9 | −2.90 ± 0.08 |
| * 56 PEG 2 kDa | 157.2 ± 29.3 | 164 ± 36.2 | +5.6 ± 5.1 | −3.55 ± 0.31 |
| DXS | 153.8 ± 28.2 | 176.5 ± 18.8 | −52.9 ± 7.62 | −0.98 ± 0.05 |
| Aminated DXS | 192.7 ± 38.2 | 168.4 ± 19.6 | −33.4 ± 5.73 | −0.34 ± 0.13 |
| Bead | K (10−13 mL/Bead) | S (105 Beads/Cell) | KS (10−8) | R2 |
|---|---|---|---|---|
| Aminated Starch | 1.0 ± 0.4 | 3.6 ± 0.6 | 3.6 ± 1.4 | 0.98 |
| 0.7 PEG | 2.2 ± 0.4 | 2.6 ± 0.15 | 5.7 ± 1.2 | 0.99 |
| Starch | 0.48 ± 0.33 | 4.5 ± 1.8 | 2.2 ± 1.3 | 0.96 |
| DXS | 0.55 ± 0.06 * | N/A | ||
| Aminated DXS | 0.54 ± 0.05 * | N/A | ||
| 23 PEG | 0.43 ± 0.01 ** | 0.99 | ||
| 56 PEG | 0.03 ± 0.01 ** | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sannidhi, A.; Zhou, C.; Choi, Y.S.; David, A.E.; Todd, P.W.; Hanley, T.R. Nanomaterial Endocytosis: Quantification of Adsorption and Ingestion Mechanisms. Magnetochemistry 2023, 9, 37. https://doi.org/10.3390/magnetochemistry9020037
Sannidhi A, Zhou C, Choi YS, David AE, Todd PW, Hanley TR. Nanomaterial Endocytosis: Quantification of Adsorption and Ingestion Mechanisms. Magnetochemistry. 2023; 9(2):37. https://doi.org/10.3390/magnetochemistry9020037
Chicago/Turabian StyleSannidhi, Abhinav, Chen Zhou, Young Suk Choi, Allan E. David, Paul W. Todd, and Thomas R. Hanley. 2023. "Nanomaterial Endocytosis: Quantification of Adsorption and Ingestion Mechanisms" Magnetochemistry 9, no. 2: 37. https://doi.org/10.3390/magnetochemistry9020037
APA StyleSannidhi, A., Zhou, C., Choi, Y. S., David, A. E., Todd, P. W., & Hanley, T. R. (2023). Nanomaterial Endocytosis: Quantification of Adsorption and Ingestion Mechanisms. Magnetochemistry, 9(2), 37. https://doi.org/10.3390/magnetochemistry9020037









