Nuclear Magnetic Resonance Spectroscopy Investigations of Naphthalene-Based 1,2,3-Triazole Systems for Anion Sensing
Abstract
:1. Introduction
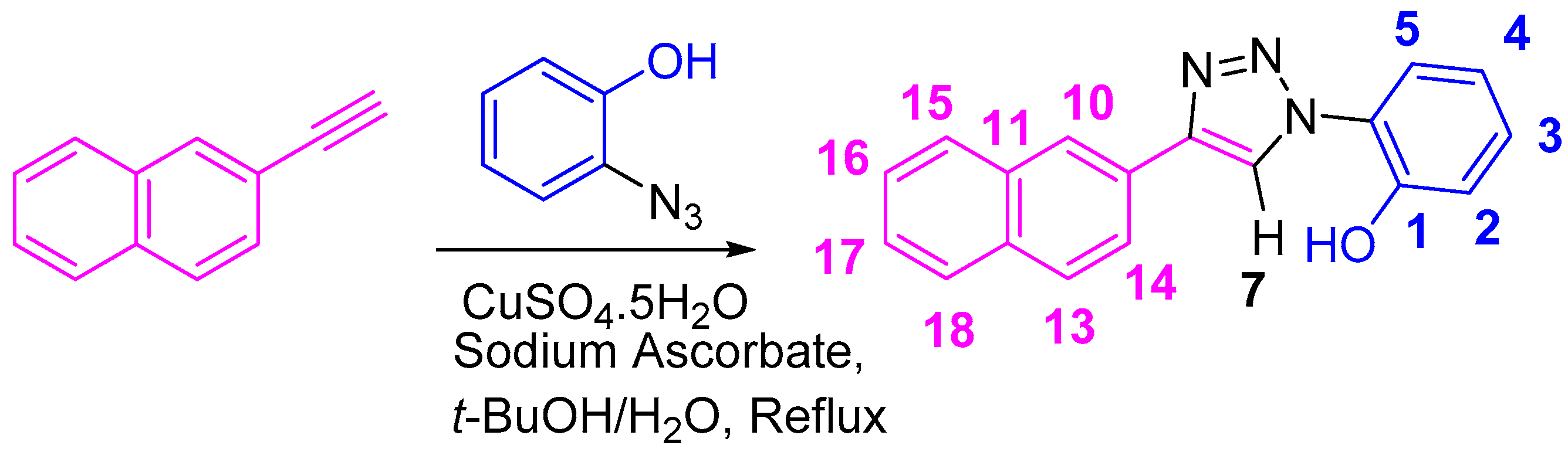
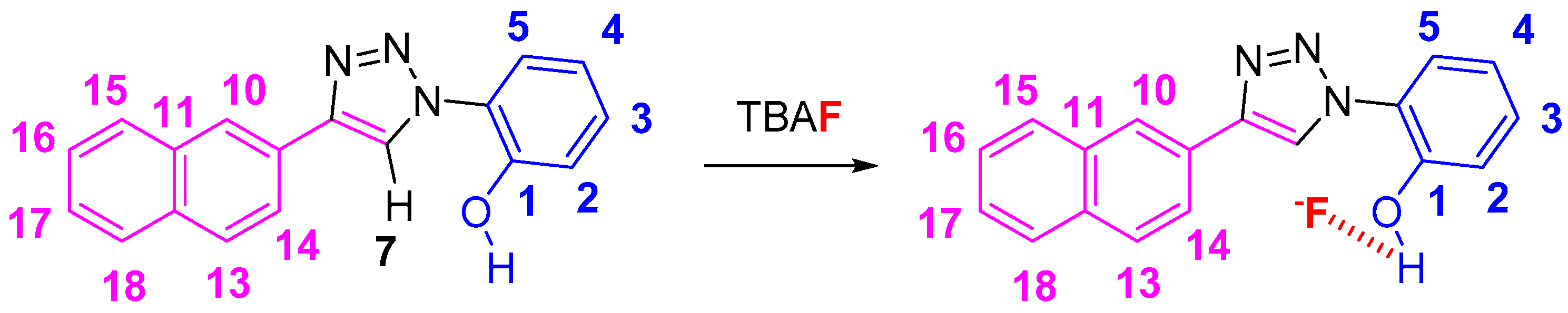
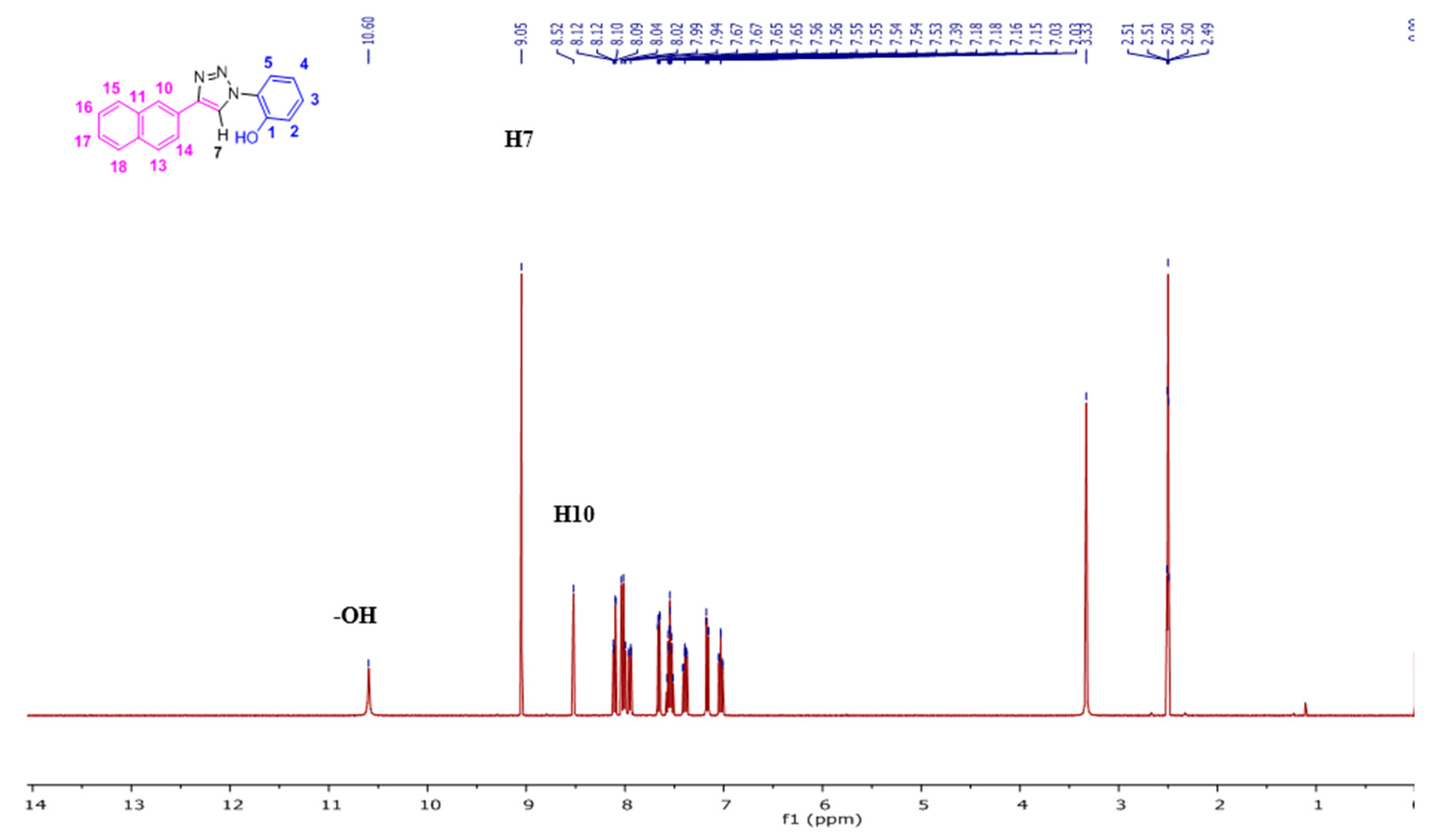
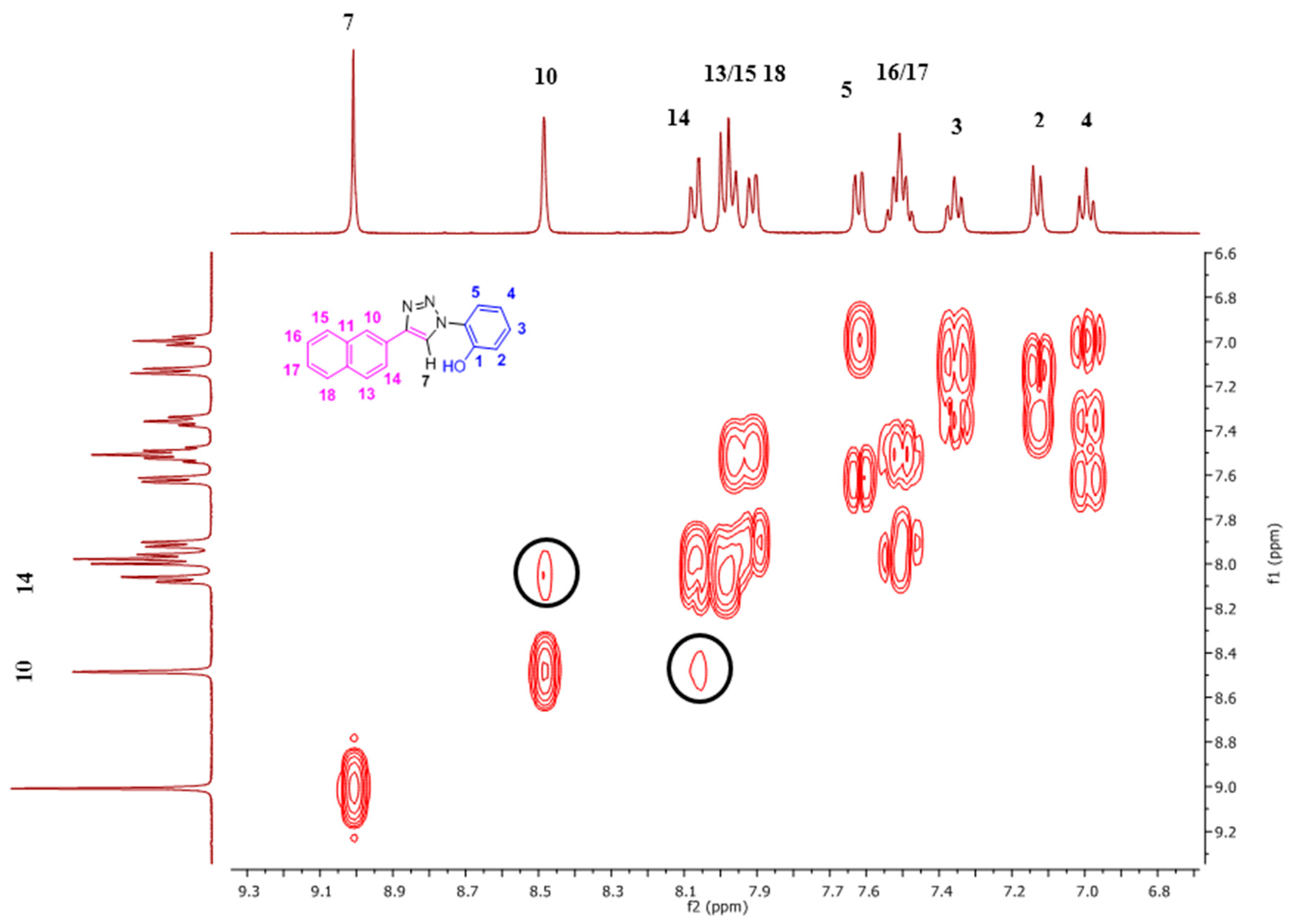
2. Results and Discussion
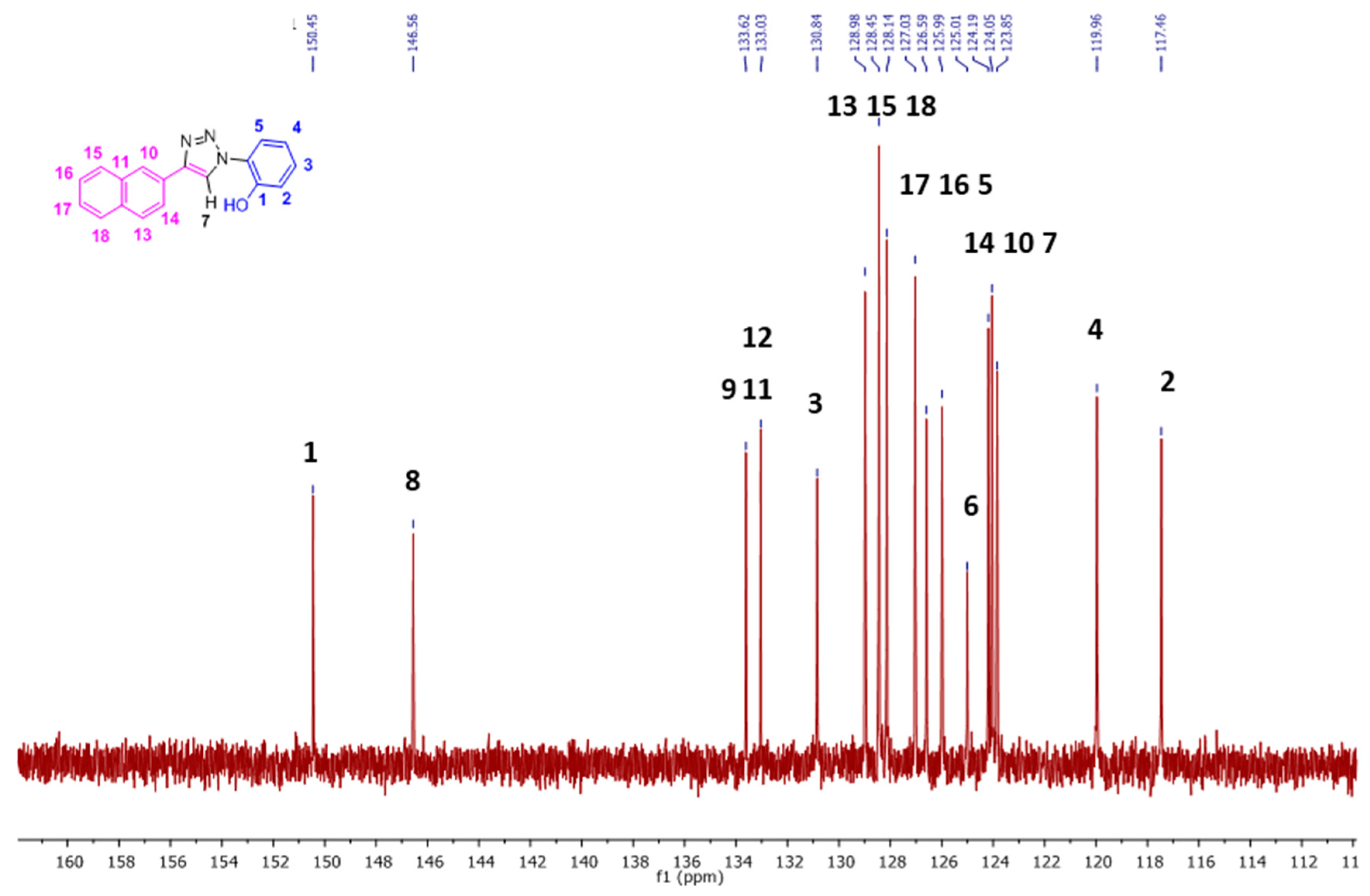
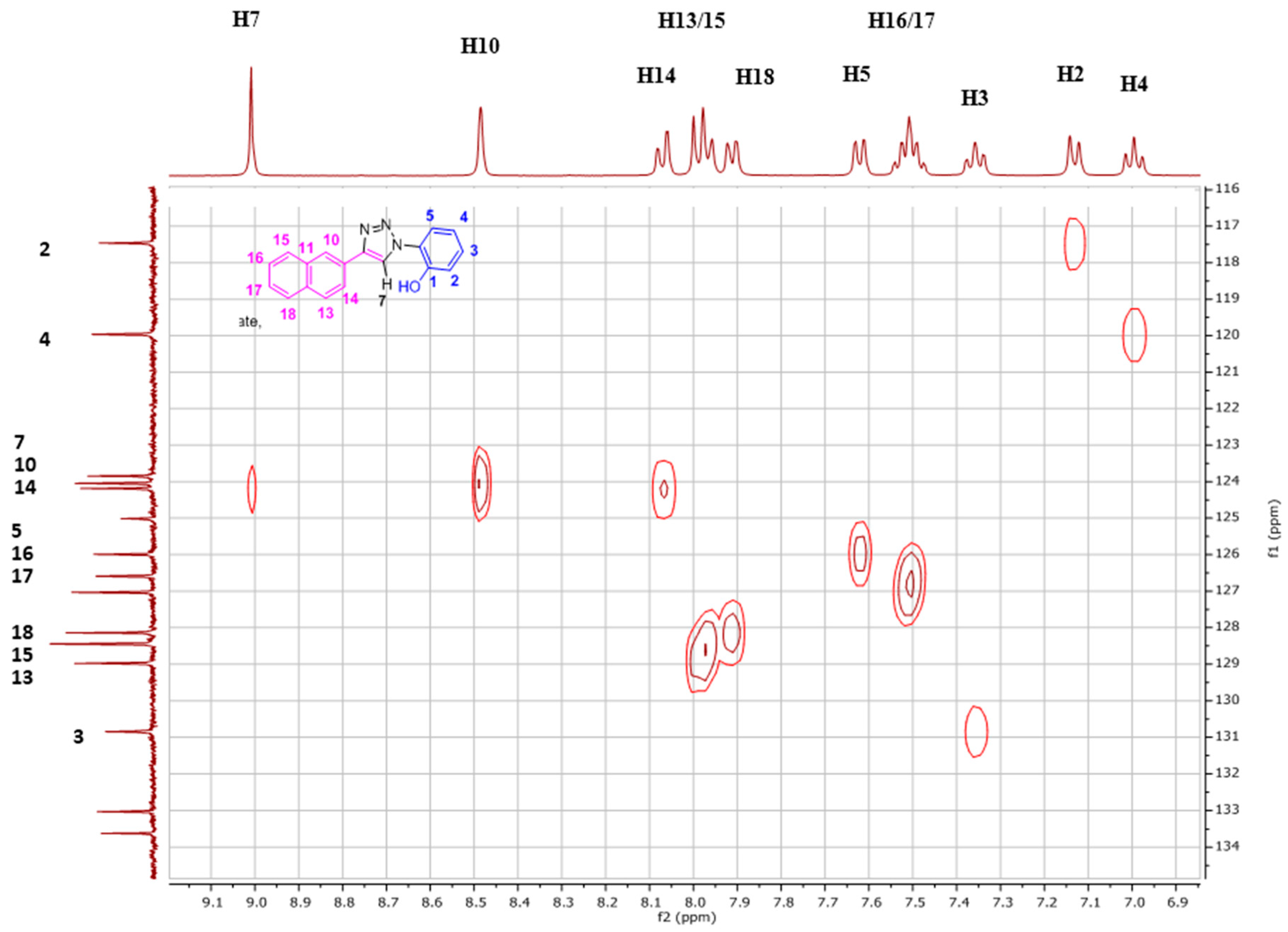
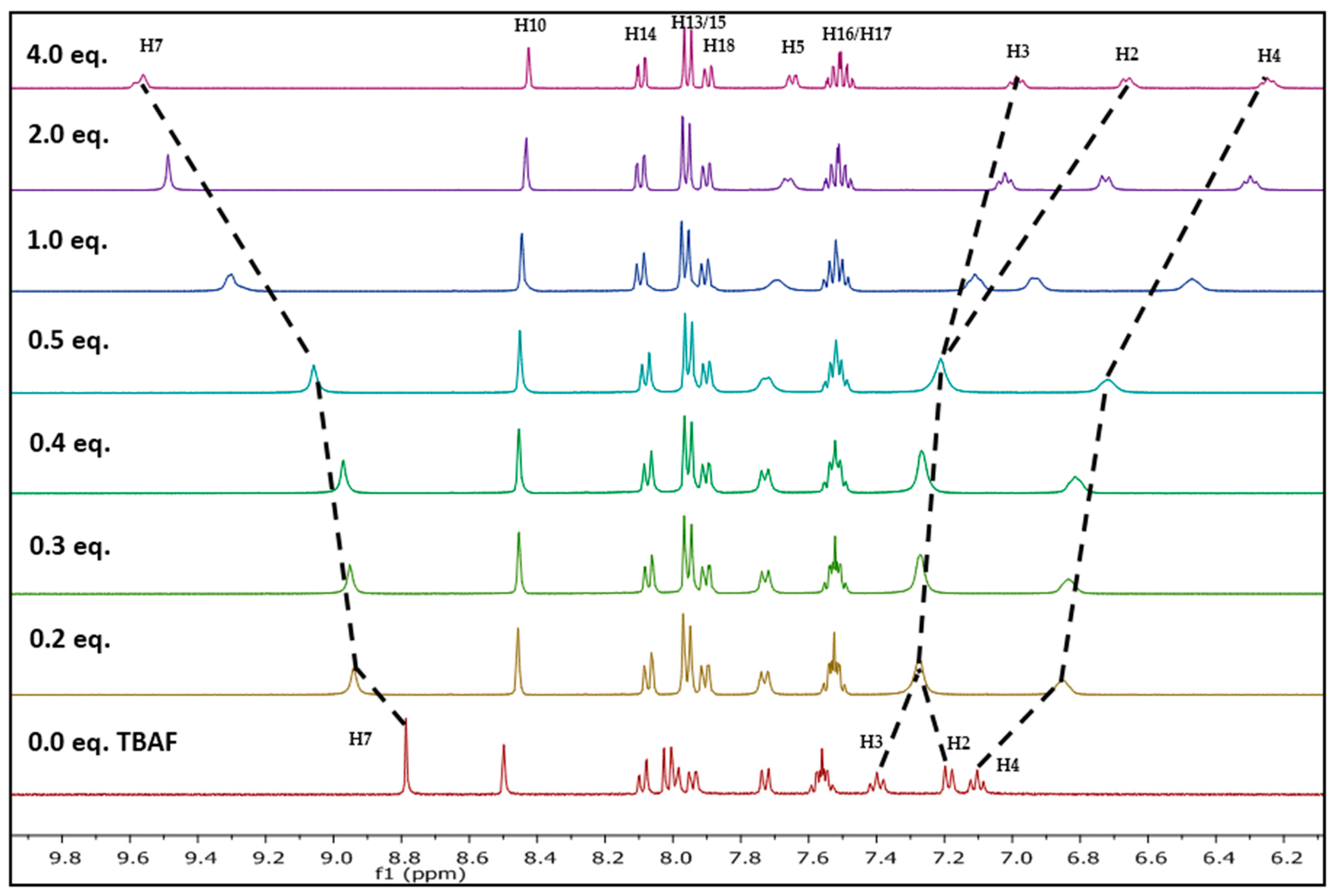
2.1. Nuclear Magnetic Resonance Spectroscopic Studies
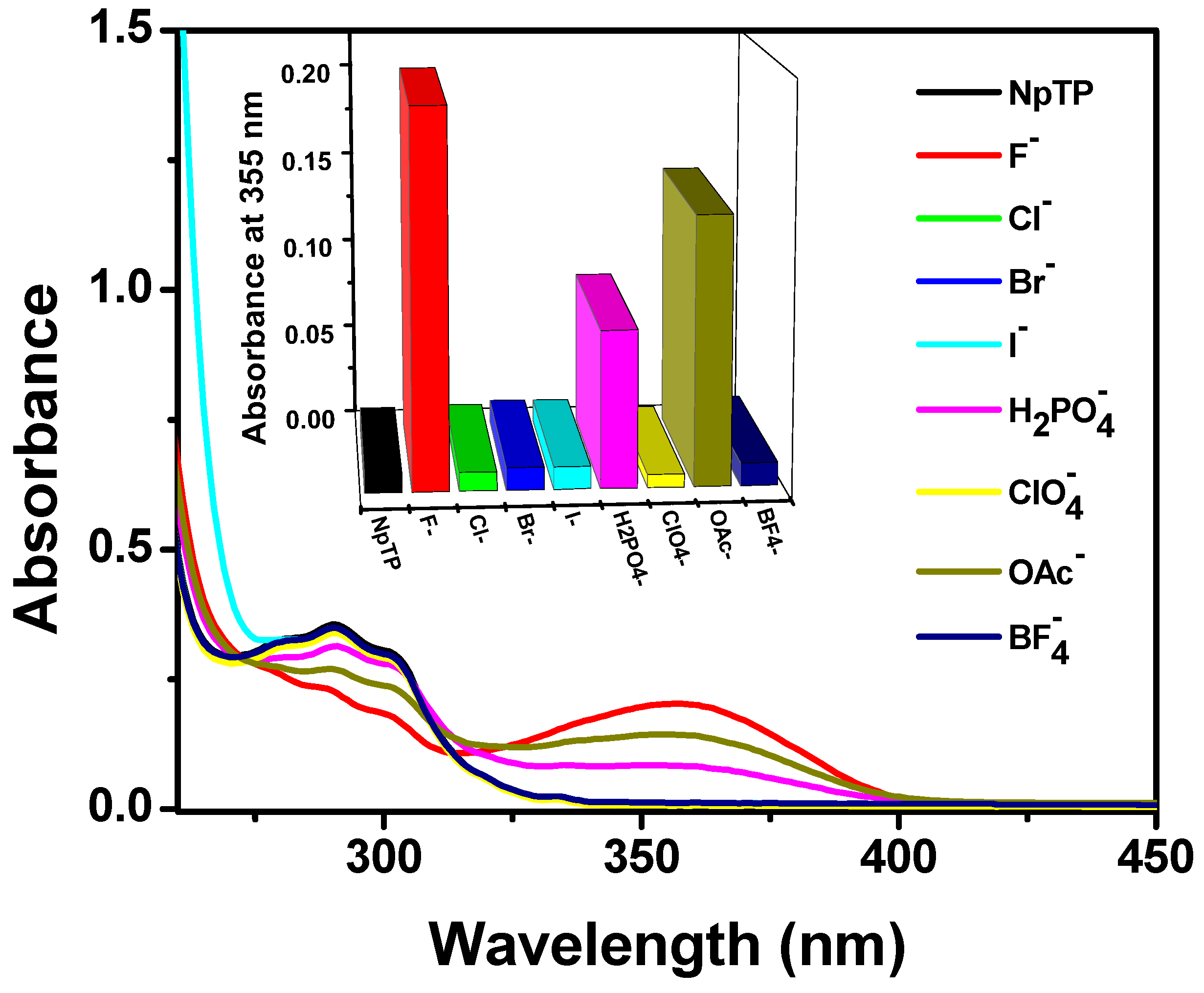
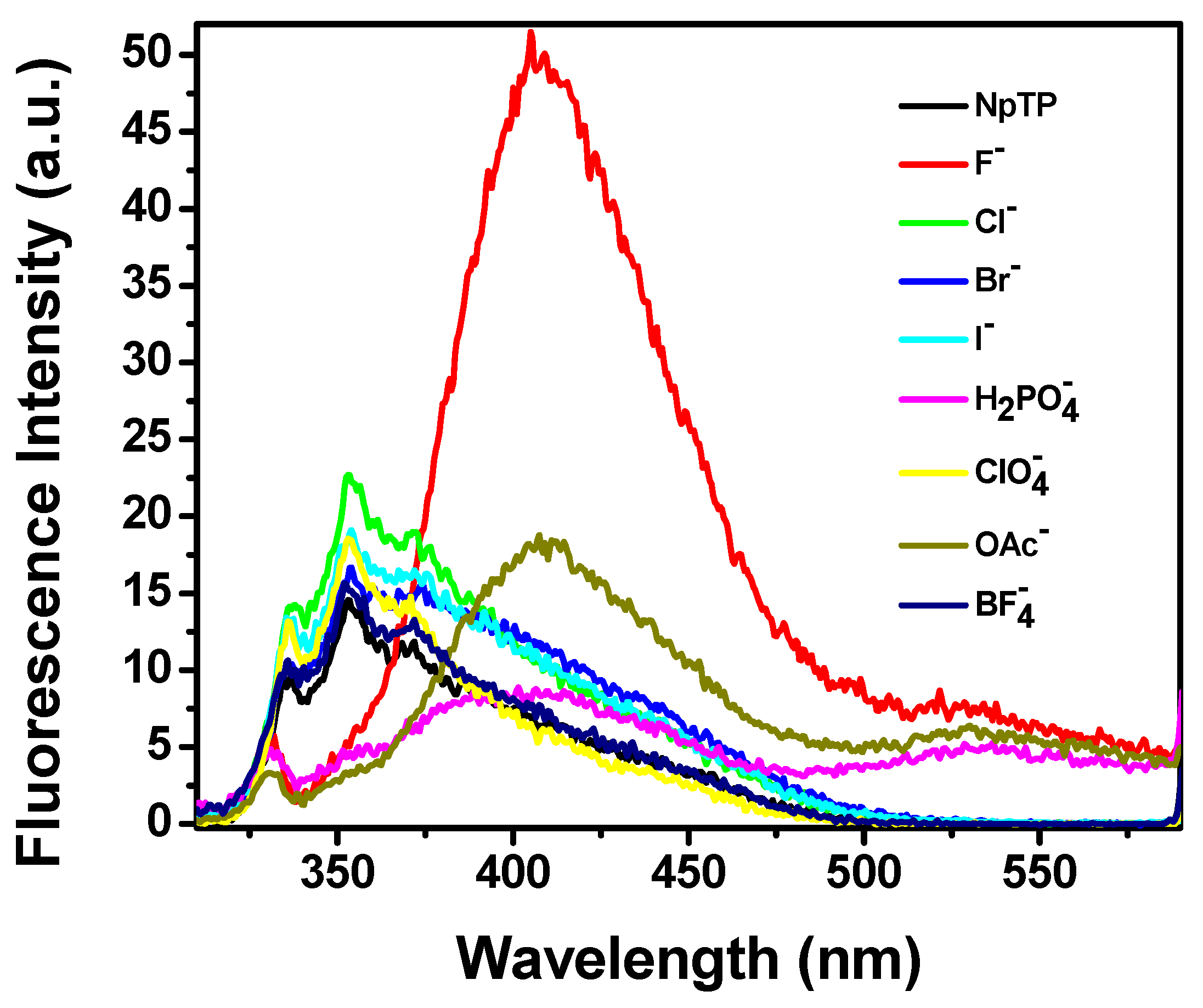
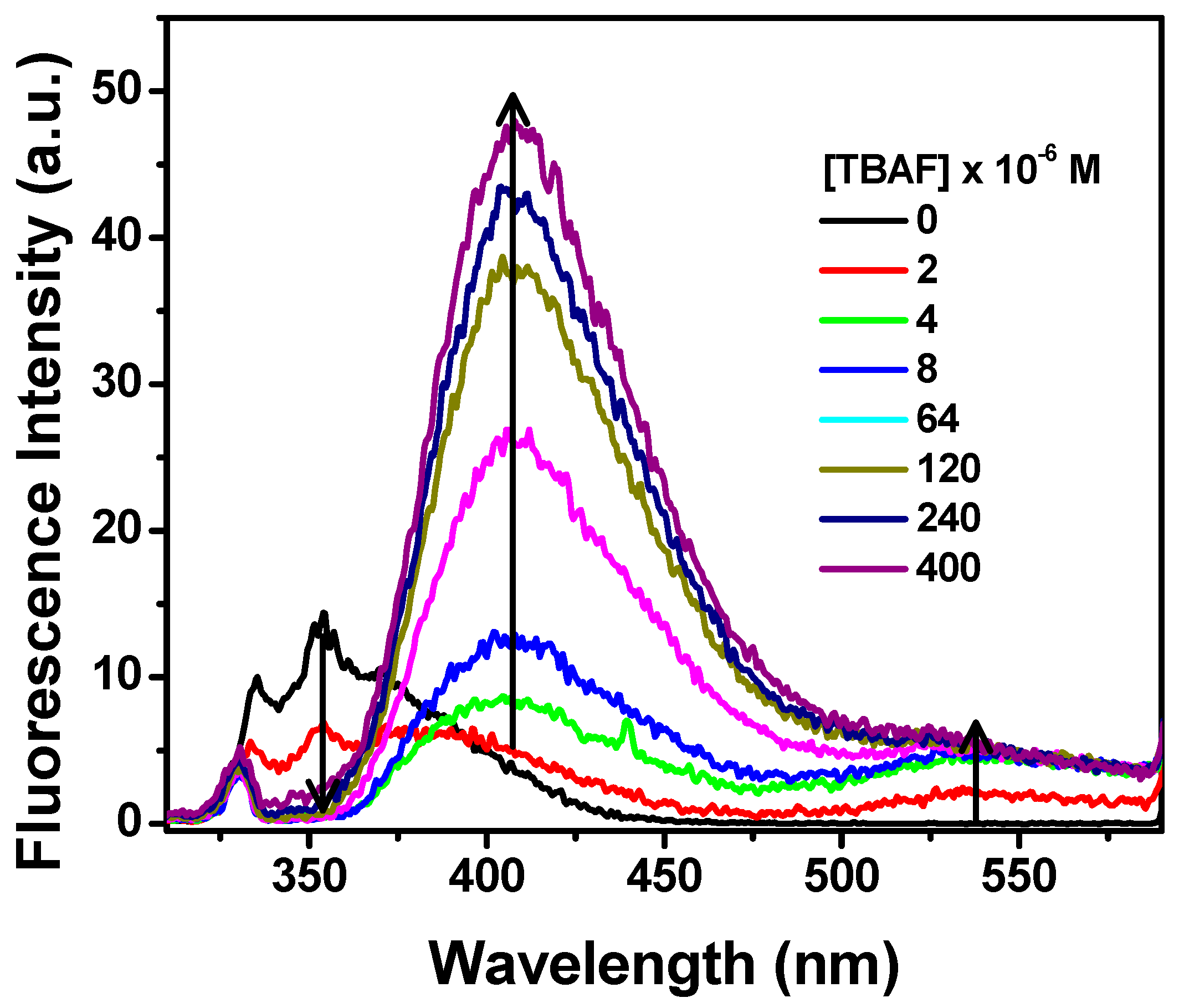
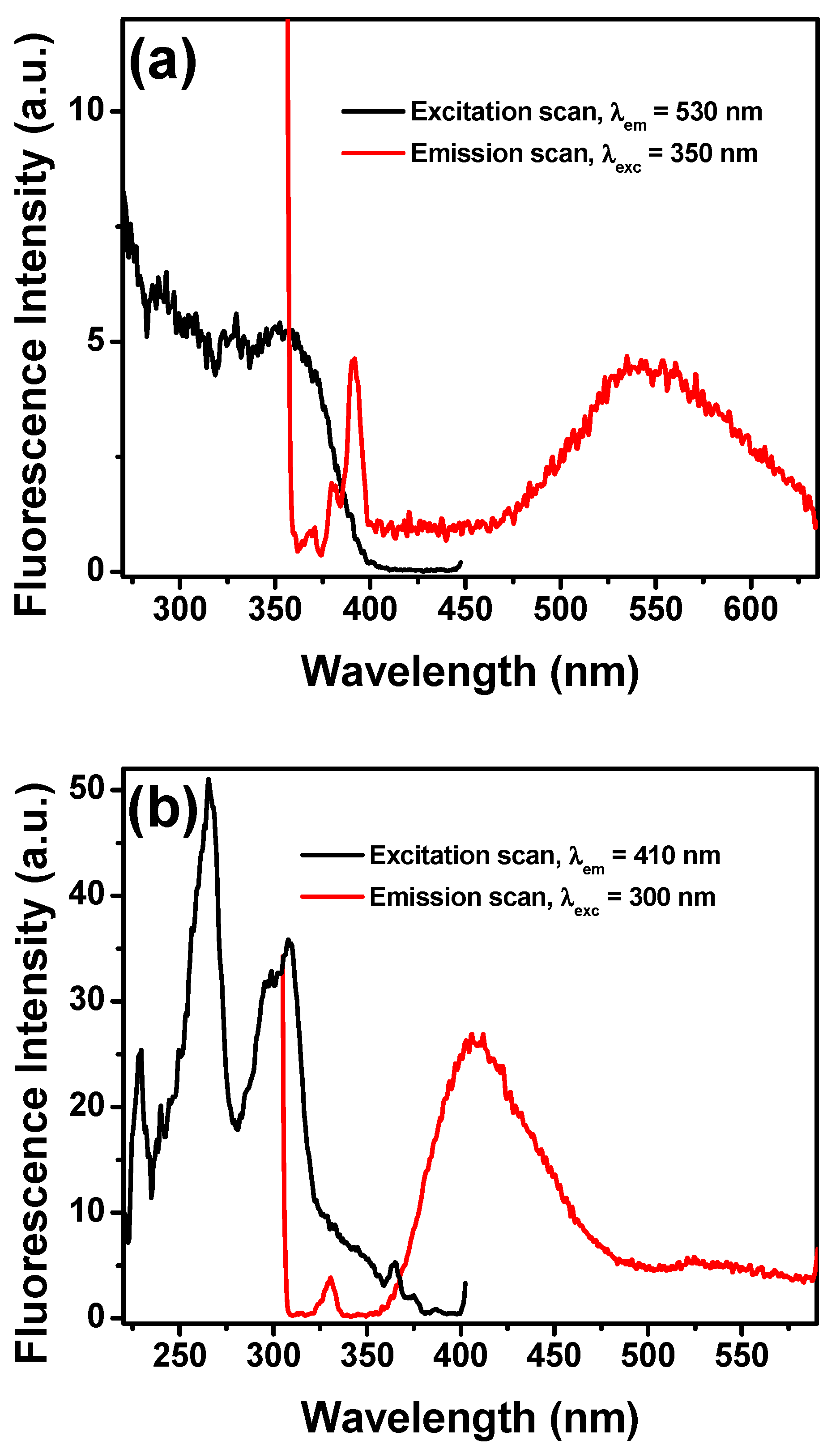
2.2. Absorption and Fluorescence Studies
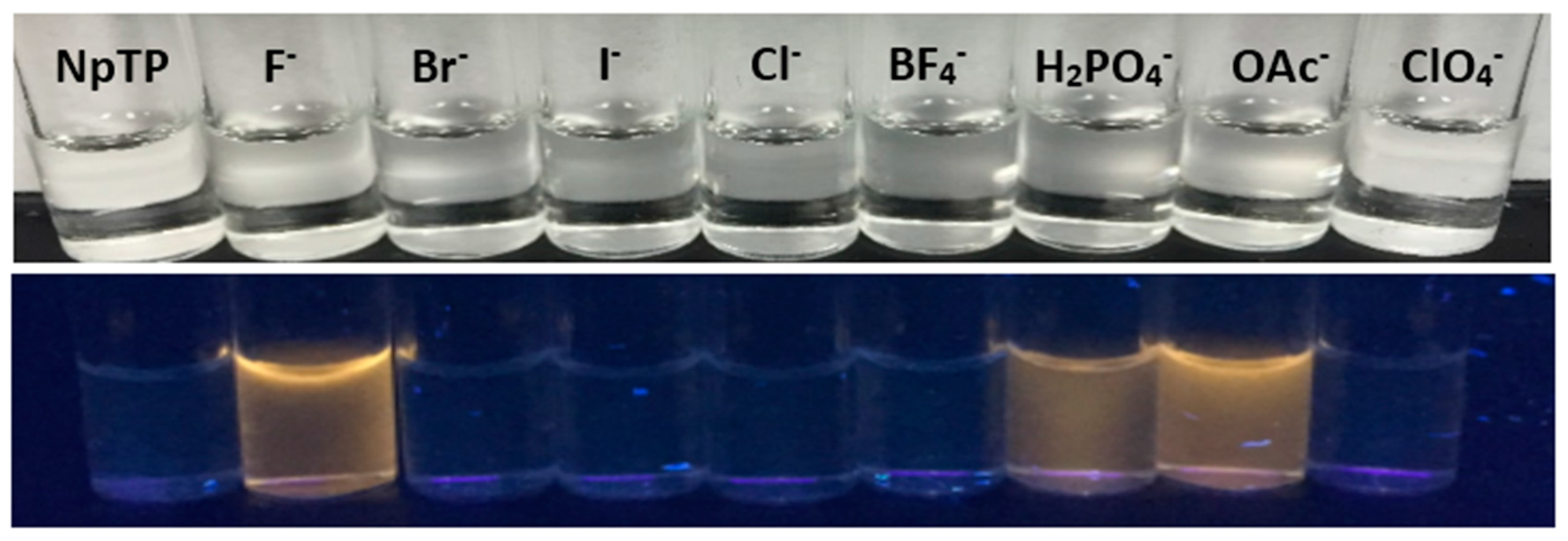
2.3. Color Studies
2.4. Single Crystal X-ray Crystallography Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lehn, J.-M. Molecular and Supramolecular Devices. In Supramolecular Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1995. [Google Scholar]
- Gopel, W.; Hesse, J.; Zemel, J.N. Sensors: A Comprehensive Survey; Wiley: Hoboken, NJ, USA, 1989. [Google Scholar]
- Zaccheroni, N.; Palomba, F.; Rampazzo, E. Luminescent Chemosensors: From Molecules to Nanostructures. In Applied Photochemistry: When Light Meets Molecules; Bergamini, G., Silvi, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Kubo, Y.; Yamamoto, M.; Ikeda, M.; Takeuchi, M.; Shinkai, S.; Yamaguchi, S.; Tamao, K. A Colorimetric and Ratiometric Fluorescent Chemosensor with Three Emission Changes: Fluoride Ion Sensing by a Triarylborane– Porphyrin Conjugate. Angew. Chem. Int. Ed. 2003, 42, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Udhayakumari, D.; Naha, S.; Velmathi, S. Colorimetric and fluorescent chemosensors for Cu2+. A comprehensive review from the years 2013–15. Anal. Methods 2017, 9, 552–578. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012, 41, 4511–4535. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Ahamad, K.U.; Nath, P. Low-Cost, Robust, and Field Portable Smartphone Platform Photometric Sensor for Fluoride Level Detection in Drinking Water. Anal. Chem. 2017, 89, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Ayoob, S.; Gupta, A.K. Fluoride in Drinking Water: A Review on the Status and Stress Effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Carey, C.M. Focus on Fluorides: Update on the Use of Fluoride for the Prevention of Dental Caries. J. Evid. Based Dent. Pract. 2014, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.T. Fluoride’s Effects on the Formation of Teeth and Bones, and the Influence of Genetics. J. Dent. Res. 2010, 90, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Singh, R.K.; Damodaran, T.; Mishra, V.K.; Sharma, D.K.; Rai, D. Fluoride in Groundwater: Toxicological Exposure and Remedies. J. Toxicol. Environ. Health 2013, 16, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Valdez Jiménez, L.; López Guzmán, O.D.; Cervantes Flores, M.; Costilla-Salazar, R.; Calderón Hernández, J.; Alcaraz Contreras, Y.; Rocha-Amador, D.O. In utero exposure to fluoride and cognitive development delay in infants. Neurotoxicology 2017, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, E.H.; De Loos-Vollebregt, M.T.C.; Wassink, A.P.; Kalter, H. Determination of trace elements in uranium by inductively coupled plasma-atomic emission spectrometry using Kalman filtering. Anal. Chem. 1992, 64, 1643–1649. [Google Scholar] [CrossRef]
- Orlov, A.A.; Tsimbalyuk, A.F.; Malyugin, R.V. Desublimation for Purification and Transporting UF6: Process Description and Modeling. Sep. Purif. Rev. 2017, 46, 81–89. [Google Scholar] [CrossRef]
- El Sayed, S.; Pascual, L.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F.; Costero, A.M.; Parra, M.; Gil, S. A Chromogenic Probe for the Selective Recognition of Sarin and Soman Mimic DFP. ChemistryOpen 2014, 3, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef] [PubMed]
- Duke, R.M.; Veale, E.B.; Pfeffer, F.M.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric and fluorescent anion sensors: an overview of recent developments in the use of 1,8-naphthalimide-based chemosensors. Chem. Soc. Rev. 2010, 39, 3936–3953. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Fabbrizzi, L.; Mosca, L.; Schmidtchen, F.-P. Urea-, Squaramide-, and Sulfonamide-Based Anion Receptors: A Thermodynamic Study. Chemistry 2011, 17, 5972–5981. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, R.; Diaz, Y.; Goldfuss, B.; Hertel, D.; Meerholz, K.; Griesbeck, A.G. Fluoride recognition by a chiral urea receptor linked to a phthalimide chromophore. Org. Biomol. Chem. 2009, 7, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Davis, A.P.; Glynn, M. Fluorescent photoinduced electron transfer (PET) sensing of anions using charge neutral chemosensors. Chem. Commun. 2001, 24, 2556–2557. [Google Scholar] [CrossRef]
- Beer, P.D. Transition-Metal Receptor Systems for the Selective Recognition and Sensing of Anionic Guest Species. Acc. Chem. Res. 1998, 31, 71–80. [Google Scholar] [CrossRef]
- Ghosh, D.; Nandi, N.; Chattopadhyay, N. Differential Förster Resonance Energy Transfer from the Excimers of Poly(N-vinylcarbazole) to Coumarin 153. J. Phys. Chem. B 2012, 116, 4693–4701. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Sarkar, D.; Chattopadhyay, N. Intramolecular charge transfer promoted fluorescence transfer: A demonstration of re-absorption of the donor fluorescence by the acceptor. J. Mol. Liq. 2010, 156, 131–136. [Google Scholar] [CrossRef]
- Loken, M.R.; Hayes, J.W.; Gohlke, J.R.; Brand, L. Excited-state proton transfer as a biological probe. Determination of rate constants by means of nanosecond fluorometry. Biochemistry 1972, 11, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, L.; Wu, F.-Y.; Jiang, Y.-B. Development of Fluorescent Sensing of Anions under Excited-State Intermolecular Proton Transfer Signaling Mechanism. Org. Lett. 2003, 5, 2667–2670. [Google Scholar] [CrossRef] [PubMed]
- Kompa, K.L.; Levine, R.D. A molecular logic gate. Proc. Natl. Acad. Sci. USA 2001, 98, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Bojinov, V.; Georgiev, N. Molecular sensors and molecular logic gates (review). J. Univ. Chem. Technol. Metall. 2011, 46, 3–26. [Google Scholar]
- Borghetti, J.; Snider, G.S.; Kuekes, P.J.; Yang, J.J.; Stewart, D.R.; Williams, R.S. /‘Memristive/’ switches enable /‘stateful/’ logic operations via material implication. Nature 2010, 464, 873–876. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.P. Molecular Logic Gates. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Landge, S.M.; Aprahamian, I. A pH Activated Configurational Rotary Switch: Controlling the E/Z Isomerization in Hydrazones. J. Am. Chem. Soc. 2009, 131, 18269–18271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dhir, A.; Bhalla, V. A Molecular Keypad Lock Based on the Thiacalix[4]arene of 1,3-Alternate Conformation. Org. Lett. 2009, 11, 2567–2570. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, K.; Khanmohammadi, H. Molecular logic circuits and a security keypad lock based on a novel colorimetric azo receptor with dual detection ability for copper(II) and fluoride ions. Supramol. Chem. 2016, 28, 256–266. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Petcu, A.R.; Rogozea, E.A.; Lazar, C.A.; Olteanu, N.L.; Meghea, A.; Mihaly, M. Specific interactions within micelle microenvironment in different charged dye/surfactant systems. Arab. J. Chem. 2016, 9, 9–17. [Google Scholar] [CrossRef]
- Park, G.J.; Jo, H.Y.; Ryu, K.Y.; Kim, C. A new coumarin-based chromogenic chemosensor for the detection of dual analytes Al3+ and F. RSC Adv. 2014, 4, 63882–63890. [Google Scholar] [CrossRef]
- Tang, S.; Meng, Q.; Sun, H.; Su, J.; Yin, Q.; Zhang, Z.; Yu, H.; Chen, L.; Gu, W.; Li, Y. Dual pH-sensitive micelles with charge-switch for controlling cellular uptake and drug release to treat metastatic breast cancer. Biomaterials 2017, 114, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lebrini, M.; Traisnel, M.; Lagrenée, M.; Mernari, B.; Bentiss, F. Inhibitive properties, adsorption and a theoretical study of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles as corrosion inhibitors for mild steel in perchloric acid. Corros. Sci. 2008, 50, 473–479. [Google Scholar] [CrossRef]
- Lu, W.; Chen, D.; Jiang, H.; Jiang, L.; Shen, Z. Polymer-based fluoride-selective chemosensor: Synthesis, sensing property, and its use for the design of molecular-scale logic devices. J. Polym. Sci. Part A 2012, 50, 590–598. [Google Scholar] [CrossRef]
- Wagner, D.B. The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Flood, A.H. Click chemistry generates privileged CH hydrogen-bonding triazoles: The latest addition to anion supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; Rutledge, P.J.; Watkinson, M.; Todd, M.H. Chemical sensors that incorporate click-derived triazoles. Chem. Soc. Rev. 2011, 40, 2848–2866. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, D.; Yu, S.; Cornilleau, T.; Ruiz, J.; Salmon, L.; Astruc, D. Design and Applications of an Efficient Amphiphilic “Click” CuI Catalyst in Water. ACS Catal. 2016, 6, 5424–5431. [Google Scholar] [CrossRef]
- Watkinson, M. Click Triazoles as Chemosensors. In Click Triazoles; Košmrlj, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Zhang, F.; Moses, J.E. Benzyne Click Chemistry with in Situ Generated Aromatic Azides. Org. Lett. 2009, 11, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Cho, E.J.; Ryu, B.J.; Lee, Y.J.; Nam, K.C. Visible Colorimetric Fluoride Ion Sensors. Org. Lett. 2005, 7, 2607–2609. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Moon, J.W.; Ko, S.W.; Lee, J.Y.; Kim, S.K.; Yoon, J.; Nam, K.C. A New Fluoride Selective Fluorescent as Well as Chromogenic Chemosensor Containing a Naphthalene Urea Derivative. J. Am. Chem. Soc. 2003, 125, 12376–12377. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Rhodes, S.; Hawkins, K.; Winder, D.; Atkinson, A.; Ming, W.; Padgett, C.; Orvis, J.; Aiken, K.; Landge, S. A simple and effective 1,2,3-triazole based “turn-on” fluorescence sensor for the detection of anions. New J. Chem. 2015, 39, 295–303. [Google Scholar] [CrossRef]
- Govan, R.D. Approaches Toward Novel 1,2,3-Triazole Sensors for the Detection of Anions and Heavy Metal Cations, Electronic Theses & Dissertations; Georgia Southern University: Statesboro, GA, USA, 2017; Available online: http://digitalcommons.georgiasouthern.edu/etd/1604 (accessed on 15 December 2017).
- Boiocchi, M.; Del Boca, L.; Gómez, D.E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. Nature of Urea−Fluoride Interaction: Incipient and Definitive Proton Transfer. J. Am. Chem. Soc. 2004, 126, 16507–16514. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wu, Y.; Fan, J.; Tian, M.; Han, K. Colorimetric and Ratiometric Fluorescence Sensing of Fluoride: Tuning Selectivity in Proton Transfer. J. Org. Chem. 2005, 70, 10524–10531. [Google Scholar] [CrossRef] [PubMed]
- Juwarker, H.; Lenhardt, J.M.; Castillo, J.C.; Zhao, E.; Krishnamurthy, S.; Jamiolkowski, R.M.; Kim, K.-H.; Craig, S.L. Anion Binding of Short, Flexible Aryl Triazole Oligomers. J. Org. Chem. 2009, 74, 8924–8934. [Google Scholar] [CrossRef] [PubMed]
- Job, P. Spectrochemical Methods of Analysis; Wiley Interscience: New York, NY, USA, 1971. [Google Scholar]
- Choi, K.; Hamilton, A.D. Selective Anion Binding by a Macrocycle with Convergent Hydrogen Bonding Functionality. J. Am. Chem. Soc. 2001, 123, 2456–2457. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Rhodes, S.; Winder, D.; Atkinson, A.; Gibson, J.; Ming, W.; Padgett, C.; Landge, S.; Aiken, K. Spectroscopic investigation of bis-appended 1,2,3-triazole probe for the detection of Cu(II) ion. J. Mol. Struct. 2017, 1134, 638–648. [Google Scholar] [CrossRef]
- Charisiadis, P.; Exarchou, V.; Troganis, A.N.; Gerothanassis, I.P. Exploring the “forgotten” -OH NMR spectral region in natural products. Chem. Commun. 2010, 46, 3589–3591. [Google Scholar] [CrossRef] [PubMed]
- Siskos, M.G.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Gerothanassis, I.P. Investigation of solute-solvent interactions in phenol compounds: Accurate ab initio calculations of solvent effects on 1H NMR chemical shifts. Org. Biomol. Chem. 2013, 11, 7400–7411. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Gupta, R.; Agarwal, M. Benzimidazole scaffold as dipodal molecular cleft for swift and efficient naked eye fluoride ion recognition via preorganized N-H and aromatic C-H in aqueous media. Indian J. Chem. 2017, 56, 513–518. [Google Scholar]
- Li, J.; Xu, X.; Shao, X.; Li, Z. A novel colorimetric fluoride sensor based on a semi-rigid chromophore controlled by hydrogen bonding. Luminescence 2015, 30, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. The Ultraviolet Absorption Spectra of Aromatic Hydrocarbons. Chem. Rev. 1943, 32, 1–46. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharyya, S.; Sharma, A. Photocatalytic Degradation of Naphthalene by Electrospun Mesoporous Carbon-Doped Anatase TiO2 Nanofiber Mats. Ind. Eng. Chem. Res. 2014, 53, 18900–18909. [Google Scholar] [CrossRef]
- Silva, A.F.; Fiedler, H.D.; Nome, F. Ionic Quenching of Naphthalene Fluorescence in Sodium Dodecyl Sulfate Micelles. J. Phys. Chem. A 2011, 115, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Maeda, T.; Mizuno, K. Absorption and Fluorescence Spectroscopic Properties of 1- and 1,4-Silyl-Substituted Naphthalene Derivatives. Molecules 2012, 17, 5108–5125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, X.; Petersen, J.L.; Li, M.; Shi, X. Synthesis and Characterization of Bis-N-2-Aryl Triazole as a Fluorophore. J. Org. Chem. 2015, 80, 3664–3669. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.; Tavassoli, S.H.; Mousavi, S.J.; Darbani, S.M.R. Measuring of naphthalene fluorescence emission in the water with nanosecond time delay laser induced fluorescence spectroscopy method. Opt. Int. J. Light Electron Opt. 2016, 127, 6218–6223. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2007. [Google Scholar]
- Harris, C.M.; Selinger, B.K. Acid-base properties of 1-naphthol. Proton-induced fluorescence quenching. J. Phys. Chem. 1980, 84, 1366–1371. [Google Scholar] [CrossRef]
- Boyer, R.; Deckey, G.; Marzzacco, C.; Mulvaney, M.; Schwab, C.; Halpern, A.M. The photophysical properties of 2-naphthol: A physical chemistry experiment. J. Chem. Ed. 1985, 62, 630. [Google Scholar] [CrossRef]
- Laws, W.R.; Brand, L. Analysis of two-state excited-state reactions. The fluorescence decay of 2-naphthol. J. Phys. Chem. 1979, 83, 795–802. [Google Scholar] [CrossRef]
- Marciniak, B.; Kozubek, H.; Paszyc, S. Estimation of pKa* in the first excited singlet state. A physical chemistry experiment that explores acid-base properties in the excited state. J. Chem. Ed. 1992, 69, 247. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Bhosale, S.V.; Kalyankar, M.B.; Langford, S.J. A Core-Substituted Naphthalene Diimide Fluoride Sensor. Org. Lett. 2009, 11, 5418–5421. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hu, S.; Liu, K.; Guo, Y.; Xu, J.; Shao, S. Oxidized Bis(indolyl)methane: A Simple and Efficient Chromogenic-Sensing Molecule Based on the Proton Transfer Signaling Mode. Org. Lett. 2006, 8, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Pirali, T.; Gatti, S.; DiBrisco, R.; Tacchi, S.; Zaninetti, R.; Brunelli, E.; Massarotti, A.; Sorba, G.; Canonico, P.L.; Moro, L.; et al. Estrogenic Analogues Synthesized by Click Chemistry. ChemMedChem 2007, 2, 437–440. [Google Scholar] [CrossRef] [PubMed]


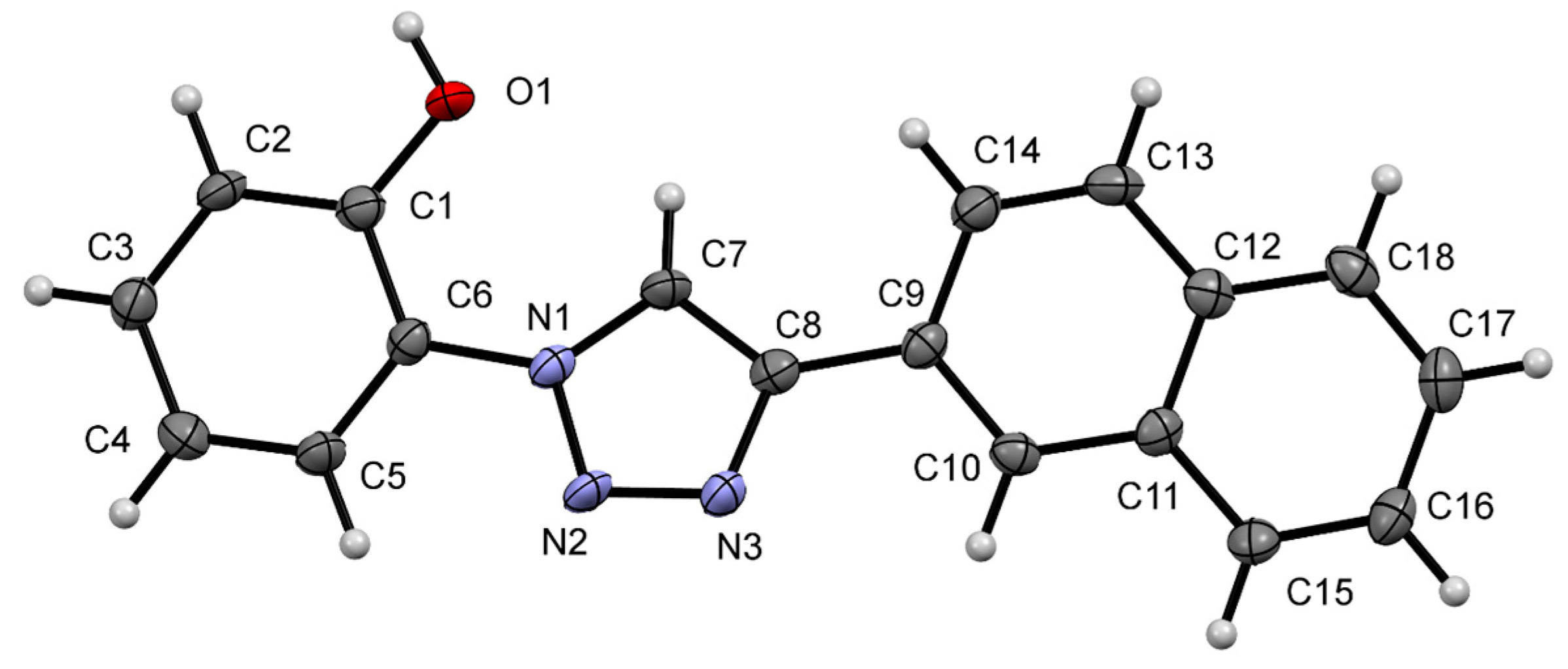
| Crystal Data for NpTP | |
| Chemical formula | C18H13N3O |
| Mr | 287.31 |
| Crystal system, space group | Tetragonal, P43 |
| Temperature (K) | 173 |
| a, b, c (Å) | 7.3806 (16), 7.3806 (16), 50.665 (16) |
| V (Å3) | 2759.9(13) |
| Z | 8 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.09 |
| Crystal size (mm) | 0.07 × 0.07 × 0.07 |
| Data collection for NpTP | |
| Diffractometer | Bruker D8 Venture Photon 100 diffractometer |
| Absorption correction | Multi-scan SADABS, Bruker |
| Tmin, Tmax | 0.883, 1.00 |
| No. of measured, independent and observed [F2 > 2.0σ(F2)] reflections | 17713, 5409, 4263 |
| Rint | 0.045 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.049, 0.096, 1.02 |
| No. of reflections | 5409 |
| No. of parameters | 398 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.16, −0.20 |
| Special treatment | Refined as a 2-component inversion twin |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiken, K.; Bunn, J.; Sutton, S.; Christianson, M.; Winder, D.; Freeman, C.; Padgett, C.; McMillen, C.; Ghosh, D.; Landge, S. Nuclear Magnetic Resonance Spectroscopy Investigations of Naphthalene-Based 1,2,3-Triazole Systems for Anion Sensing. Magnetochemistry 2018, 4, 15. https://doi.org/10.3390/magnetochemistry4010015
Aiken K, Bunn J, Sutton S, Christianson M, Winder D, Freeman C, Padgett C, McMillen C, Ghosh D, Landge S. Nuclear Magnetic Resonance Spectroscopy Investigations of Naphthalene-Based 1,2,3-Triazole Systems for Anion Sensing. Magnetochemistry. 2018; 4(1):15. https://doi.org/10.3390/magnetochemistry4010015
Chicago/Turabian StyleAiken, Karelle, Jessica Bunn, Steven Sutton, Matthew Christianson, Domonique Winder, Christian Freeman, Clifford Padgett, Colin McMillen, Debanjana Ghosh, and Shainaz Landge. 2018. "Nuclear Magnetic Resonance Spectroscopy Investigations of Naphthalene-Based 1,2,3-Triazole Systems for Anion Sensing" Magnetochemistry 4, no. 1: 15. https://doi.org/10.3390/magnetochemistry4010015
APA StyleAiken, K., Bunn, J., Sutton, S., Christianson, M., Winder, D., Freeman, C., Padgett, C., McMillen, C., Ghosh, D., & Landge, S. (2018). Nuclear Magnetic Resonance Spectroscopy Investigations of Naphthalene-Based 1,2,3-Triazole Systems for Anion Sensing. Magnetochemistry, 4(1), 15. https://doi.org/10.3390/magnetochemistry4010015




