Glutathione and Magnetic Nanoparticle-Modified Nanochannels for the Detection of Cadmium (II) in Cereal Grains
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Apparatus
2.2. Modification of Porous AAO with GSH
2.3. Surface Modification of Fe3O4 Nanoparticles with GSH
2.4. Preparation of the GSH@Fe3O4/GSH@AAO Membrane
2.5. Preparation of GSH@Fe3O4/GSH@AAO/SPCE
2.6. Preparation and Pretreatment of Real Samples
3. Results and Discussion
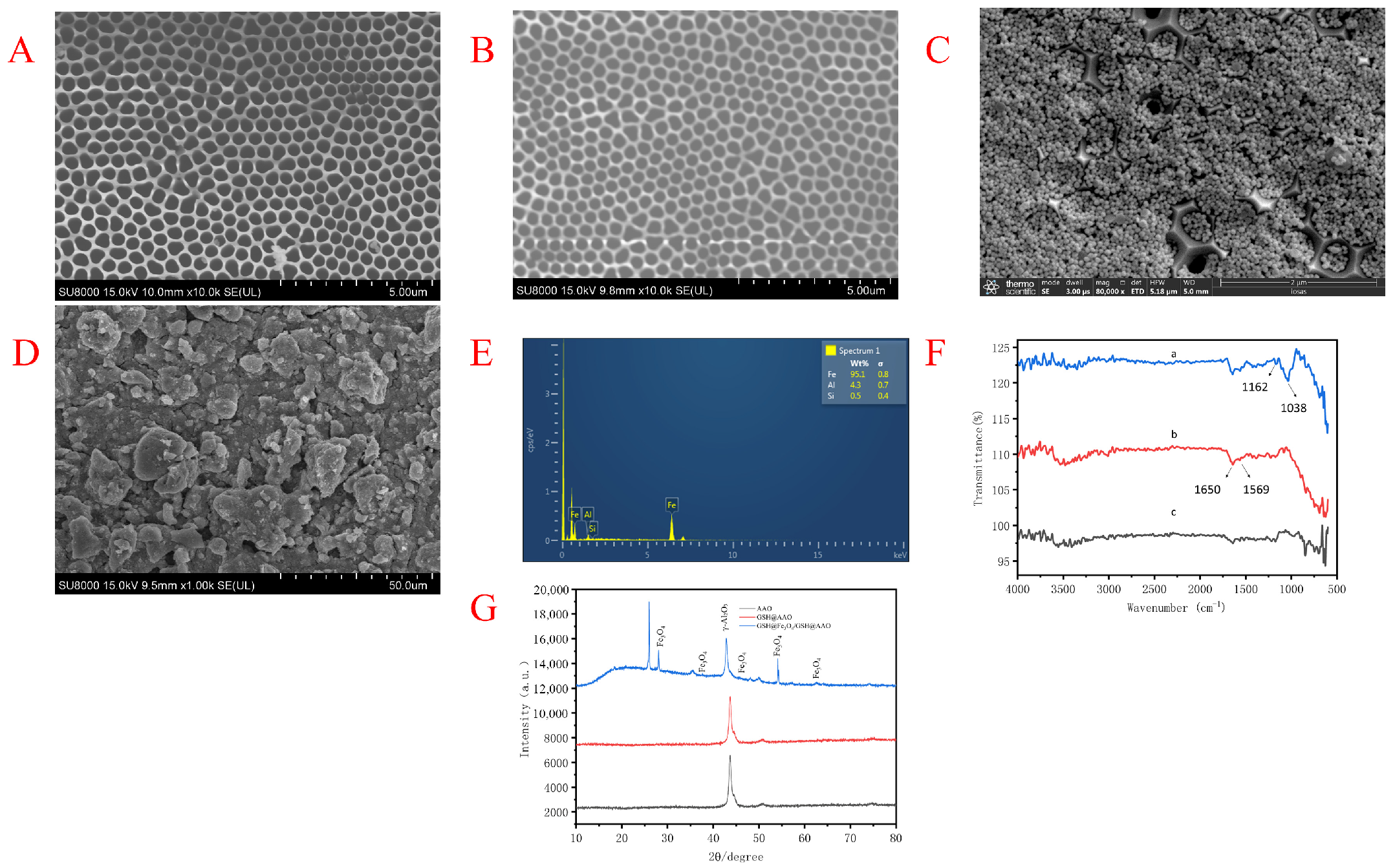
3.1. Characterization of the GSH@Fe3O4/GSH@AAO Membrane
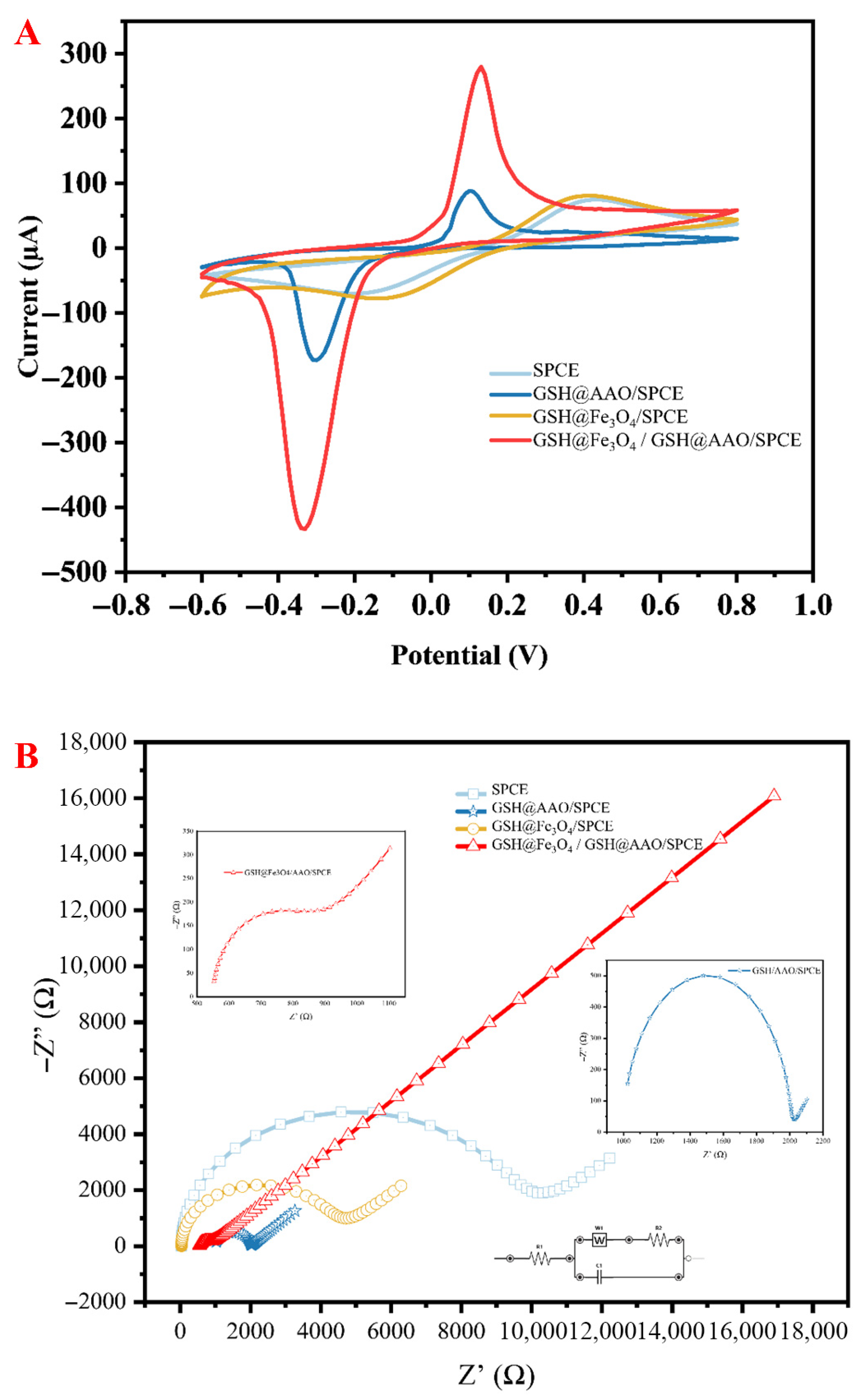
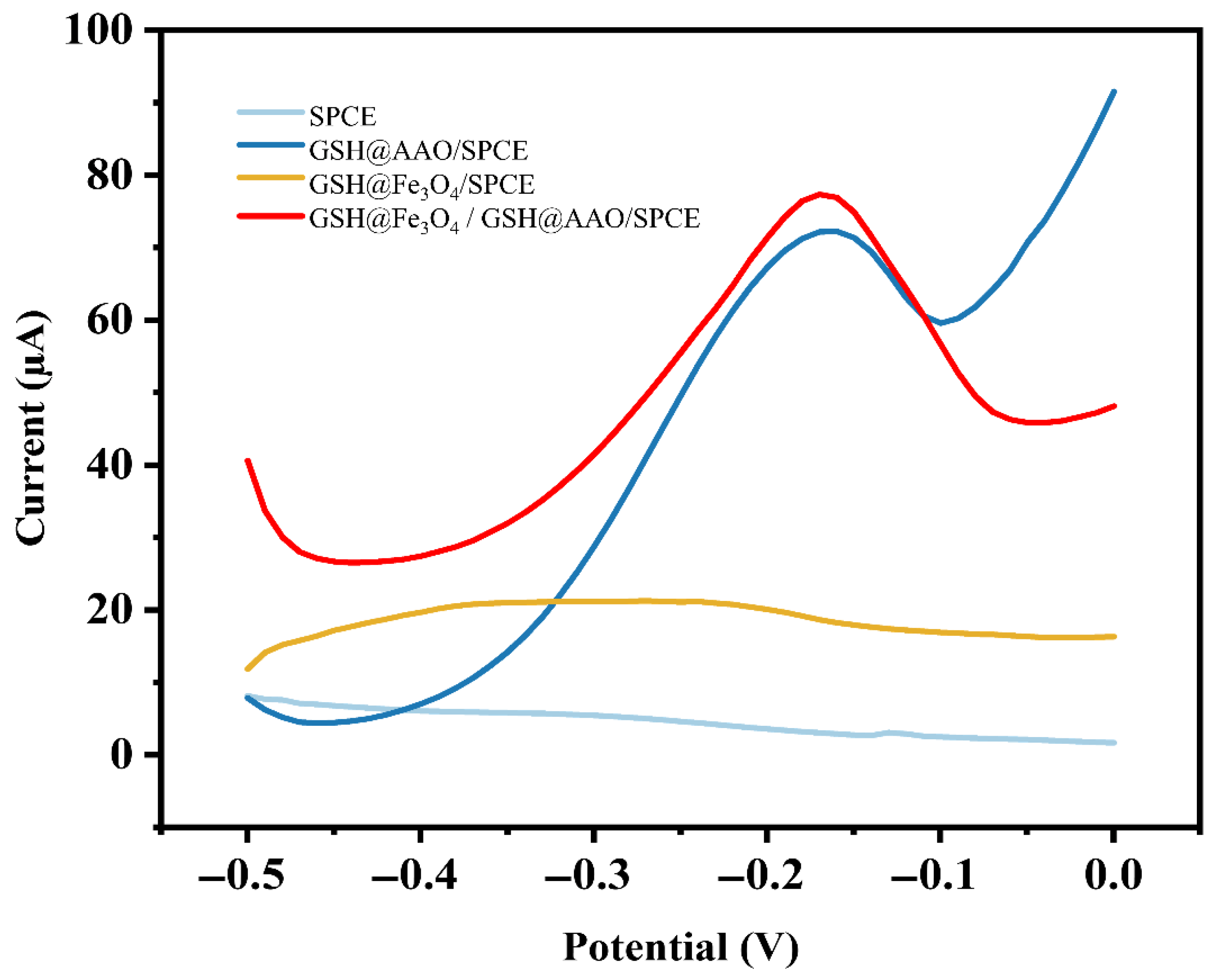
3.2. Electrochemical Characterization of the GSH@Fe3O4/GSH@AAO/SPCE Sensor
3.3. Electroanalytical Characteristics of Cd2+ on Different Electrodes
3.4. Optimization of Experimental Parameters for the GSH@Fe3O4/GSH@AAO/SPCE Electrochemical Sensor
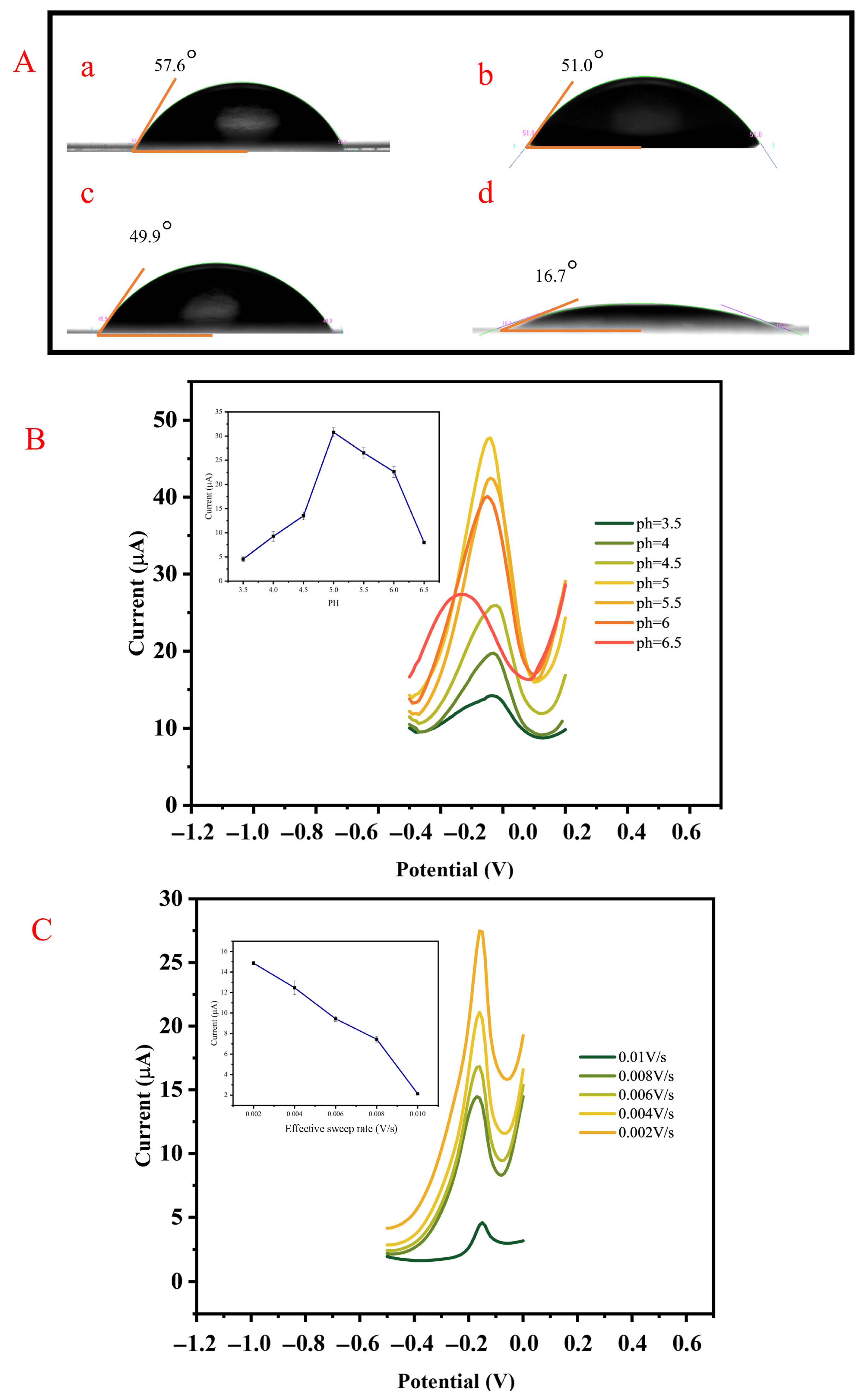
3.4.1. Optimization of Hydrophilicity of the AAO Membrane
3.4.2. Optimization of pH for DPV
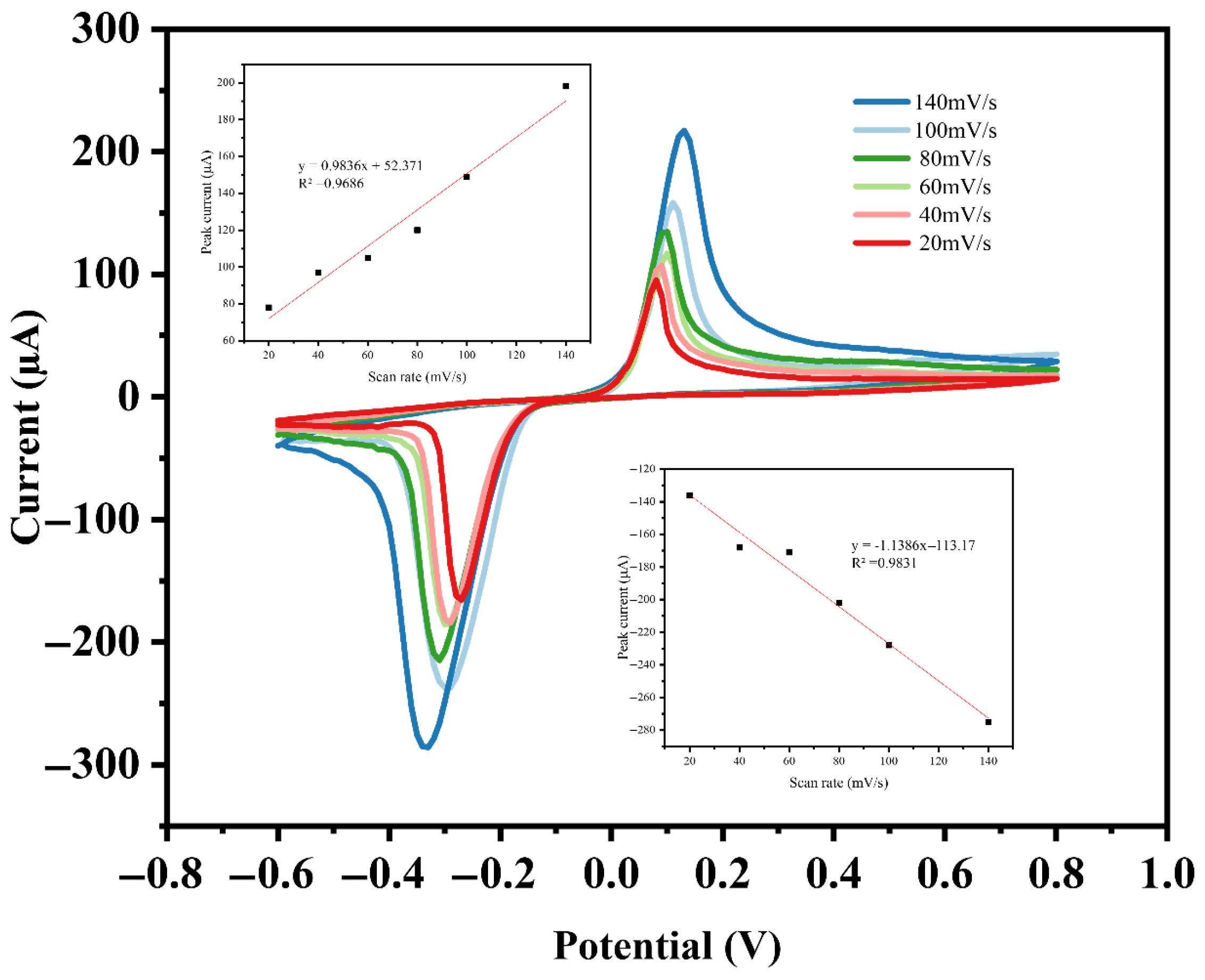
3.4.3. Optimization of the Effective Sweep Rate for DPV
3.5. Detection of Cd2+ Using the GSH@Fe3O4/GSH@AAO/SPCE Electrochemical Sensor
3.6. Analysis of Real Samples
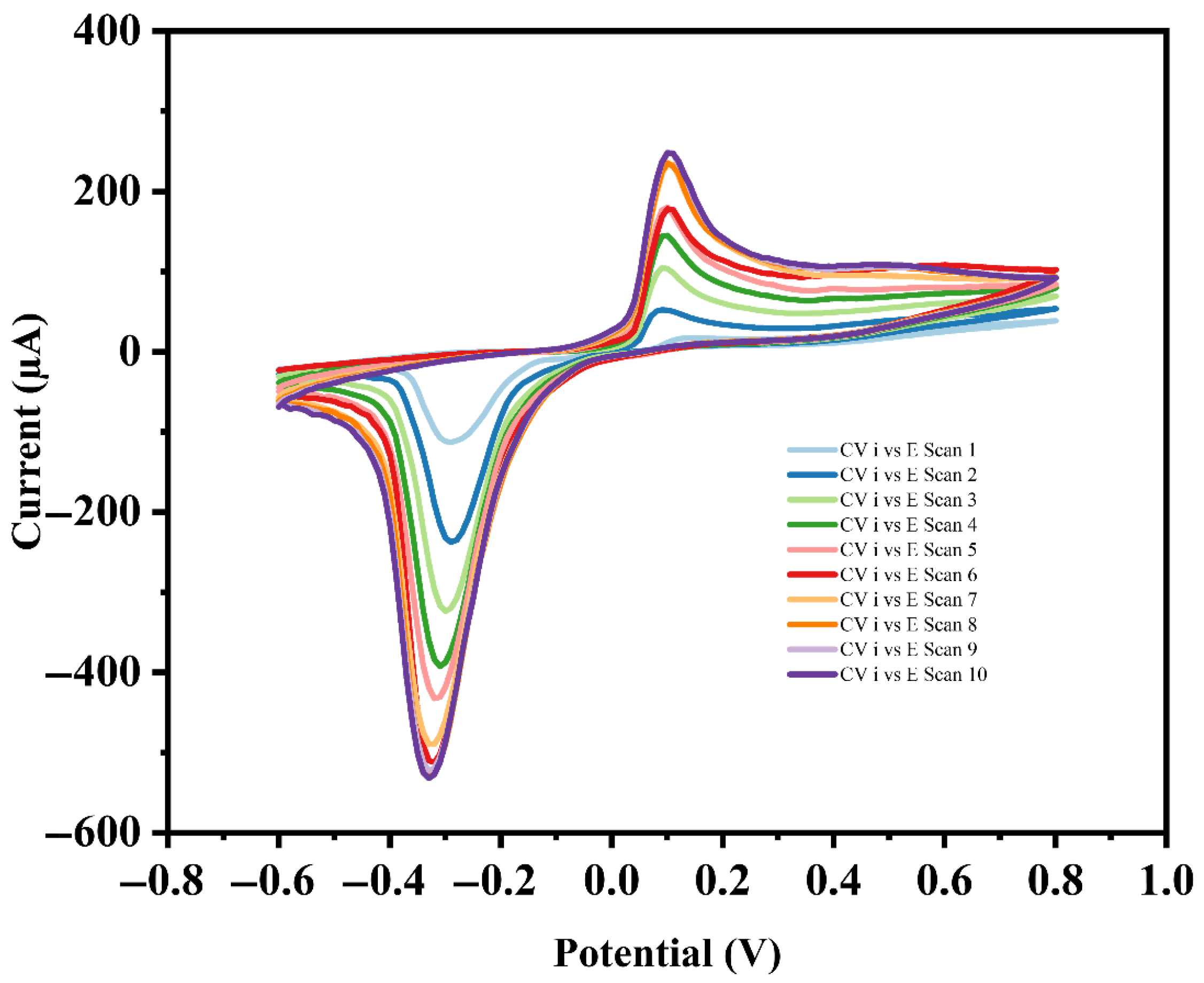
3.7. Scalability and Reproducibility Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Z.; Gong, T.; Liang, P. Heavy Metal Exposure and Cardiovascular Disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef]
- Wu, M.; Xu, J.; Nie, Z.; Shi, H.; Liu, H.; Zhang, Y.; Li, C.; Zhao, P.; Liu, H. Physiological, biochemical and transcriptomic insights into the mechanisms by which molybdenum mitigates cadmium toxicity in Triticum aestivum L. J. Hazard Mater. 2024, 472, 134516. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Johns, C.E.; Gattu, M.; Camilli, S.; Desaraju, A.; Kolliputi, N.; Galam, L. The Cd/Zn Axis: Emerging concepts in cellular fate and cytotoxicity. Biomolecules 2023, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Pereira, É.R.; de Quadros, D.P.C.; Welz, B.; Carasek, E.; de Andrade, J.B.; del Campo Menoyo, J.; Feldmann, J. Investigation of chemical modifiers for the determination of cadmium and chromium in fish oil and lipoid matrices using HR-CS GF AAS and a simple ‘dilute-and-shoot’ approach. Microchem. J. 2017, 133, 175–181. [Google Scholar] [CrossRef]
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated metallothionein-bound cadmium concentrations in urine from bladder carcinoma patients, investigated by size exclusion chromatography-inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2009, 631, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wen, L.; Zhao, D.; Yang, H.; Zhao, J.; Hu, Z.; Ma, Y.; Hou, C.; Huo, D. Flexible carbon fiber cloth supports decorated with cerium metal- organic frameworks and multi-walled carbon nanotubes for simultaneous on-site detection of Cd2+ and Pb2+ in food and water samples. Food Chem. 2023, 418, 135869. [Google Scholar] [CrossRef] [PubMed]
- Jiokeng, S.L.Z.; Ntep, T.J.M.M.; Fetzer, M.N.A.; Strothmann, T.; Fotsop, C.G.; Tonle, I.K.; Janiak, C. Efficient Electrochemical Lead Detection by a Histidine-Grafted Metal-Organic Framework MOF-808 Electrode Material. ACS Appl. Mater. Interfaces 2024, 16, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jiang, D.; Chen, C.; Zhou, Y.; Zhang, S.; Zhang, N.; Wang, L. Advances in nanochannel electrochemical sensing technology for food safety detection. Trends Food Sci. Technol. 2025, 161, 104983. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, R.; Yang, C.; Zhou, Z.; Yuan, G.; Zhou, H.; Hu, S. Surface diffusion enhanced ion transport through two-dimensional nanochannels. Sci. Adv. 2023, 9, eadi8493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Li, Z.; Zhang, Q.; Li, Y.; Ying, Y.; Fu, Y. Nanoconfinement Effect for Signal Amplification in Electrochemical Analysis and Sensing. Small 2021, 17, e2101665. [Google Scholar] [CrossRef] [PubMed]
- Grommet, A.B.; Feller, M.; Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 2020, 15, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, G.; Yang, W.; Dai, X.; Huang, Y.; Ni, J.; Wang, Q. Portable anti-fouling electrochemical sensor for soil heavy metal ions detection based on the screen-printed carbon electrode modified with silica isoporous membrane. J. Electroanal. Chem. 2023, 930, 117141. [Google Scholar] [CrossRef]
- Tang, L.W.; Alias, Y.; Jiwanti, P.K.; Woi, P.M. Recent advances in amino acid-based electrode fabrication strategies for enhanced electrochemical detection of metal ions. Trends Environ. Anal. Chem. 2024, 41, e0025. [Google Scholar] [CrossRef]
- Yang, S.; Liu, P.; Wang, Y.; Guo, Z.; Tan, R.; Qu, L. Electrochemical sensor using poly-(l-cysteine) functionalized CuO nanoneedles/N-doped reduced graphene oxide for detection of lead ions. RSC Adv. 2020, 10, 18526–18532. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xiong, B.; Wang, Y.; Wang, L.; Wang, H. Determination of Trace Lead and Cadmium in Decorative Material Using Disposable Screen-Printed Electrode Electrically Modified with Reduced Graphene Oxide/L-Cysteine/Bi-Film. Sensors 2020, 20, 1322. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Glutathione modified screen-printed carbon nanofiber electrode for the voltammetric determination of metal ions in natural samples. Talanta 2016, 155, 8–13. [Google Scholar] [CrossRef]
- Zhou, S.F.; Han, X.J.; Liu, Y.Q. SWASV performance toward heavy metal ions based on a high-activity and simple magnetic chitosan sensing nanomaterials. J. Alloys Compd. 2016, 684, 1–7. [Google Scholar] [CrossRef]
- Fan, H.L.; Zhou, S.F.; Gao, J.; Liu, Y.Z. Continuous preparation of Fe3O4 nanoparticles through Impinging Stream-Rotating Packed Bed reactor and their electrochemistry detection toward heavy metal ions. J. Alloys Compd. 2016, 671, 354–359. [Google Scholar] [CrossRef]
- Yantasee, W.; Hongsirikarn, K.; Warner, C.L.; Choi, D.; Sangvanich, T.; Toloczko, M.B.; Warner, M.G.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C. Direct detection of Pb in urine and Cd, Pb, Cu, and Ag in natural waters using electrochemical sensors immobilized with DMSA functionalized magnetic nanoparticles. Analyst 2008, 133, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Zhou, W.Y.; Jiang, M.; Guo, Z.; Liu, J.H.; Zhang, L.; Huang, X.J. Surface Fe (II)/Fe (III) cycle promoted ultra-highly sensitive electrochemical sensing of arsenic (III) with dumbbell-like Au/Fe3O4 nanoparticles. Anal. Chem. 2018, 90, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Lu, X.Y.; Kong, F.Y.; Li, H.Y.; Wang, Z.X.; Wang, W. A reduced graphene oxide supported Au-Bi bimetallic nanoparticles as an enhanced sensing platform for simultaneous voltammetric determination of Pb (II) and Cd (II). Microchem. J. 2022, 175, 107078. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, H.; Donley, C.; Liang, J.; Yang, S.; Xu, S. Thiolation and characterization of regenerated Bombyx mori silk fibroin films with reduced glutathione. BMC Chem. 2019, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Baghayeri, M.; Amiri, A.; Maleki, B.; Alizadeh, Z.; Reiser, O. A simple approach for simultaneous detection of cadmium (II) and lead (II) based on glutathione coated magnetic nanoparticles as a highly selective electrochemical probe. Sens. Actuators B Chem. 2018, 273, 1442–1450. [Google Scholar] [CrossRef]
- Chow, E.; Hibbert, D.B.; Gooding, J.J. Voltammetric detection of cadmium ions at glutathione-modified gold electrodes. Analyst 2005, 130, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Sacara, A.M.; Pitzalis, F.; Salis, A.; Turdean, G.L.; Muresan, L.M. Glassy carbon electrodes modified with ordered mesoporous silica for the electrochemical detection of cadmium ions. ACS Omega 2019, 4, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, I.; Hogan, A.; Alatawi, H.; Alsefri, S.; Moore, E. A novel comparative study for simultaneous determination of Cd (II) and Pb (II) based on ruthenium complex-nanoparticles-nafion modified screen-printed gold electrode. Sens. Actuators B Chem. 2023, 380, 133273. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.W.; Wang, J. Graphene Aerogel-Metal-Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, J.; Luo, J.; Mamat, X.; Sambasivam, S.; Li, Y.; Hu, X.; Wågberg, T.; Hu, G. Selective voltammetric determination of Cd(II) by using N,S-codoped porous carbon nanofibers. Mikrochim. Acta 2018, 185, 282. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, J.; Wang, G.; Wang, X.; Zhang, J.; Sun, B.; He, D.; Suo, H.; Zhao, C. A novel electrode for simultaneous detection of multiple heavy metal ions without pre-enrichment in food samples. Food Chem. 2024, 448, 138994. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, I.; Hogan, A.; Alatawi, H.; Moore, E. A sensitive electrochemical analysis for cadmium and lead based on Nafion-Bismuth film in a water sample. Sens. Bio-Sens. Res. 2021, 34, 100454. [Google Scholar] [CrossRef]
- Tapia, M.A.; Pérez-Ràfols, C.; Gusmão, R.; Serrano, N.; Sofer, Z.; Díaz-Cruz, J.M. Enhanced voltammetric determination of metal ions by using a bismuthene-modified screen-printed electrode. Electrochim. Acta. 2020, 362, 137144. [Google Scholar] [CrossRef]
- Costa, M.; Di Masi, S.; Garcia-Cruz, A.; Piletsky, S.A.; Malitesta, C. Disposable electrochemical sensor based on ion imprinted polymeric receptor for Cd (II) ion monitoring in waters. Sens. Actuators B Chem. 2023, 383, 133559. [Google Scholar] [CrossRef]
- Lu, S.M.; Vannoy, K.J.; Dick, J.E.; Long, Y.T. Multiphase chemistry under nanoconfinement: An electrochemical perspective. J. Am. Chem. Soc. 2023, 145, 25043–25055. [Google Scholar] [CrossRef] [PubMed]
- Jaugstetter, M.; Blanc, N.; Kratz, M.; Tschulik, K. Electrochemistry under confinement. Chem. Soc. Rev. 2022, 51, 2491–2543. [Google Scholar] [CrossRef] [PubMed]



| Electrodes | Technique | Linear Range (μg L−1) | LOD (μgL−1) | Reference |
|---|---|---|---|---|
| Ru-GO/Nafion screen-printed gold electrode | SWASV | 50~350 | 4.2 | [28] |
| Ru-Au/Nafion screen-printed gold electrode | SWASV | 10~300 | 12.01 | [28] |
| UiO-66-NH2/GA | DPSV | 18.5~925 | 6.2 | [29] |
| CeMOF@MWCNTs/CC | DPASV | 10~1200 | 2.2 | [8] |
| RGO/Au-Bi | DPASV | 0.1~300 | 0.02 | [23] |
| N, S-PCNFs | DPASV | 2~500 | 0.7 | [30] |
| Mo-WO3/CC | DPV | 0.1~100 | 0.017 | [31] |
| SPGE-Nafion/Bi | SWASV | 20~300 | 4 | [32] |
| BiSP/SPE | DPASV | 0.9~25.0 | 0.3 | [33] |
| GSH@Fe3O4/GSH@AAO/SPCE | DPV | 5~120 | 0.1 | This work |
| Sample | Quantity Added (μg/kg) | Measured Value (μg/kg) | Measured by AAS (μg/kg) | Recovery Rate (%) |
|---|---|---|---|---|
| Barley | 50 | 48.5 ± 1.5 | 47.5 | 97 |
| 100 | 97.5 ± 2 | 96.5 | 97.5 | |
| Wheat | 50 | 54 ± 1 | 53 | 108 |
| 100 | 106 ± 1.5 | 103.5 | 106 | |
| Corn | 50 | 52.5 ± 1.5 | 51 | 105 |
| 100 | 102 ± 1 | 99 | 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Xiang, X.; Jiang, D.; Zhang, N.; Wang, L. Glutathione and Magnetic Nanoparticle-Modified Nanochannels for the Detection of Cadmium (II) in Cereal Grains. Magnetochemistry 2025, 11, 61. https://doi.org/10.3390/magnetochemistry11070061
Hu W, Xiang X, Jiang D, Zhang N, Wang L. Glutathione and Magnetic Nanoparticle-Modified Nanochannels for the Detection of Cadmium (II) in Cereal Grains. Magnetochemistry. 2025; 11(7):61. https://doi.org/10.3390/magnetochemistry11070061
Chicago/Turabian StyleHu, Wei, Xinyue Xiang, Donglei Jiang, Na Zhang, and Lifeng Wang. 2025. "Glutathione and Magnetic Nanoparticle-Modified Nanochannels for the Detection of Cadmium (II) in Cereal Grains" Magnetochemistry 11, no. 7: 61. https://doi.org/10.3390/magnetochemistry11070061
APA StyleHu, W., Xiang, X., Jiang, D., Zhang, N., & Wang, L. (2025). Glutathione and Magnetic Nanoparticle-Modified Nanochannels for the Detection of Cadmium (II) in Cereal Grains. Magnetochemistry, 11(7), 61. https://doi.org/10.3390/magnetochemistry11070061





