Electronic Influence of Trifluoromethyl Substituents on Benzoate Ligands in Paddlewheel-Type Diruthenium(II,II) Naphthyridine Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
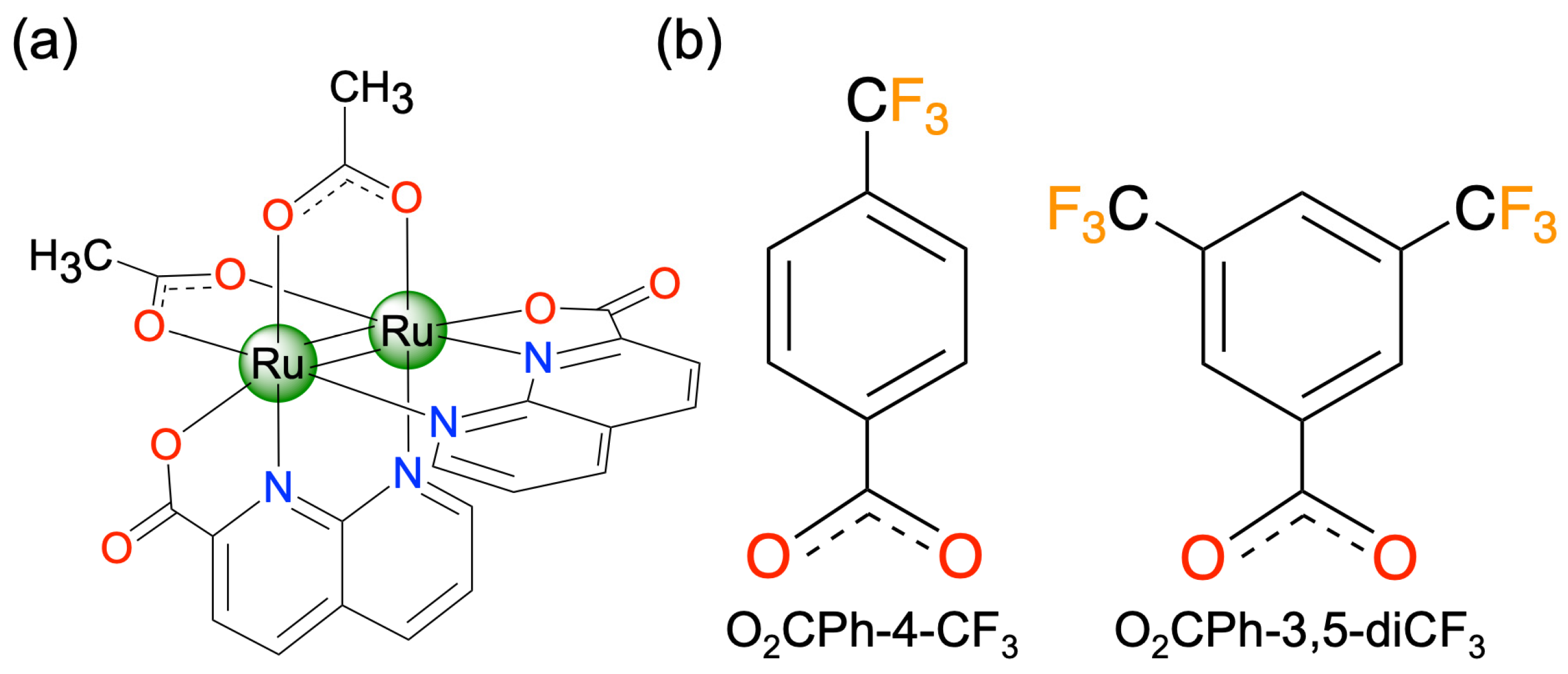
2.2. Synthesis of [Ru2(npc)2(O2CPh-4-CF3)2] (4)
2.3. Synthesis of [Ru2(npc)2(O2CPh-3,5-diCF3)2] (5)
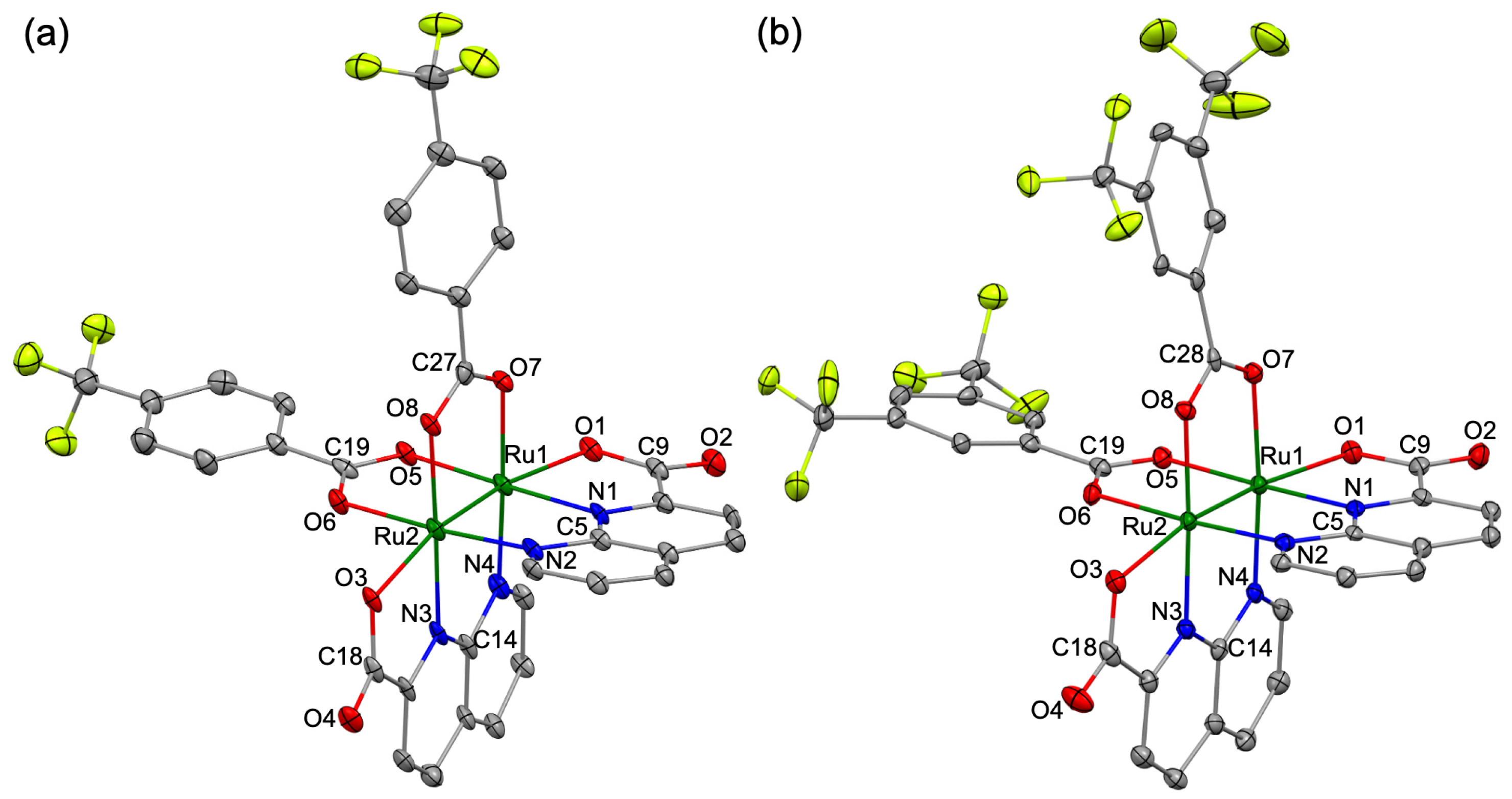
2.4. Single Crystal X-Ray Diffraction Analysis
2.5. Quantum Chemical Calculation Methods
3. Results
3.1. Synthesis and Characterization
3.2. Crystal Structures
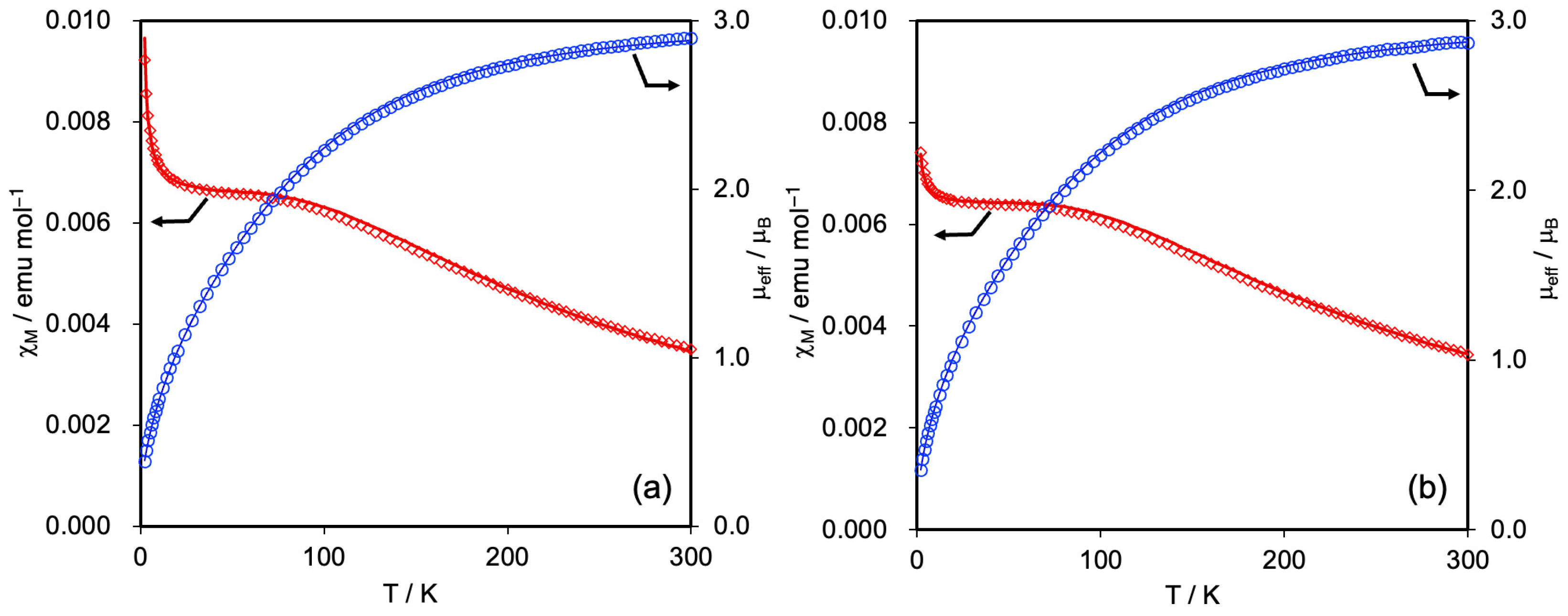
3.3. Magnetic Susceptibilities
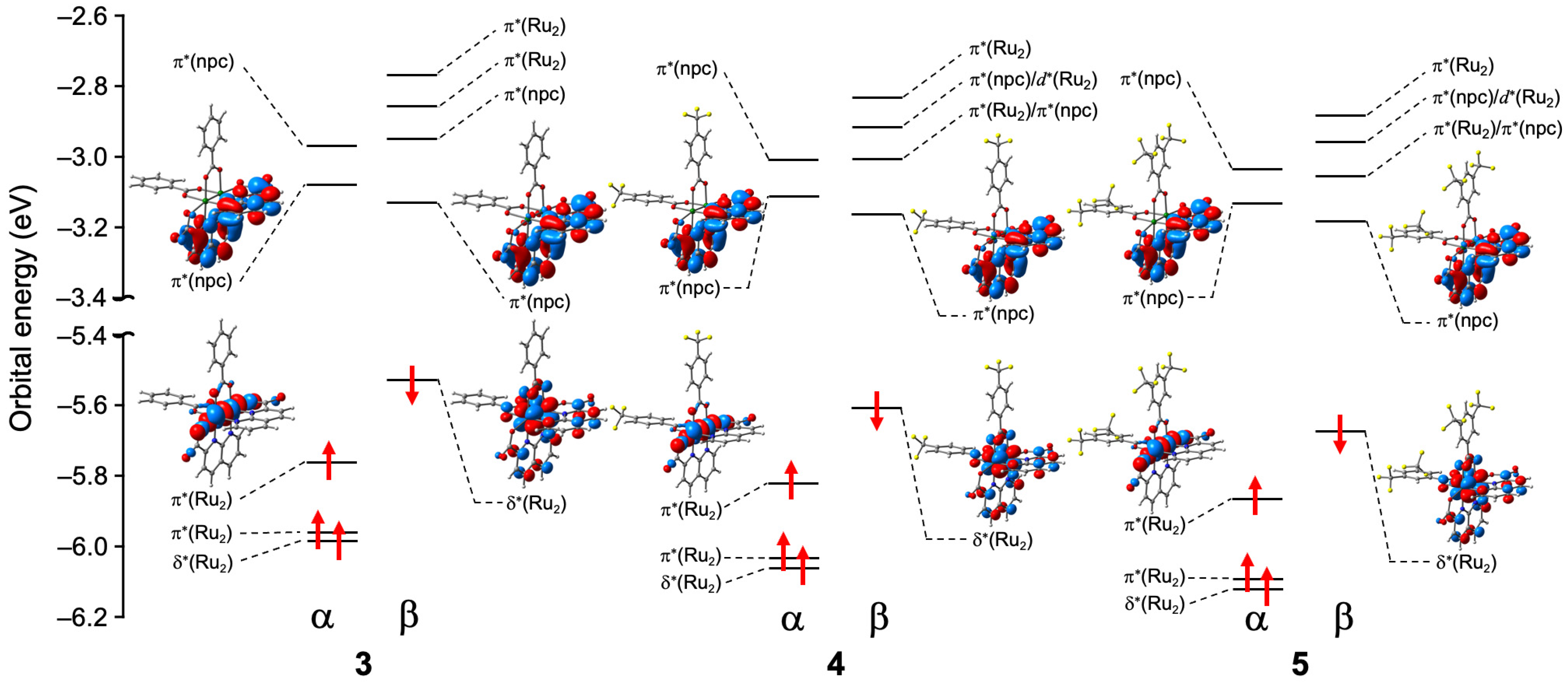
3.4. Optimized Geometries, Spin Density Distributions, and Electronic Structures
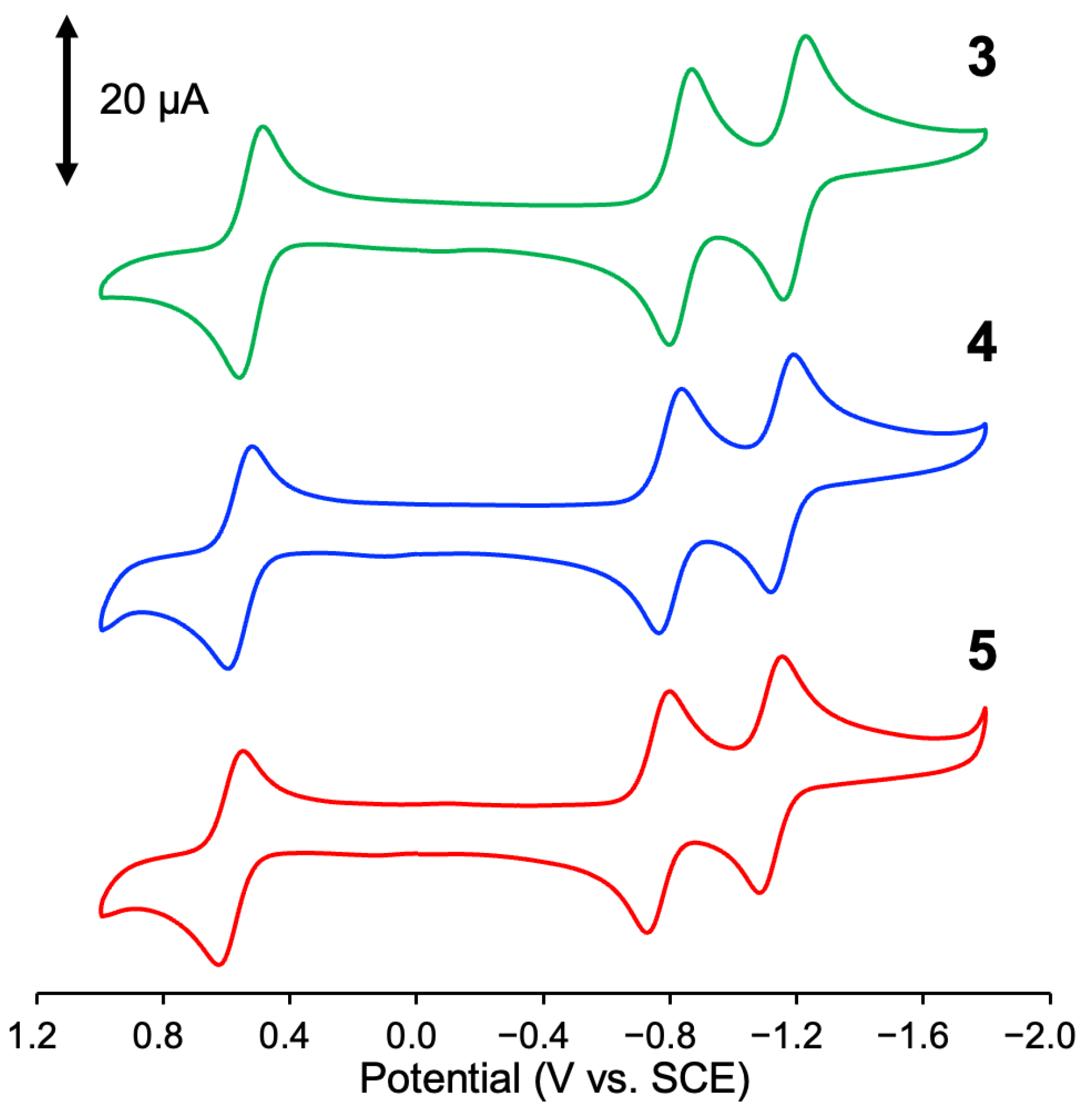
3.5. Electrochemical Properties
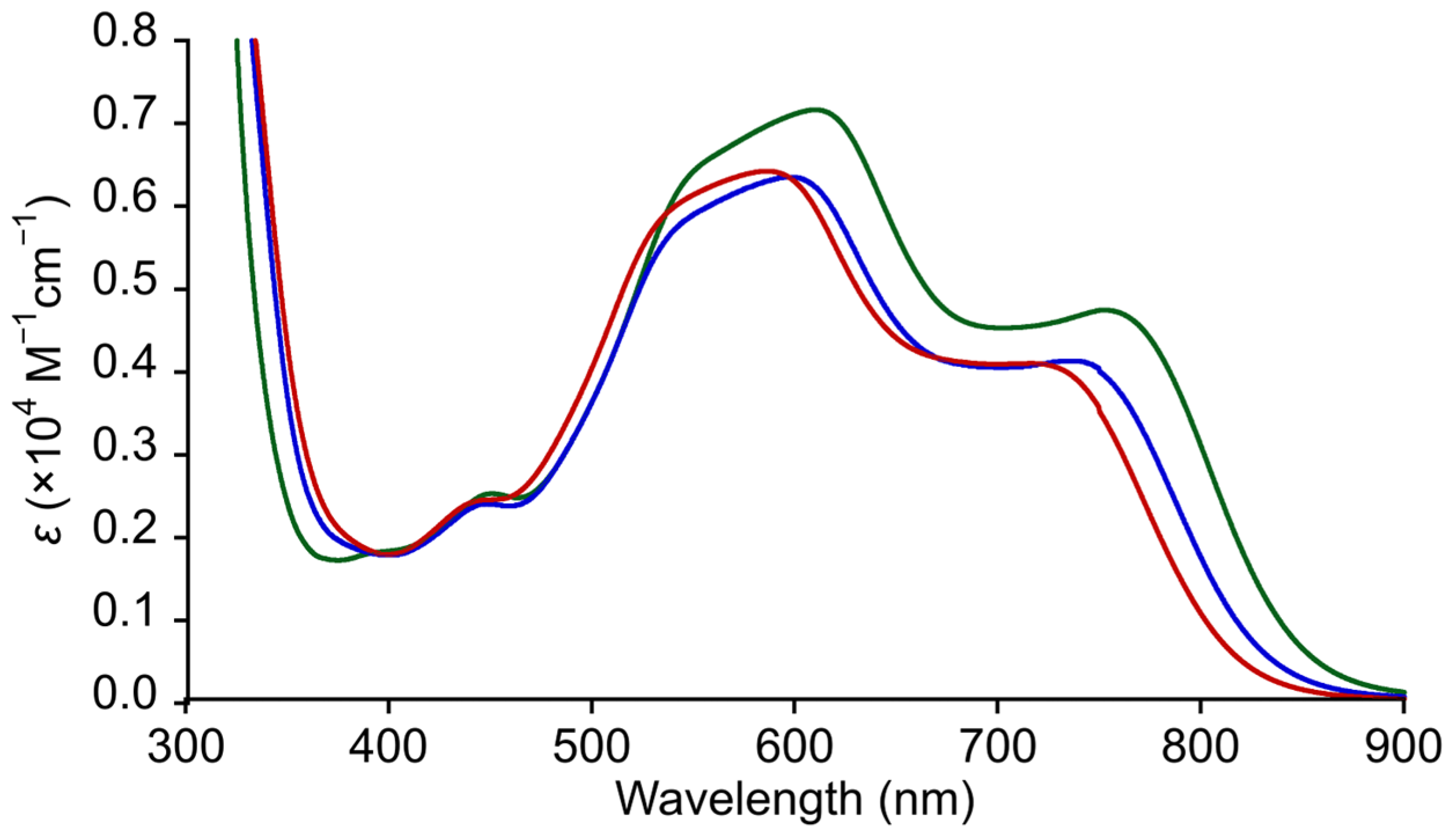
3.6. Absorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds Between Metal Atoms, 3rd ed.; Springer Science and Business Media: New York, NY, USA, 2005. [Google Scholar]
- Cotton, F.A.; Lin, C.; Murillo, C.A. Supramolecular Arrays Based on Dimetal Building Units. Acc. Chem. Res. 2001, 34, 759–771. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Patmore, N.J. Studies of Electronic Coupling and Mixed Valency in Metal-Metal Quadruply Bonded Complexes Linked by Dicarboxylate and Closely Related Ligands. Acc. Chem. Res. 2007, 40, 19–27. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Diruthenium and diosmium tetracarboxylates: Synthesis, physical properties, and applications. Coord. Chem. Rev. 1998, 170, 141–202. [Google Scholar] [CrossRef]
- Van Caemelbecke, E.; Phan, T.; Osterloh, W.R.; Kadish, K.M. Electrochemistry of metal-metal bonded diruthenium complexes. Coord. Chem. Rev. 2021, 434, 213706. [Google Scholar] [CrossRef]
- Cortijo, M.; González-Prieto, R.; Herrero, S.; Priego, J.L.; Jiménez-Aparicio, R. The use of amidinate ligands in paddlewheel diruthenium chemistry. Coord. Chem. Rev. 2019, 400, 213040. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Mikuriya, M.; Handa, M. Coordination polymers and metal–organic frameworks based on paddlewheel-type dirhodium(II) tetracarboxylates. Coord. Chem. Rev. 2022, 472, 214796. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Mikuriya, M.; Handa, M. Paddlewheel-type dirhodium complexes with N,N’-bridging ligands. Coord. Chem. Rev. 2023, 479, 214997. [Google Scholar] [CrossRef]
- Ren, T. Substituent effects in dinuclear paddlewheel compounds: Electrochemical and spectroscopic investigations. Coord. Chem. Rev. 1998, 175, 43–58. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Recent developments in the synthesis and properties of diruthenium tetracarboxylates. Coord. Chem. Rev. 2004, 248, 1025–1045. [Google Scholar] [CrossRef]
- Miyasaka, H. Charge Manipulation in Metal-Organic Frameworks: Toward Designer Functional Molecular Materials. Bull. Chem. Soc. Jpn. 2021, 94, 2929–2955. [Google Scholar] [CrossRef]
- Hansen, J.; Davies, H.M.L. High symmetry dirhodium(II) paddlewheel complexes as chiral catalysts. Coord. Chem. Rev. 2008, 252, 545–555. [Google Scholar] [CrossRef]
- Fiori, K.W.; Du Bois, J. Catalytic Intermolecular Amination of C−H Bonds: Method Development and Mechanistic Insights. J. Am. Chem. Soc. 2007, 129, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Chifotides, H.T.; Dunbar, K.R. Interactions of Metal−Metal-Bonded Antitumor Active Complexes with DNA Fragments and DNA. Acc. Chem. Res. 2005, 38, 146–156. [Google Scholar] [CrossRef]
- Uemura, K. One-dimensional complexes extended by unbridged metal–metal bonds based on a HOMO–LUMO interaction at the dz2 orbital between platinum and heterometal atoms. Dalton Trans. 2017, 46, 5474–5492. [Google Scholar] [CrossRef]
- Knöfel, N.D.; Schoo, C.; Seifert, T.P.; Roesky, P.W. A dimolybdenum paddlewheel as a building block for heteromultimetallic structures. Dalton Trans. 2020, 49, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Mori, W.; Sato, T.; Nozaki Kato, C.; Takei, T.; Ohmura, T. Discovery and development of microporous metal carboxylates. Chem. Rec. 2005, 5, 336–351. [Google Scholar] [CrossRef]
- Chipman, J.A.; Berry, J.F. Paramagnetic Metal–Metal Bonded Heterometallic Complexes. Chem. Rev. 2020, 120, 2409–2447. [Google Scholar] [CrossRef]
- Liao, Y.; Shum, W.W.; Miller, J.S. Synthesis and Magnetic Properties of 3-D [RuII/III2(O2CMe)4]3[MIII(CN)6] (M = Cr, Fe, Co). J. Am. Chem. Soc. 2002, 124, 9336–9337. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yoshioka, D.; Handa, M. Magnetic interactions in one-, two-, and three-dimensional assemblies of dinuclear ruthenium carboxylates. Coord. Chem. Rev. 2006, 250, 2194–2211. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Matsunaga, S.; Yamashita, M.; Sugimoto, K.; Mori, T.; Toyota, N.; Dunbar, K.R. Control of Charge Transfer in a Series of Ru22+/TCNQ Two-Dimensional Networks by Tuning the Electron Affinity of TCNQ Units: A Route to Synergistic Magnetic/Conducting Materials. J. Am. Chem. Soc. 2010, 132, 1532–1544. [Google Scholar] [CrossRef]
- Liu, B.; Jin, J.; Liu, X.-M.; Hu, H.-M.; Ding, T.; Zhang, N.; Jia, Y.-Y.; Xue, G.-L. A diruthenium soft ferromagnet showing Tc = 3.0 K: Mn4(H2O)16H[Ru2(CO3)4]2[Ru2(CO3)4(H2O)2]·11H2O. Dalton Trans. 2012, 41, 4748–4750. [Google Scholar] [CrossRef] [PubMed]
- Trenerry, M.J.; Wallen, C.M.; Brown, T.R.; Park, S.V.; Berry, J.F. Spontaneous N2 formation by a diruthenium complex enables electrocatalytic and aerobic oxidation of ammonia. Nat. Chem. 2021, 13, 1221–1227. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Masuda, K.; Tanaka, H.; Naito, S.; Mori, W. Photocatalytic hydrogen production from water using porous material [Ru2(p-BDC)2]n. Energy Environ. Sci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- Villalobos, L.; Barker Paredes, J.E.; Cao, Z.; Ren, T. tert-Butyl Hydroperoxide Oxygenation of Organic Sulfides Catalyzed by Diruthenium(II,III) Tetracarboxylates. Inorg. Chem. 2013, 52, 12545–12552. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Suzuki, T.; Kumagai, Y.; Takizawa, K.; Kikuchi, T.; Kato, S.; Onoda, A.; Hayashi, T.; Kamei, Y.; Kamiyama, F.; et al. Chiral paddle-wheel diruthenium complexes for asymmetric catalysis. Nat. Catal. 2020, 3, 851–858. [Google Scholar] [CrossRef]
- Tolbatov, I.; Barresi, E.; Taliani, S.; La Mendola, D.; Marzo, T.; Marrone, A. diruthenium(II,III) paddlewheel complexes: Effects of bridging and axial ligands on anticancer properties. Inorg. Chem. Front. 2023, 10, 2226–2238. [Google Scholar] [CrossRef]
- Terán, A.; Ferraro, G.; Sánchez-Peláez, A.E.; Herrero, S.; Merlino, A. Effect of Equatorial Ligand Substitution on the Reactivity with Proteins of Paddlewheel Diruthenium Complexes: Structural Studies. Inorg. Chem. 2023, 62, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Imasaki, N.; Yano, N.; Mitsumi, M.; Handa, M. Redox-triggered reversible modulation of intense near-infrared and visible absorption using paddlewheel-Type diruthenium(III) complex. Dalton Trans. 2021, 50, 9547–9553. [Google Scholar] [CrossRef]
- Lindsay, A.J.; Wilkinson, G.; Motevalli, M.; Hursthouse, M.B. The synthesis, magnetic, electrochemical, and spectroscopic properties of diruthenium(II,II) tetra-µ-carboxylates and their adducts. X-ray Structures of Ru2(O2CR)4L2 (R = Me, L = H2O or Tetrahydrofuran; R = Et, L = Me2CO). J. Chem. Soc. Dalton Trans. 1985, 11, 2321–2326. [Google Scholar] [CrossRef]
- Furukawa, S.; Kitagawa, S. Neutral Paddlewheel Diruthenium Complexes with Tetracarboxylates of Large π-Conjugated Substituents: Facile One-Pot Synthesis, Crystal Structures, and Electrochemical Studies. Inorg. Chem. 2004, 43, 6464–6472. [Google Scholar] [CrossRef]
- Kataoka, Y.; Tada, N.; Masamori, N.; Yano, N.; Moriyoshi, C.; Handa, M. Paddlewheel-type and half-paddlewheel-type diruthenium(II,II) complexes with 1,8-naphthyridine-2-carboxylate. Dalton Trans. 2025, 54, 3047–3056. [Google Scholar] [CrossRef]
- Kataoka, Y.; Tada, N.; Omaki, J.; Matsubara, K.; Yano, N.; Handa, M. Paddlewheel-Type Diruthenium(II) Naphthyridine Complex with Electron-Withdrawing Trifluoroacetate Ligands. Chemistry 2025, 7, 72. [Google Scholar] [CrossRef]
- Tada, N.; Yano, N.; Mizogami, Y.; Matsubara, K.; Handa, M.; Kataoka, Y. Paddlewheel-Type Diruthenium(II,II) Naphthyridine Complexes with π-Conjugated Aromatic Carboxylate Ligands. Cryst. Growth Des. 2025, 25, 4458–4465. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Atsuumi, R.; Kamo, H.; Asai, Y.; Yamashita, M. Tuning of the ionization potential of paddlewheel diruthenium(II,II) complexes with fluorine atoms on the benzoate ligands. Dalton Trans. 2011, 40, 673–682. [Google Scholar] [CrossRef]
- Kosaka, W.; Watanabe, Y.; Aliyah, K.H.; Miyasaka, H. Role of intramolecular hydrogen bonding in the redox chemistry of hydroxybenzoate-bridged paddlewheel diruthenium(II,II) complexes. Dalton Trans. 2022, 51, 85–94. [Google Scholar] [CrossRef]
- Delgado-Martínez, P.; Elvira-Bravo, A.; González-Prieto, R.; Priego, J.L.; Jimenez-Aparicio, R.; Torres, M.R. Synthesis of Ru2Br(μ-O2CC6H4–R)4 (R = o-Me, m-Me, p-Me) Using Microwave Activation: Structural and Magnetic Properties. Inorganics 2014, 2, 524–536. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.43.108a; Rigaku Oxford Diffraction: Oxford, UK, 2018.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Becke, A.D. Density–functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chem. Acc. 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with time-dependent density functional theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Campos-Fernández, C.S.; Thomson, L.M.; Galán-Mascarós, J.R.; Ouyang, X.; Dunbar, K.R. Homologous Series of Redox-Active, Dinuclear Cations [M2(O2CCH3)2(pynp)2]2+ (M = Mo, Ru, Rh) with the Bridging Ligand 2-(2-Pyridyl)-1,8-naphthyridine (pynp). Inorg. Chem. 2002, 41, 1523–1533. [Google Scholar] [CrossRef]
- Sharma, R.P.; Kumar, S.; Venugopalan, P.; Ferretti, V.; Tarushi, A.; Psomas, G.; Witwicki, M. New copper(II) complexes of the anti-inflammatory drug mefenamic acid: A concerted study including synthesis, physicochemical characterization and their biological evaluation. RSC Adv. 2016, 6, 88546–88558. [Google Scholar] [CrossRef]
- Miyasaka, H.; Clérac, R.; Campos-Fernández, C.S.; Dunbar, K.R. The first crystal structure of a one-dimensional chain of linked RuII–RuII units. J. Chem. Soc. Dalton Trans. 2001, 858–861. [Google Scholar] [CrossRef]
- Chen, W.-Z.; Cotton, F.A.; Dalal, N.S.; Murillo, C.A.; Ramsey, C.M.; Ren, T.; Wang, X. Proof of Large Positive Zero-Field Splitting in a Ru25+ Paddlewheel. J. Am. Chem. Soc. 2005, 127, 12691–12696. [Google Scholar] [CrossRef]
- Shum, W.W.; Liao, Y.; Miller, J.S. Zero-Field Splitting, Field-Dependent Magnetization of Mixed-Valent S = 3/2 Diruthenium(II,III) Tetracarboxylates. J. Phys. Chem. A 2004, 108, 7460–7462. [Google Scholar] [CrossRef]
- Cotton, F.A.; Miskowski, V.M.; Zhong, B. Chemistry, Structure and Bonding in Diruthenium(II) Tetracarboxylates. J. Am. Chem. Soc. 1989, 111, 6177–6182. [Google Scholar] [CrossRef]
- Cotton, F.A.; Ren, T.; Eglin, J.L. Further Investigation of Molecular, Magnetic, and Electronic Structures of 2-Hydroxypyridinate Complexes of Diruthenium(II). Inorg. Chem. 1991, 30, 2552–2558. [Google Scholar] [CrossRef]
- Cukiernik, F.D.; Giroud-Godquin, A.-M.; Maldivi, P.; Marchon, J.-C. Pyrazine-mediated antiferromagnetic intermolecular exchange in mixed-valent diruthenium tetracarboxylates. Inorg. Chim. Acta 1994, 215, 203–207. [Google Scholar] [CrossRef]

| 4 | 5 | |
|---|---|---|
| Chemical formula | C40H29F6N7O9Ru2 | C36H18F12N4O9Ru2 |
| Formula weight | 1067.84 | 1080.68 |
| Crystal system | monoclinic | orthorhombic |
| Space group | P 21/c | P na21 |
| a [Å] | 14.3266(2) | 22.9594(3) |
| b [Å] | 10.25320(10) | 16.2295(2) |
| c [Å] | 27.8368(3) | 10.00890(10) |
| α [°] | 90 | 90 |
| β [°] | 99.0220(10) | 90 |
| γ [°] | 90 | 90 |
| V [Å3] | 4038.46(8) | 3729.51(8) |
| Z | 4 | 4 |
| Dcalc(g cm−3) | 1.756 | 1.925 |
| μ [mm−1] | 6.889 | 7.685 |
| F(000) | 2128.0 | 2120.0 |
| R1 (I > 2σ(I)) | 0.0692 | 0.0368 |
| wR2 (I > 2σ(I)) | 0.1723 | 0.0970 |
| R1 (all data) | 0.0703 | 0.0369 |
| wR2 (all data) | 0.1727 | 0.0971 |
| GOF on F2 | 1.240 | 1.077 |
| Complex | E1/2: V vs. SCE (ΔE: mV) |
|---|---|
| 1 | 0.43 (62), −0.92 (64), −1.19 (64) |
| 2 | 0.72 (68), −0.67 (63), −1.10 (56) |
| 3 | 0.52 (66), −0.83 (56), −1.19 (57) |
| 4 | 0.56 (72), −0.80 (63), −1.15 (64) |
| 5 | 0.58 (67), −0.76 (64), −1.12 (64) |
| Complex | Wavelengths (nm) [ε (M−1 cm−1)] |
|---|---|
| 1 | 781 (4313), 621 (6421), 552 (5245) |
| 2 | 686 (3780) *, 555 (6599), 514 (6090) * |
| 3 | 771 (4528), 620 (7164), 548 (6374) |
| 4 | 736 (4135), 598 (6352), 540 (5683) |
| 5 | 717 (4103), 586 (6424), 531 (5760) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, N.; Yano, N.; Handa, M.; Kataoka, Y. Electronic Influence of Trifluoromethyl Substituents on Benzoate Ligands in Paddlewheel-Type Diruthenium(II,II) Naphthyridine Complexes. Magnetochemistry 2025, 11, 104. https://doi.org/10.3390/magnetochemistry11120104
Tada N, Yano N, Handa M, Kataoka Y. Electronic Influence of Trifluoromethyl Substituents on Benzoate Ligands in Paddlewheel-Type Diruthenium(II,II) Naphthyridine Complexes. Magnetochemistry. 2025; 11(12):104. https://doi.org/10.3390/magnetochemistry11120104
Chicago/Turabian StyleTada, Nozomi, Natsumi Yano, Makoto Handa, and Yusuke Kataoka. 2025. "Electronic Influence of Trifluoromethyl Substituents on Benzoate Ligands in Paddlewheel-Type Diruthenium(II,II) Naphthyridine Complexes" Magnetochemistry 11, no. 12: 104. https://doi.org/10.3390/magnetochemistry11120104
APA StyleTada, N., Yano, N., Handa, M., & Kataoka, Y. (2025). Electronic Influence of Trifluoromethyl Substituents on Benzoate Ligands in Paddlewheel-Type Diruthenium(II,II) Naphthyridine Complexes. Magnetochemistry, 11(12), 104. https://doi.org/10.3390/magnetochemistry11120104







