Physiological and Gene Expression Analysis of Herbaceous Peony Resistance to Alternaria tenuissima Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
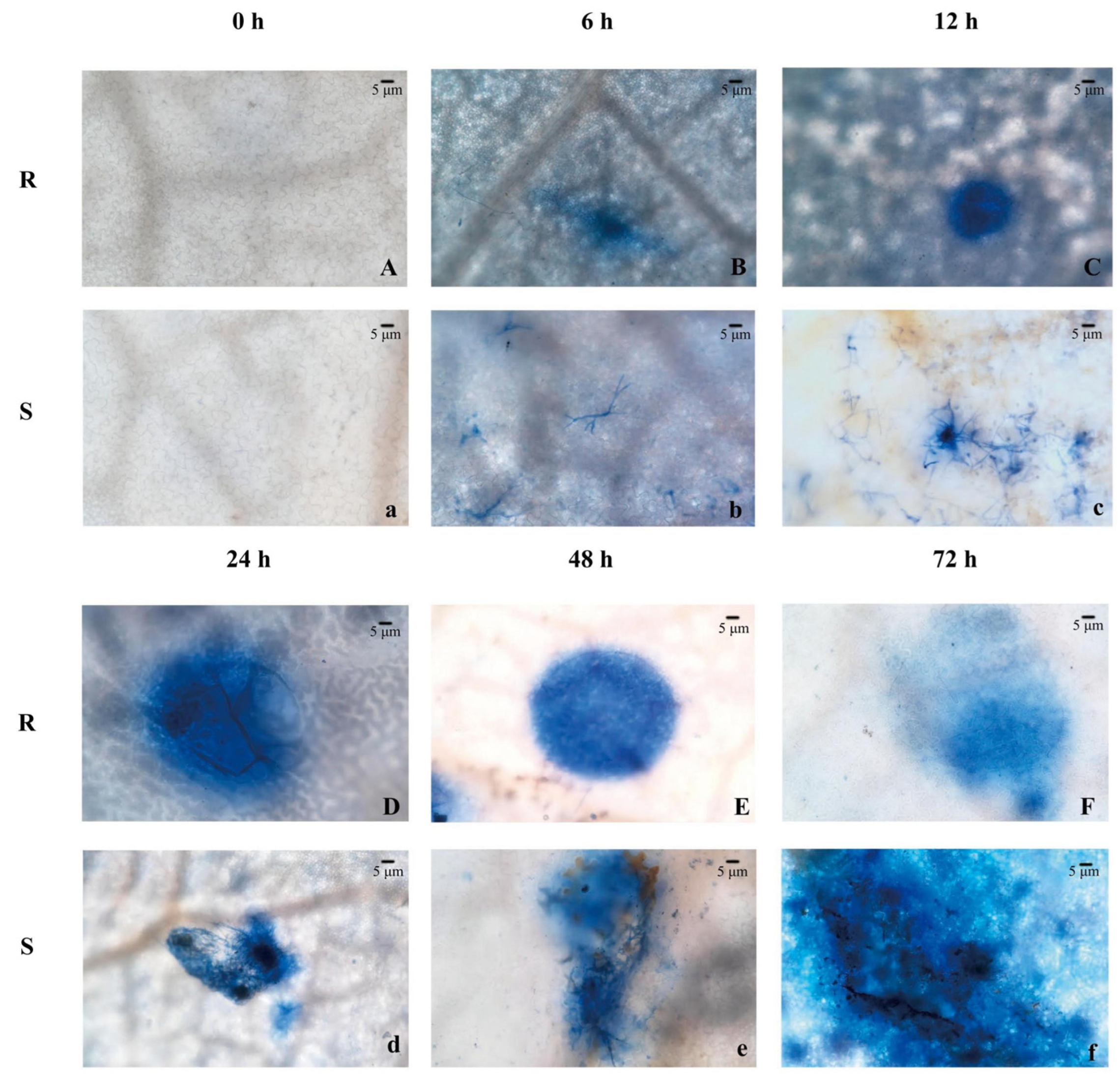
2.2. Observation of Hypersensitive Response
2.3. Determination of Malondialdehyde Content and Defensive Enzyme Activity
2.4. Analysis of Gene Expression
2.5. Data Analysis
3. Results
3.1. Hypersensitive Response
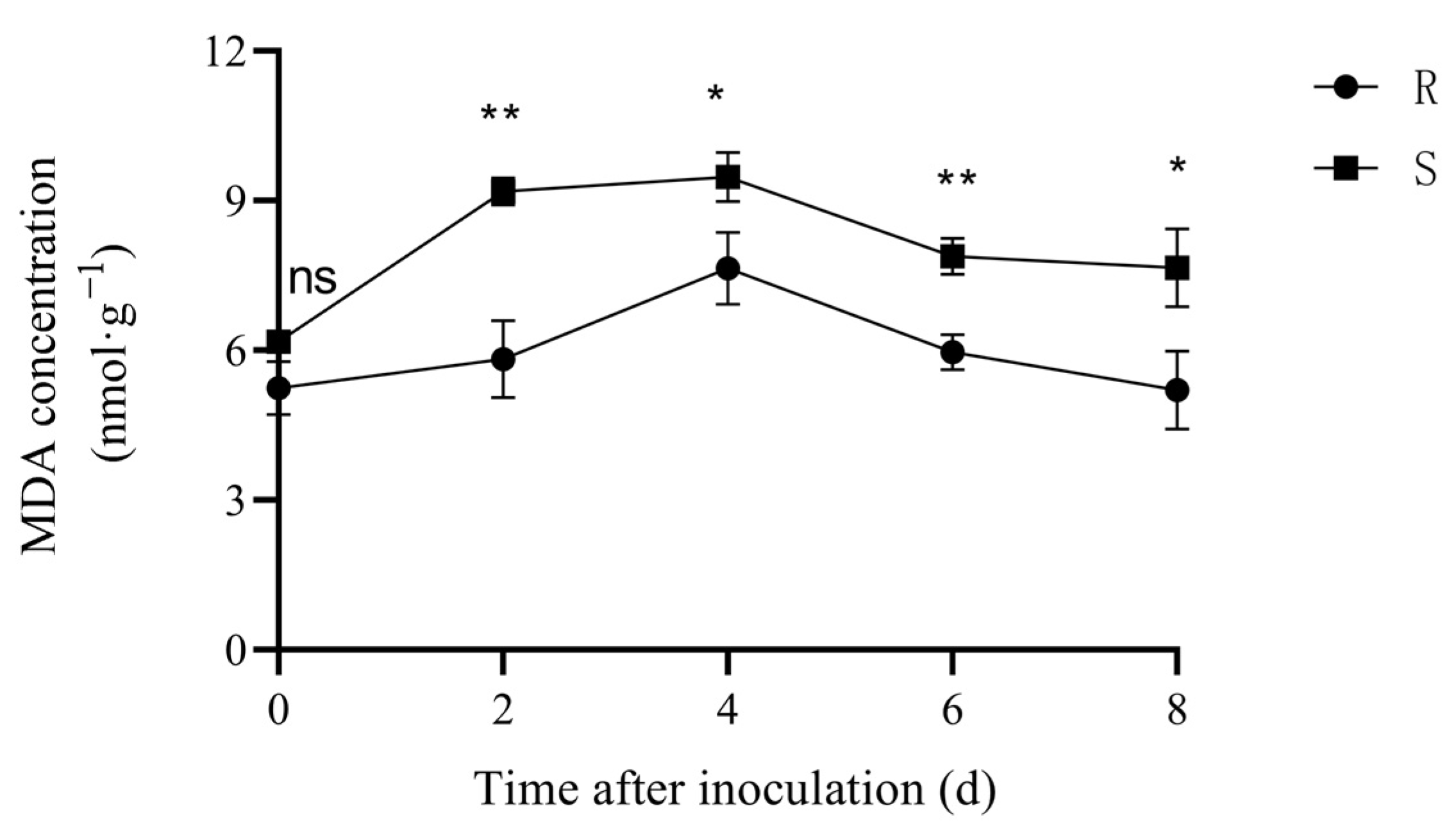
3.2. MDA Content and Activity of Related Enzymes
3.2.1. MDA Content
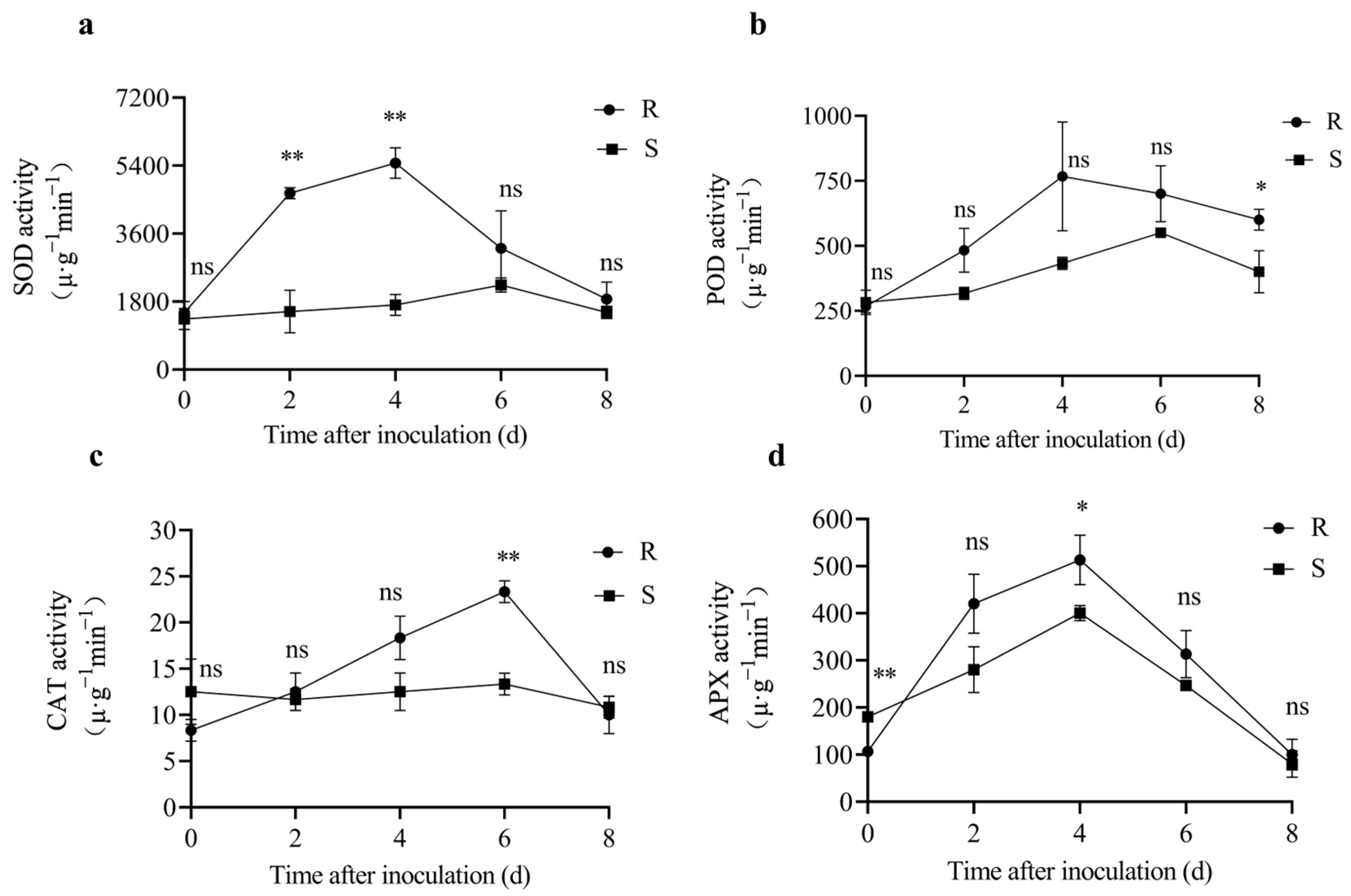
3.2.2. Activities of SOD, POD, CAT, and APX
3.2.3. Activities of PPO and PAL
3.3. Expression of the Pathogen-Related Genes
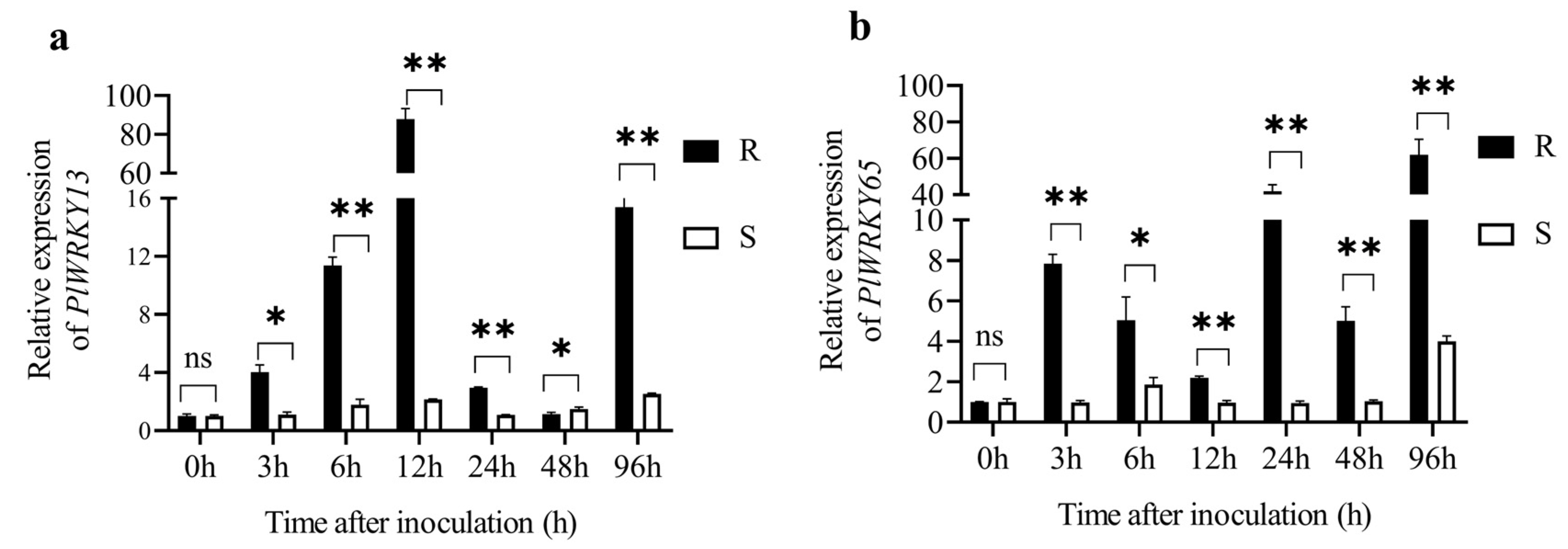
3.3.1. Expression of PlWRKY13 and PlWRKY65
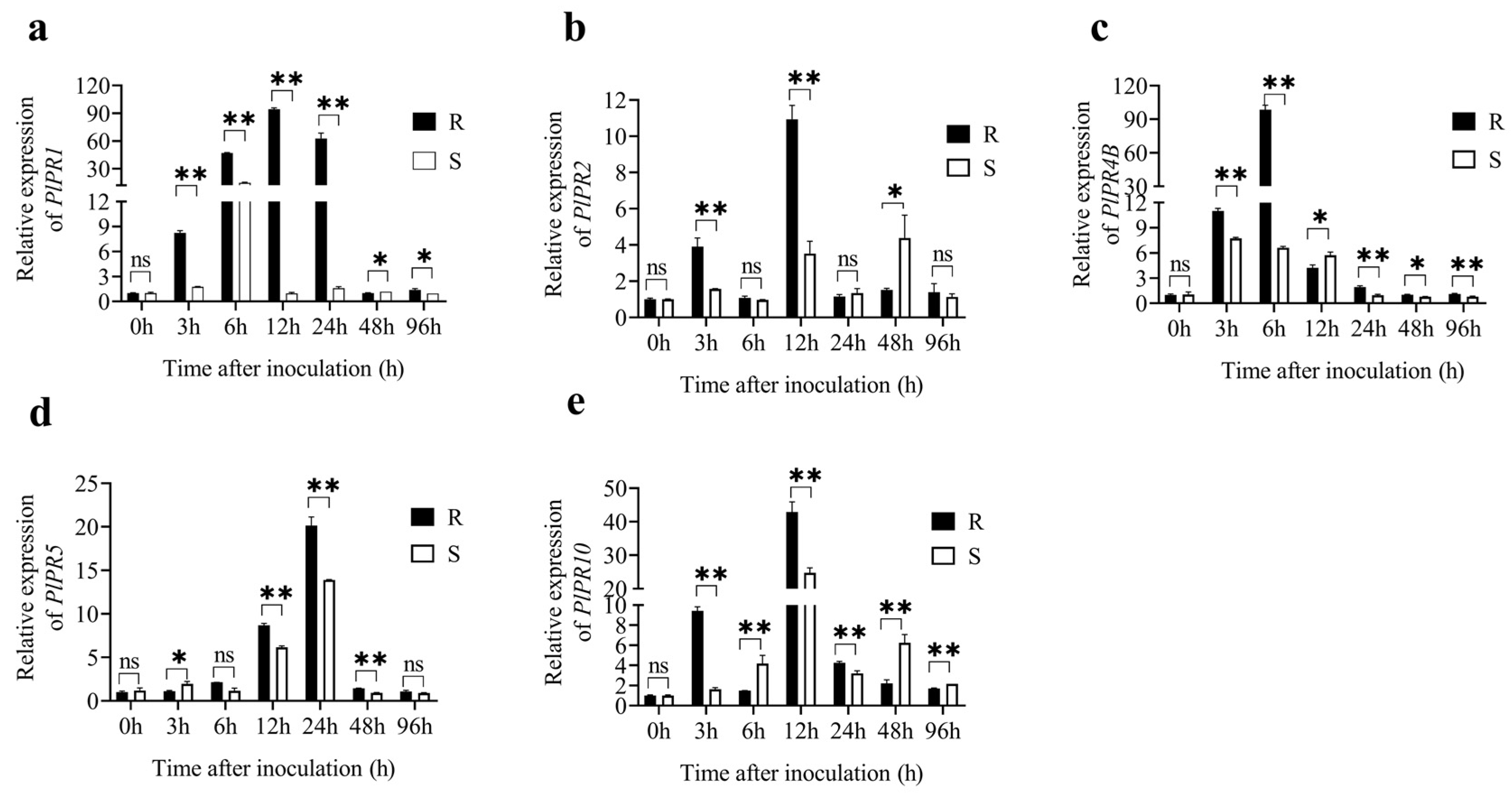
3.3.2. Expression of PlPRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, F.Y.; Zhong, Y.; Long, F.; Yu, X.N.; Kamenetsky-Goldstein, R. Chinese herbaceous peonies: Cultivar selection for forcing culture and effects of chilling and gibberellin (GA3) on plant development. Isr. J. Plant Sci. 2009, 57, 357–367. [Google Scholar] [CrossRef]
- Sun, X.M.; Huang, J.G. First report of Alternaria tenuissima causing red leaf spot disease on Paeonia lactiflora in China. Plant Dis. 2017, 101, 1322. [Google Scholar] [CrossRef]
- Li, L.; Song, S.X.; Liu, H.X.; Guo, X.F. Identification of red spot pathogens on peony in Shandong province (in Chinese, abstract in English). Yuan Yi Xue Bao 2016, 43, 365–372. [Google Scholar] [CrossRef]
- Staskawicz, B.J.; Ausubel, F.M.; Baker, J.B.; Ellis, J.G.; Jones, J.D.G. Molecular genetics of plant disease resistance. Science 1995, 268, 661–667. [Google Scholar] [CrossRef]
- Vleeshouwers, V.G.A.A.; van Dooijeweert, W.; Govers, F.; Kamoun, S.; Colon, L.T. The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta 2000, 210, 853–864. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Fayez, K.A.; Mahmoud, S.Y.; Lu, G. Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiol. Plant. 2010, 32, 891–904. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Li, B.; Zhang, Q.; Liang, W.; Wang, C. Salicylic acid confers enhanced resistance to Glomerella leaf spot in apple. Plant Physiol. Biochem. 2016, 106, 64–72. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Jun, S.Y.; Sattler, S.A.; Cortez, G.S.; Vermerris, W.; Sattler, S.E.; Kang, C. Biochemical and structural analysis of substrate specificity of a phenylalanine ammonia-lyase. Plant Physiol. 2018, 176, 1452–1468. [Google Scholar] [CrossRef]
- Jia, Y.; Martin, G.B. Rapid transcript accumulation of pathogenesis-related genes during an incompatible interaction in bacterial speck disease-resistant tomato plants. Plant Mol. Biol. 1999, 40, 455–465. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-related genes of PR1, PR2, PR4, and PR5 families are involved in the response to Fusarium infection in garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of pathogenesis-related (PR) proteins in plant defense mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 265–281. [Google Scholar] [CrossRef]
- dos Santos, C.; Franco, O.L. Pathogenesis-related proteins (PRs) with enzyme activity activating plant defense responses. Nat. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Jiang, J.J.; Ma, S.H.; Ye, N.H.; Cao, J.S.; Zhang, J.H.; Jiang, M. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushtun, P.J.; Schmelzer, E.; Hahlbrock, K.; Somssich, I.E. Early nuclear events in plant defence signalling: Rapid gene activation by WRKY transcription factors. EMBO J. 1999, 18, 4689–4699. [Google Scholar] [CrossRef]
- Jimmy, J.L.; Babu, S. Role of OsWRKY transcription factors in rice disease resistance. Trop. Plant Pathol. 2015, 40, 355–361. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.J.; Guo, J.; Qiao, Q.; Guo, X.F.; Ma, Y. The WRKY transcription factor PlWRKY65 enhances the resistance of Paeonia lactiflora (herbaceous peony) to Alternaria tenuissima. Hortic. Res. 2020, 7, 57. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.J.; Guo, X.F.; Ma, Y.; Qiao, Q.; Guo, J. PlWRKY13: A transcription factor involved in abiotic and biotic stress responses in Paeonia lactiflora. Int. J. Mol. Sci. 2019, 20, 5953. [Google Scholar] [CrossRef]
- Xiao, S.; Brown, S.; Patrick, E.; Brearley, C.; Turner, J.G. Enhanced transcription of the Arabidopsis disease resistance genes RPW8.1 and RPW8.2 via a salicylic acid-dependent amplification circuit is required for hypersensitive cell death. Plant Cell 2003, 15, 33–45. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts I kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kagan, V.; Jaworski, A.W.; Brown, S.K. Enzymatic browning in relation to phenolic compounds and polyphenoloxidase activity among various peach cultivars. J. Agric. Food Chem. 1990, 38, 99–101. [Google Scholar] [CrossRef]
- Dickerson, D.P.; Pascholati, S.F.; Hagerman, A.E.; Butler, L.G.; Nicholson, R.L. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Plant Pathol. 1984, 25, 111–123. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, Y.; Liu, G.; Gao, Z.; Li, M.; Su, Z.; Zhang, Z. Inhibition on anthracnose and induction of defense response by nitric oxide in pitaya fruit. Sci. Hortic. 2019, 245, 224–230. [Google Scholar] [CrossRef]
- Chan, Z.; Tian, S. Induction of H2O2-metabolizing enzymes and total protein synthesis by antagonistic yeast and salicylic acid in harvested sweet cherry fruit. Postharvest Biol. Technol. 2006, 39, 314–320. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef]
- Tian, S.; Wan, Y.; Qin, G.; Xu, Y. Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Appl. Microbiol. Biotechnol. 2006, 70, 729–734. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Eulgem, T.; Somssich, I.E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007, 10, 366–371. [Google Scholar] [CrossRef]
- Jimmy, J.L.; Babu, S. Gene network mediated by WRKY13 to regulate resistance against sheath infecting fungi in rice (Oryza sativa L.). Plant Sci. 2019, 280, 269–282. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Chen, Q.; Deng, B.; Deng, L.; Zeng, K. Transcription factor CsWRKY65 participates in the establishment of disease resistance of citrus fruits to Penicillium digitatum. J. Agric. Food Chem. 2021, 69, 5671–5682. [Google Scholar] [CrossRef]
- Rockenbach, M.F.; Velho, A.C.; Alaniz, S.M.; Stadnik, M.J. Resistance of apple leaves to infection by Colletotrichum fructicola acts independently of hypersensitive reaction and PR-1 and PR-10 gene expression. Trop. Plant Pathol. 2018, 43, 360–370. [Google Scholar] [CrossRef]
- Park, C.J.; Kim, K.J.; Shin, R.; Park, J.M.; Shin, Y.C.; Paek, K.H. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J. 2004, 37, 186–198. [Google Scholar] [CrossRef]
- Xie, Y.R.; Chen, Z.Y.; Brown, R.L.; Bhatnagar, D. Expression and functional characterization of two pathogenesis-related protein 10 genes from Zea mays. J. Plant Physiol. 2010, 167, 121–130. [Google Scholar] [CrossRef]
- Hou, M.; Xu, W.; Bai, H.; Liu, Y.; Li, L.; Liu, L.; Liu, B.; Liu, G. Characteristic expression of rice pathogenesis-related proteins in rice leaves during interactions with Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 2012, 31, 895–904. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Odjakova, M.; Hadjiivanova, C. The complexity of pathogen defense in plants. Bulg. J. Plant Physiol. 2001, 27, 101–109. [Google Scholar]
- Boccardo, N.A.; Segretin, M.E.; Hernandez, I.; Mirkin, F.G.; Chacón, O.; Lopez, Y.; Borrás-Hidalgo, O.; Bravo-Almonacid, F.F. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci. Rep. 2019, 9, 2791. [Google Scholar] [CrossRef]
- Besbes, F.; Franz-Oberdorf, K.; Schwab, W. Phosphorylation-dependent ribonuclease activity of Fra a 1 proteins. J. Plant Physiol. 2019, 233, 1–11. [Google Scholar] [CrossRef]
- van Verk, M.C.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J.M. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar] [CrossRef]
- Choi, C.; Hwang, S.H.; Fang, I.R.; Kwon, S.I.; Park, S.R.; Ahn, I.; Kim, J.B.; Hwang, D.J. Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol. 2015, 208, 846–859. [Google Scholar] [CrossRef]
- Yin, L.; Zou, Y.; Ke, X.; Liang, D.; Du, X.; Zhao, Y.; Zhang, Q.; Ma, F. Phenolic responses of resistant and susceptible Malus plants induced by Diplocarpon mali. Sci. Hortic. 2013, 164, 17–23. [Google Scholar] [CrossRef]
- Silva, L.C.; Debona, D.; Aucique-Pérez, C.E.; Oliveira, J.R.; Júnior, J.I.R.; Brás, V.V.; Rodrigues, F.A. Physiological and antioxidant insights into common bean resistance to common bacterial blight. Physiol. Mol. Plant Pathol. 2020, 111, 101505. [Google Scholar] [CrossRef]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Lu, Z.; Zhu, Y.; Guo, X. Physiological and Gene Expression Analysis of Herbaceous Peony Resistance to Alternaria tenuissima Infection. Horticulturae 2023, 9, 862. https://doi.org/10.3390/horticulturae9080862
Wu Y, Lu Z, Zhu Y, Guo X. Physiological and Gene Expression Analysis of Herbaceous Peony Resistance to Alternaria tenuissima Infection. Horticulturae. 2023; 9(8):862. https://doi.org/10.3390/horticulturae9080862
Chicago/Turabian StyleWu, Yang, Zhonghua Lu, Yongfang Zhu, and Xianfeng Guo. 2023. "Physiological and Gene Expression Analysis of Herbaceous Peony Resistance to Alternaria tenuissima Infection" Horticulturae 9, no. 8: 862. https://doi.org/10.3390/horticulturae9080862
APA StyleWu, Y., Lu, Z., Zhu, Y., & Guo, X. (2023). Physiological and Gene Expression Analysis of Herbaceous Peony Resistance to Alternaria tenuissima Infection. Horticulturae, 9(8), 862. https://doi.org/10.3390/horticulturae9080862



