Effects of Sulfate on the Physiology, Biochemistry, and Activity of Group 1 Sulfate Transporters in Seedlings of Brassica pekinensis
Abstract
1. Introduction
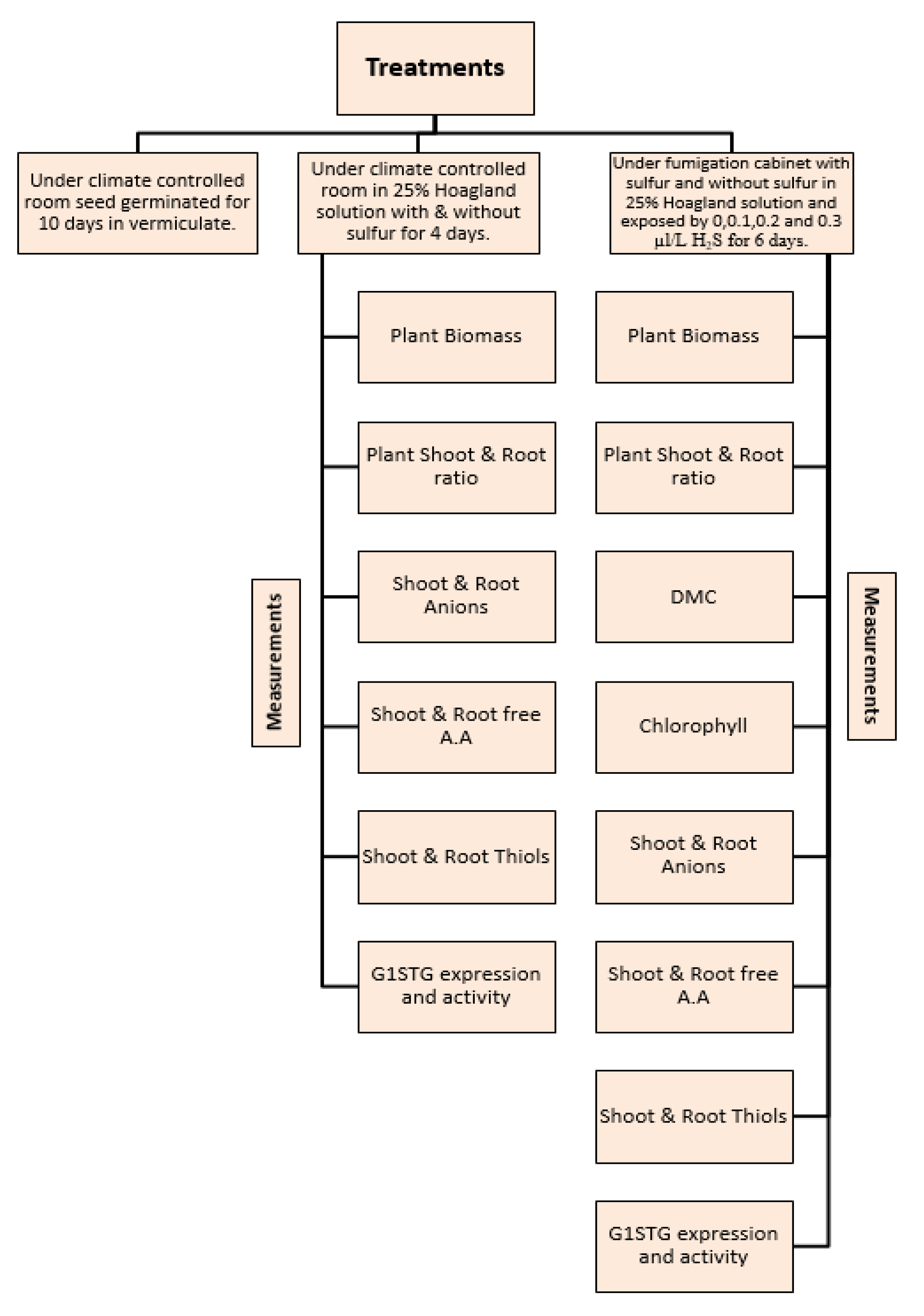
2. Materials and Methods
2.1. Plant Materials and Growth Conditions in a Climate-Controlled Room
2.2. H2S Exposure and Growth Conditions in Fumigation Cabinets
2.3. Pigments
2.4. Anions
2.5. Water-Soluble Non-Protein Thiols
2.6. Free Amino Acids
2.7. RNA Extraction
2.8. Relative Expression of Sultr1;1, Sultr1;2 by Real-Time Quantitative PCR
2.9. Sulfate-Uptake Capacity
2.10. Statistical Analysis
3. Results
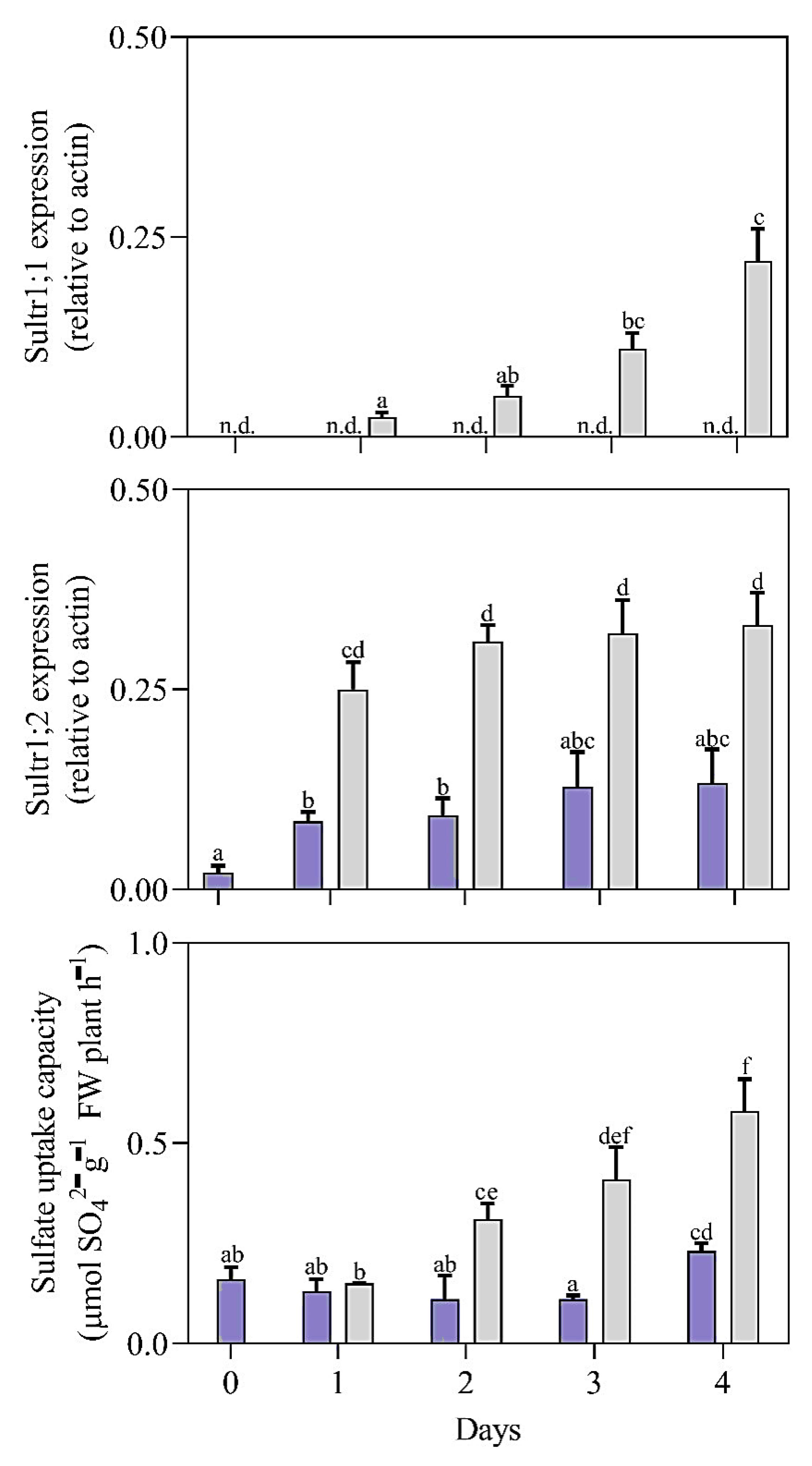
3.1. Impact of Sulfur on Plant Growth, Physico-Chemical Parameters, and Sulfate Transporter Gene Expression
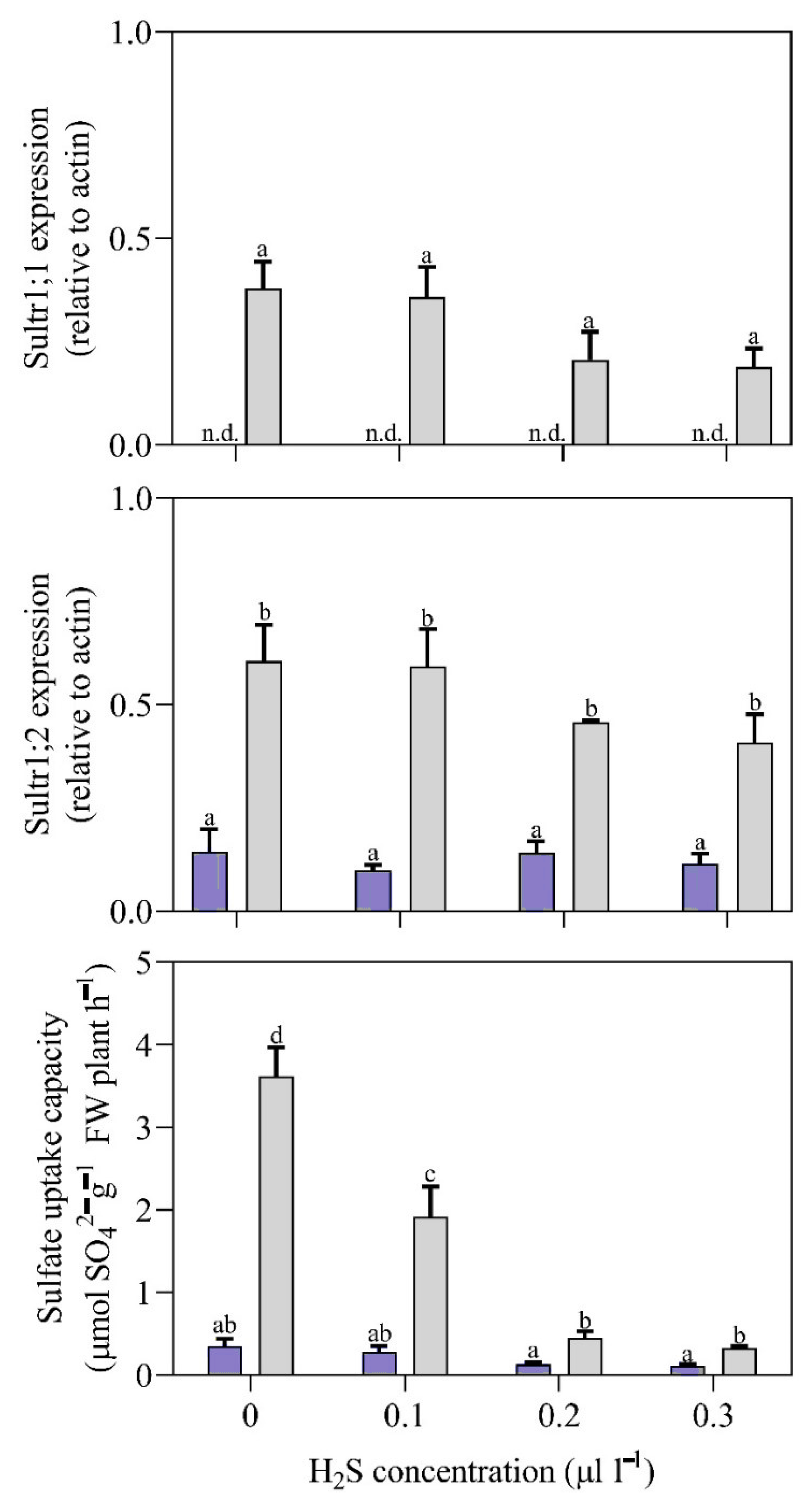
3.2. Effects of H2S Treatment on Growth, Physico-Chemical Parameters, and Sulfate Transporter Gene Expression in Plants Grown without and with Sulfate
3.3. Effects of H2S Treatment on Sulfate Transporter Gene Expression in Plants Grown without and with Sulfate
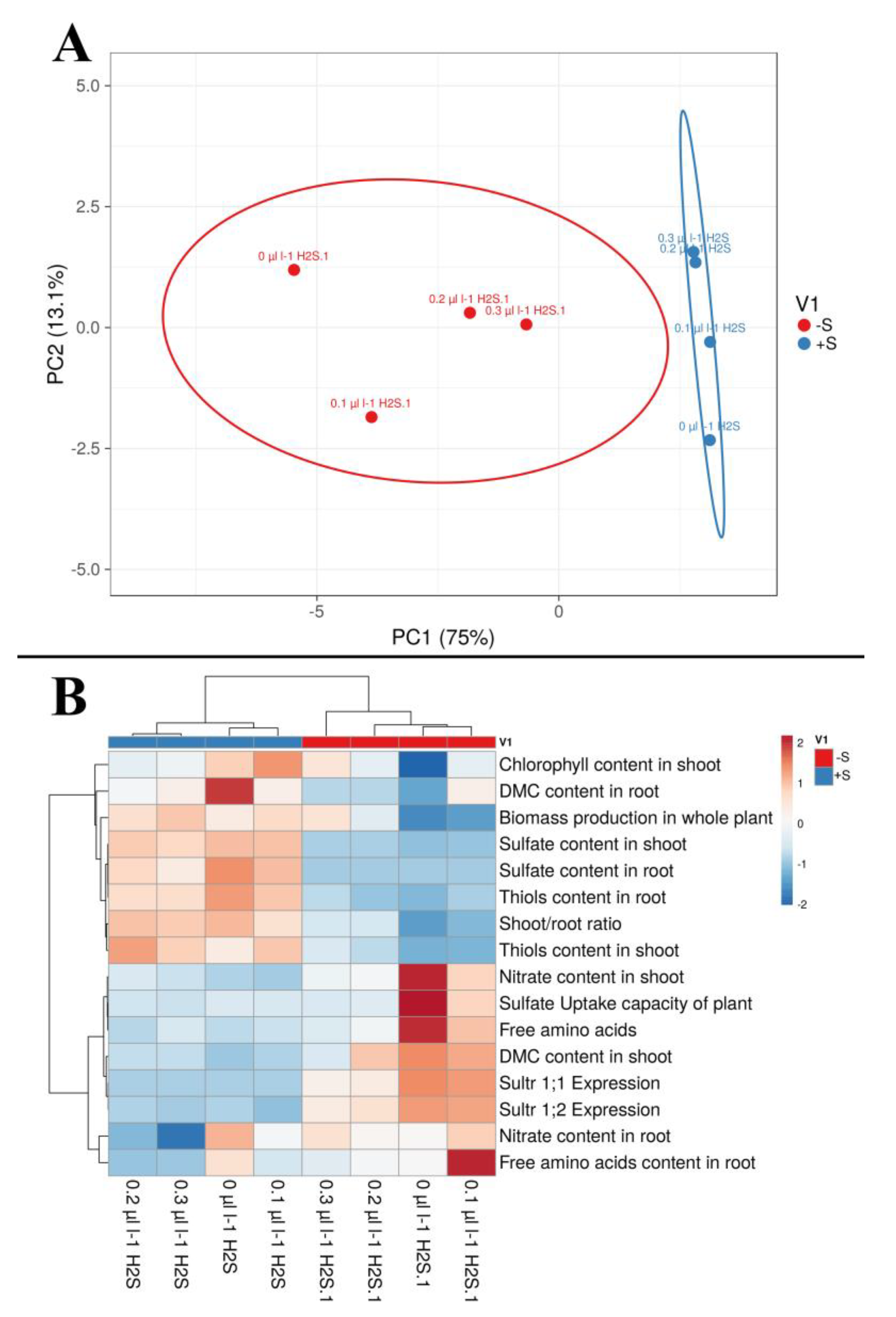
3.4. Multivariate Cluster Analysis, Non-Metric Multidimensional Scaling, and Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Quispe, C.; Butnariu, M.; Sarac, I.; Marmouzi, I.; Kamle, M.; Tripathi, V.; Kumar, P.; Bouyahya, A.; Capanoglu, E.; et al. Phytotherapy and food applications from Brassica genus. Phytother. Res. 2021, 35, 3590–3609. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.; Shahbaz, M.; Prajapati, D.H.; Parmar, S.; Hawkesford, M.J.; De Kok, L.J. Interactions of sulfate with other nutrients as revealed by H2S fumigation of Chinese cabbage. Front. Plant Sci. 2016, 7, 541. [Google Scholar] [CrossRef] [PubMed]
- De Kok, L.J.; Hawkesford, M.J.; Haneklaus, S.H.; Schnug, E. Sulfur Metabolism in Higher Plants-Fundamental, Environmental and Agricultural Aspects, (Proceedings of the International Plant Sulfur Workshop); Springer International Publishing: Cham, Switzerland, 2017; p. 243. [Google Scholar]
- Liu, T.; Chen, J.A.; Wang, W.; Simon, M.; Wu, F.; Hu, W.; Chen, J.B.; Zheng, H. A combined proteomic and transcriptomic analysis on sulfur metabolism pathways of Arabidopsis thaliana under simulated acid rain. PLoS ONE 2014, 9, e90120. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, T.A.; Prajapati, D.H.; Burow, M. Copper toxicity affects indolic glucosinolates and gene expression of key enzymes for their biosynthesis in Chinese cabbage. Arch. Agron. Soil Sci. 2019, 66, 1288–1301. [Google Scholar] [CrossRef]
- Qian, Y.L.; Hua, G.K.H.; Scott, J.C.; Dung, J.K.; Qian, M.C. Evaluation of Sulfur-Based Biostimulants for the Germination of Sclerotium cepivorum Sclerotia and Their Interaction with Soil. J. Agric. Food Chem. 2022, 70, 15038–15045. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants; Marschner, P., Ed.; Elsevier: London, UK, 2023; pp. 201–281. [Google Scholar]
- Kovács, S.; Kutasy, E.; Csajbók, J. The Multiple Role of Silicon Nutrition in Alleviating Environmental Stresses in Sustainable Crop Production. Plants 2022, 11, 1223. [Google Scholar] [CrossRef]
- Shafiq, B.A.; Nawaz, F.; Majeed, S.; Aurangzaib, M.; Al Mamun, A.; Ahsan, M.; Ahmad, K.S.; Shehzad, M.A.; Ali, M.; Hashim, S.; et al. Sulfate-based fertilizers regulate nutrient uptake, photosynthetic gas exchange, and enzymatic antioxidants to increase sunflower growth and yield under drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2229–2241. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.A.; Prajapati, D.H.; Burow, M. Differential partitioning of thiols and glucosinolates between shoot and root in Chinese cabbage upon excess zinc exposure. J. Plant Physiol. 2020, 244, 153088. [Google Scholar] [CrossRef]
- Shahzad, R.; Harlina, P.W.; Ayaad, M.; Ewas, M.; Nishawy, E.; Fahad, S.; Subthain, H.; Amar, M.H. Dynamic roles of microRNAs in nutrient acquisition and plant adaptation under nutrient stress: A review. Plant Omics 2018, 11, 58–79. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Yang, A. Sulfur homeostasis in plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef]
- Ferrari, M.; Cozza, R.; Marieschi, M.; Torelli, A. Role of Sulfate Transporters in Chromium Tolerance in Scenedesmus acutus M. (Sphaeropleales). Plants 2022, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Heidari, P.; Hasanzadeh, S.; Faraji, S.; Ercisli, S.; Mora-Poblete, F. Genome-Wide Characterization of the Sulfate Transporter Gene Family in Oilseed Crops: Camelina sativa and Brassica napus. Plants 2023, 12, 628. [Google Scholar] [CrossRef]
- Kiefer, C.; Willing, E.M.; Jiao, W.B.; Sun, H.; Piednoël, M.; Hümann, U.; Hartwig, B.; Koch, M.A.; Schneeberger, K. Interspecies association mapping links reduced CG to TG substitution rates to the loss of gene-body methylation. Nat. Plants 2019, 5, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Cai, M.L.; Chen, S.H.; Huang, X.Y.; Zhao, F.J.; Wang, P. High-Affinity Sulfate Transporter Sultr1;2 Is a Major Transporter for Cr(VI) Uptake in Plants. Environ. Sci. Technol. 2021, 55, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Damiani, I.; Drain, A.; Guichard, M.; Balzergue, S.; Boscari, A.; Boyer, J.C.; Brunaud, V.; Cottaz, S.; Rancurel, C.; Da Rocha, M.; et al. Nod Factor Effects on Root Hair-Specific Transcriptome of Medicago truncatula: Focus on Plasma Membrane Transport Systems and Reactive Oxygen Species Networks. Front. Plant Sci. 2016, 7, 794. [Google Scholar] [CrossRef] [PubMed]
- Koralewska, A.; Posthumus, F.S.; Stuiver, C.E.E.; Buchner, P.; Hawkesford, M.J.; De Kok, L.J. The characteristic high sulfate content in Brassica oleracea is controlled by the expression and activity of sulfate transporters. Plant Biol. 2007, 9, 654–661. [Google Scholar] [CrossRef]
- Wei, X.; Lyu, S.; Yu, Y.; Wang, Z.; Liu, H.; Pan, D.; Chen, J. Phylloremediation of air pollutants: Exploiting the potential of plant leaves and leaf-associated microbes. Front. Plant Sci. 2017, 8, 1318. [Google Scholar] [CrossRef]
- Ausma, T.; De Kok, L.J. Atmospheric H2S: Impact on plant functioning. Front. Plant Sci. 2019, 10, 743. [Google Scholar] [CrossRef]
- Kuhn, R.; Bryant, I.M.; Jensch, R.; Böllmann, J. Applications of environmental nanotechnologies in remediation, wastewater treatment, drinking water treatment, and agriculture. Appl. Nano 2022, 3, 54–90. [Google Scholar] [CrossRef]
- Ausma, T.; Mulder, J.; Polman, T.R.; van der Kooi, C.J.; De Kok, L.J. Atmospheric H2S exposure does not affect stomatal aperture in maize. Planta 2020, 252, 63. [Google Scholar] [CrossRef]
- Taha, A.; Patón, M.; Rodríguez, J. Model-based design and operation of biotrickling filters for foul air H2S removal at wastewater networks. J. Environ. Chem. Eng. 2022, 10, 107372. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.; Hawkesford, M.J.; De Kok, L.J. The significance of glucosinolates for sulfur storage in Brassicaceae seedlings. Front. Plant Sci. 2014, 5, 704. [Google Scholar] [CrossRef]
- Bharwana, S.A.; Ali, S.; Farooq, M.A.; Ali, B.; Iqbal, N.; Abbas, F.; Ahmad, M.S.A. Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ. Sci. Pollut. Res. 2014, 21, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, T.; Hawkesford, M.J.; De Kok, L.J. Atmospheric H2S and SO2 as sulfur sources for Brassica juncea and Brassica rapa: Regulation of sulfur uptake and assimilation. Environ. Exp. Bot. 2016, 124, 10. [Google Scholar] [CrossRef]
- Koprivova, A.; Kopriva, S. Molecular mechanisms of regulation of sulfate assimilation: First steps on a long road. Front. Plant Sci. 2014, 5, 589. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Chai, X. Genome-wide identification and expression analysis of the MYC transcription factor family and its response to sulfur stress in cabbage (Brassica oleracea L.). Gene 2022, 814, 146116. [Google Scholar] [CrossRef] [PubMed]
- Ausma, T.; De Kok, L.J. Regulation of sulfate uptake and assimilation in Barley (Hordeum vulgare) as affected by rhizospheric and atmospheric sulfur nutrition. Plants 2020, 9, 1283. [Google Scholar] [CrossRef]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, l12, 275–283. [Google Scholar] [CrossRef]
- Zuidersma, E.I.; Ausma, T.; Stuiver, C.E.E.; Prajapati, D.H.; Hawkesford, M.J.; De Kok, L.J. Molybdate toxicity in Chinese cabbage is not the direct consequence of changes in sulphur metabolism. Plant Biol. 2020, 22, 331–336. [Google Scholar] [CrossRef]
- Durenkamp, M.; De Kok, L.J. The Impact of Atmospheric H2S on Growth and Sulfur Metabolism of Allium cepa L. Phyton Ann. Rei Bot. 2002, 42, 55–63. [Google Scholar]
- Shahbaz, M.; Tseng, M.H.; Stuiver, C.E.; Koralewska, A.; Posthumus, F.S.; Venema, J.H.; Parmar, S.; Schat, H.; Hawkesford, M.J.; De Kok, L.J. Copper exposure interferes with the regulation of the uptake, distribution and metabolism of sulfate in Chinese cabbage. J. Plant Physiol. 2010, 167, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Patel, M.; Vurukonda, S.S.K.P.; Patel, A. Multi-trait Halotolerant Plant Growth-promoting Bacteria Mitigate Induced Salt Stress and Enhance Growth of Amaranthus viridis. J. Soil Sci. Plant Nutr. 2023, 23, 1860–1883. [Google Scholar] [CrossRef]
- Harris, K.S.; Durek, T.; Kaas, Q.; Poth, A.G.; Gilding, E.K.; Conlan, B.F.; Saska, I.; Daly, N.L.; Van Der Weerden, N.L.; Craik, D.J.; et al. Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase. Nat. Commun. 2015, 6, 10199. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Ejaz, H.; Bibi, E.; Ali, W.; Ahmad, I.; Lashari, A.; Faiz, H.; Nazar, W. Sulphur and particulate matter affecting on soil and underground plants. J. Agric. Appl. Biol. 2022, 3, 40–49. [Google Scholar] [CrossRef]
- Patani, A.; Prajapati, D.; Ali, D.; Kalasariya, H.; Yadav, V.K.; Tank, J.; Bagatharia, S.; Joshi, M.; Patel, A. Evaluation of the growth-inducing efficacy of various Bacillus species on the salt-stressed tomato (Lycopersicon esculentum Mill.). Front. Plant Sci. 2023, 14, 1168155. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Chen, L.; Wang, C. The Role of Hydrogen Sulfide in Plant Roots during Development and in Response to Abiotic Stress. Int. J. Mol. Sci. 2022, 23, 1024. [Google Scholar] [CrossRef]
- Etienne, P.; Sorin, E.; Maillard, A.; Gallardo, K.; Arkoun, M.; Guerrand, J.; Cruz, F.; Yvin, J.C.; Ourry, A. Assessment of Sulfur Deficiency under Field Conditions by Single Measurements of Sulfur, Chloride and Phosphorus in Mature Leaves. Plants 2018, 7, 37. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Revisiting Sulfur-The once neglected nutrient: It’s roles in plant growth, metabolism, stress tolerance and crop production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Lentz, C.; Mühling, K.H. Crosstalk between selenium and sulfur is associated with changes in primary metabolism in lettuce plants grown under Se and S enrichment. Plants 2022, 11, 927. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulou, S.N.; Bouranis, D.L. The Role of Sulfur in Agronomic Biofortification with Essential Micronutrients. Plants 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2022, 2030082. [Google Scholar] [CrossRef] [PubMed]
- Przybyla-Toscano, J.; Roland, M.; Gaymard, F.; Couturier, J.; Rouhier, N. Roles and maturation of iron-sulfur proteins in plastids. J. Biol. Inorg. Chem. 2018, 23, 545–566. [Google Scholar] [CrossRef]
- Aarabi, F.; Naake, T.; Fernie, A.R.; Hoefgen, R. Coordinating Sulfur Pools under Sulfate Deprivation. Trends Plant Sci. 2020, 25, 1227–1239. [Google Scholar] [CrossRef]
- Chung, J.S.; Kim, H.C.; Yun, S.M.; Kim, H.J.; Kim, C.S.; Lee, J.J. Metabolite analysis of lettuce in response to sulfur nutrition. Horticulturae 2022, 8, 734. [Google Scholar] [CrossRef]
- Schmidt, F.; De Bona, F.D.; Monteiro, F.A. Sulfur limitation increases nitrate and amino acid pools in tropical forages. Crop Pasture Sci. 2013, 64, 51–60. [Google Scholar] [CrossRef]
- Ristova, D.; Kopriva, S. Sulfur signaling and starvation response in Arabidopsis. iScience 2022, 25, 104242. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Y.; Wang, H.; Jin, Z.; Liu, Z.; Qiao, Z.; Fang, H.; Zhang, Y. Hydrogen Sulfide Alleviates Cadmium-Induced Cell Death through Restraining ROS Accumulation in Roots of Brassica rapa L. ssp. pekinensis. Oxid. Med. Cell. Longev. 2015, 2015, 804603. [Google Scholar] [CrossRef]
- Jobe, T.O.; Sung, D.Y.; Akmakjian, G.; Pham, A.; Komives, E.A.; Mendoza-Cózatl, D.G.; Schroeder, J.I. Feedback inhibition by thiols outranks glutathione depletion: A luciferase-based screen reveals glutathione-deficient γ-ECS and glutathione synthetase mutants impaired in cadmium-induced sulfate assimilation. Plant J. 2012, 70, 783–795. [Google Scholar] [CrossRef]
- Takahashi, H. Sulfate transport systems in plants: Functional diversity and molecular mechanisms underlying regulatory coordination. J. Exp. Bot. 2019, 70, 4075–4087. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Stappenbeck, T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 2013, 8, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, G.A.; Nocito, F.F. Plant Sulfate Transporters in the Low Phytic Acid Network: Some Educated Guesses. Plants 2019, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.I.; Prajapati, D.H.; Parmar, S.; Aghajanzadeh, T.A.; Hawkesford, M.J.; De Kok, L.J. Manganese toxicity hardly affects sulfur metabolism in Brassica rapa. In Sulfur Metabolism in Higher Plants-Fundamental, Environmental and Agricultural Aspects; De Kok, L.J., Hawkesford, M.J., Haneklaus, S.H., Schnug, E., Eds.; Springer: Cham, Switzerland, 2017; pp. 155–162. [Google Scholar] [CrossRef]

| Primer Sequence (5′-3′) | ||
|---|---|---|
| Gene | Forward | Reverse |
| Sultr1;1 | TGGCCATAGTGGTAGCTC | AGACCAAGGACGGCTGAT |
| Sultr1;2 | GCAACAGACGGTGGAGAT | GCCCCCAATCGAAAACC |
| Actin | AGCAGCATGAAGATCAAGGT | GCTGAGGGATGC |
| Initial | 1 Day | 2 Days | 3 Days | 4 Days | |||||
|---|---|---|---|---|---|---|---|---|---|
| +S | −S | +S | −S | +S | −S | +S | −S | ||
| Plant | |||||||||
| Biomass | 0.08 ± 0.00 a | 0.08 ± 0.00 a | 0.09 ± 0.01 b | 0.11 ± 0.00 ac | 0.11 ± 0.01 bc | 0.19 ± 0.03 d | 0.18 ± 0.01 d | 0.23 ± 0.02 e | 0.22 ± 0.03 de |
| Shoot/root ratio | 3.7 ± 0.9 acd | 3.3 ± 0.3 ad | 3.1 ± 0.4 a | 3.8 ± 0.6 cd | 3.6 ± 0.5 ad | 4.2 ± 0.4 ce | 4.1± 0.2 c | 5.1 ± 0.8 b | 4.5 ± 0.3 be |
| Shoot | |||||||||
| Sulfate | 12.9 ± 1.7 c | 13.2 ± 0.8 c | 11.1 ± 0.7 c | 13.3 ± 0.8 c | 6.3 ± 1.1 c | 14.0 ± 1.3 b | 3.6 ± 0.7 c | 14.3 ± 1.7 b | 1.1 ± 0.4 a |
| Nitrate | 0.3 ± 0.1 a | 8.3 ± 0.5 b | 8.5 ± 0.3 bc | 15.7 ± 3.7 cd | 26.4 ± 5.1 de | 41.0 ± 3.2 ef | 49.9 ± 2.9 f | 49.5 ± 9.3 efg | 65.8 ± 4.0 g |
| Thiols | 0.28 ± 0.02 b | 0.46 ± 0.01 c | 0.43 ± 0.03 c | 0.46 ± 0.04 c | 0.40 ± 0.03 cd | 0.61 ± 0.08 c | 0.31 ± 0.02 bd | 0.48 ± 0.04 c | 0.11 ± 0.03 a |
| Free amino acids | 3.9 ± 0.8 a | 20.9 ± 1.0 b | 23.6 ± 0.9 b | 19.9 ± 5.4 b | 24.1 ± 6.5 b | 21.8 ± 2.2 b | 25.0 ± 2.9 b | 27.0 ± 3.9 b | 27.8 ± 7.6 b |
| Root | |||||||||
| Sulfate | 9.0 ± 0.5 f | 9.5 ± 0.0 f | 7.5 ± 0.2 e | 10.1 ± 0.5 cf | 5.6 ± 0.3 d | 11.5 ± 0.4 g | 3.9 ± 0.2 b | 12.5 ± 0.8 g | 2.7 ± 0.4 a |
| Nitrate | 3.1 ± 1.1 a | 18.1 ± 1.2 b | 19.6 ± 0.4 b | 23.7 ± 0.9 c | 27.2 ± 1.8 cd | 24.4 ± 2.5 bcd | 27.5 ± 1.1 d | 24.6 ± 3.7 bc | 30.4 ± 2.3 d |
| Thiols | 0.30 ± 0.03 b | 0.32 ± 0.07 ab | 0.30 ± 0.04 ab | 0.31 ± 0.01 b | 0.33 ± 0.03 b | 0.33 ± 0.02 b | 0.38 ± 0.03 b | 0.44 ± 0.08 b | 0.19 ± 0.02 a |
| Free amino acids | 9.7 ± 1.8 a | 17.1 ± 2.6 abc | 14.0 ± 1.8 abc | 16.0 ± 2.4 abc | 15.5 ± 2.3 abc | 15.2 ± 0.6 b | 17.0 ± 1.5 bc | 18.8 ± 1.2 c | 19.3 ± 2.7 bc |
| 0 µL l−1 H2S | 0.1 µL l−1 H2S | 0.2 µL l−1 H2S | 0.3 µL l−1 H2S | |||||
|---|---|---|---|---|---|---|---|---|
| +S | −S | +S | −S | +S | −S | +S | −S | |
| Plant | ||||||||
| Biomass production | 1.86± 0.58 bc | 1.21 ± 0.23 a | 1.96 ± 0.32 c | 1.27 ± 0.26 a | 1.94± 0.34 c | 1.63 ± 0.39 b | 2.03 ± 0.37 c | 1.91 ± 0.33 c |
| Shoot/root ratio | 5.5 ± 0.8 c | 2.9 ± 0.7 a | 5.0 ± 0.8 c | 3.2 ± 0.6 ab | 5.4 ± 1.2 c | 3.8 ± 1.2 b | 5.3 ± 0.9 c | 3.8 ± 0.6 b |
| Shoot | ||||||||
| DMC | 8.2± 0.3 a | 10.1 ± 0.4 b | 8.3 ± 0.3 a | 9.9 ± 0.5 b | 8.4± 0.3 a | 9.7 ± 0.5 b | 8.4 ± 0.3 a | 8.6 ± 0.2 a |
| Chlorophyll | 0.68 ± 0.07 b | 0.44 ± 0.03 a | 0.72 ± 0.03 b | 0.59 ± 0.04 b | 0.59 ± 0.06 b | 0.59 ± 0.04 b | 0.60 ± 0.03 b | 0.65 ± 0.06 b |
| Sulfate | 14.3 ± 1.3 b | 2.0 ± 0.2 a | 14.0 ± 0.5 b | 2.3 ± 0.6 a | 13.6 ± 0.3 b | 2.9 ± 0.7 a | 12.7 ± 0.8 b | 3.0 ± 0.3 a |
| Nitrate | 56.4 ± 4.0 a | 106.0 ± 4.3 b | 55.1 ± 3.3 a | 84.3 ± 14.0 b | 62.6 ± 2.7 ab | 71.1 ± 2.9 a | 59.2 ± 7.8 a | 69.0 ± 15.5 a |
| Thiols | 0.36 ± 0.06 b | 0.13 ± 0.06 a | 0.44 ± 0.05 abc | 0.14 ± 0.03 a | 0.49 ± 0.01 c | 0.20 ± 0.04 a | 0.43 ± 0.01 bc | 0.24 ± 0.02 a |
| Free amino acids | 18.6 ± 0.7 a | 68.8 ± 3.3 e | 20.7 ± 1.8 a | 49.8 ± 6.56 d | 18.5 ± 0.2 a | 31.7 ± 3.4 bc | 23.5 ± 4.5 ab | 24.9 ± 2.5 ab |
| Root | ||||||||
| DMC | 6.9 ± 0.9 b | 5.7± 0.1 a | 6.3 ± 0.6 ab | 6.3 ± 0.3 ab | 6.2± 0.3 ab | 5.9 ± 0.4 a | 6.3 ± 0.5 ab | 5.9 ± 0.5 a |
| Sulfate | 16.8 ± 3.5 c | 1.1 ± 0.2 a | 14.1 ± 1.1 bc | 1.2 ± 0.4 a | 12.4 ± 1.3 b | 1.2 ± 0.1 a | 10.0 ± 1.4 b | 1.1 ± 0.4 a |
| Nitrate | 65.1 ± 7.2 a | 57.9 ± 8.8 abc | 57.5 ± 3.2 ab | 63.4 ± 7.5 ad | 49.3 ± 3.4 bc | 58.3 ± 2.8 ac | 44.5 ± 5.1 bc | 61.6 ± 2.7 ad |
| Thiols | 0.37 ± 0.05 b | 0.18 ± 0.05 a | 0.34 ± 0.04 b | 0.20 ± 0.02 a | 0.32 ± 0.01 b | 0.19 ± 0.00 a | 0.32 ± 0.00 b | 0.21 ± 0.02 a |
| Free amino acids | 21.2 ± 1.8 ab | 19.6 ± 2.0 ab | 17.8 ± 0.4 a | 25.4 ± 1.9 b | 16.7 ± 1.2 a | 19.5 ± 1.8 ab | 16.8 ± 1.3 a | 18.6 ± 4.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajapati, D.; Patani, A.; Patel, M.; Ali, D.; Alarifi, S.; Yadav, V.K.; Tank, J.; Patel, A. Effects of Sulfate on the Physiology, Biochemistry, and Activity of Group 1 Sulfate Transporters in Seedlings of Brassica pekinensis. Horticulturae 2023, 9, 821. https://doi.org/10.3390/horticulturae9070821
Prajapati D, Patani A, Patel M, Ali D, Alarifi S, Yadav VK, Tank J, Patel A. Effects of Sulfate on the Physiology, Biochemistry, and Activity of Group 1 Sulfate Transporters in Seedlings of Brassica pekinensis. Horticulturae. 2023; 9(7):821. https://doi.org/10.3390/horticulturae9070821
Chicago/Turabian StylePrajapati, Dharmendra, Anil Patani, Margi Patel, Daoud Ali, Saud Alarifi, Virendra Kumar Yadav, Jigna Tank, and Ashish Patel. 2023. "Effects of Sulfate on the Physiology, Biochemistry, and Activity of Group 1 Sulfate Transporters in Seedlings of Brassica pekinensis" Horticulturae 9, no. 7: 821. https://doi.org/10.3390/horticulturae9070821
APA StylePrajapati, D., Patani, A., Patel, M., Ali, D., Alarifi, S., Yadav, V. K., Tank, J., & Patel, A. (2023). Effects of Sulfate on the Physiology, Biochemistry, and Activity of Group 1 Sulfate Transporters in Seedlings of Brassica pekinensis. Horticulturae, 9(7), 821. https://doi.org/10.3390/horticulturae9070821






