Composts Obtained by Mixing Hop Leaves with Wheat Straw or Farmyard Manure Improved Soil Properties and Increased Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Trials
2.2. Soil Analysis
2.3. DNA Extraction, PCR Amplification, and Sequences Analysis
2.4. Data Analysis
3. Results
3.1. Soil Chemical and Microbiological Evaluation
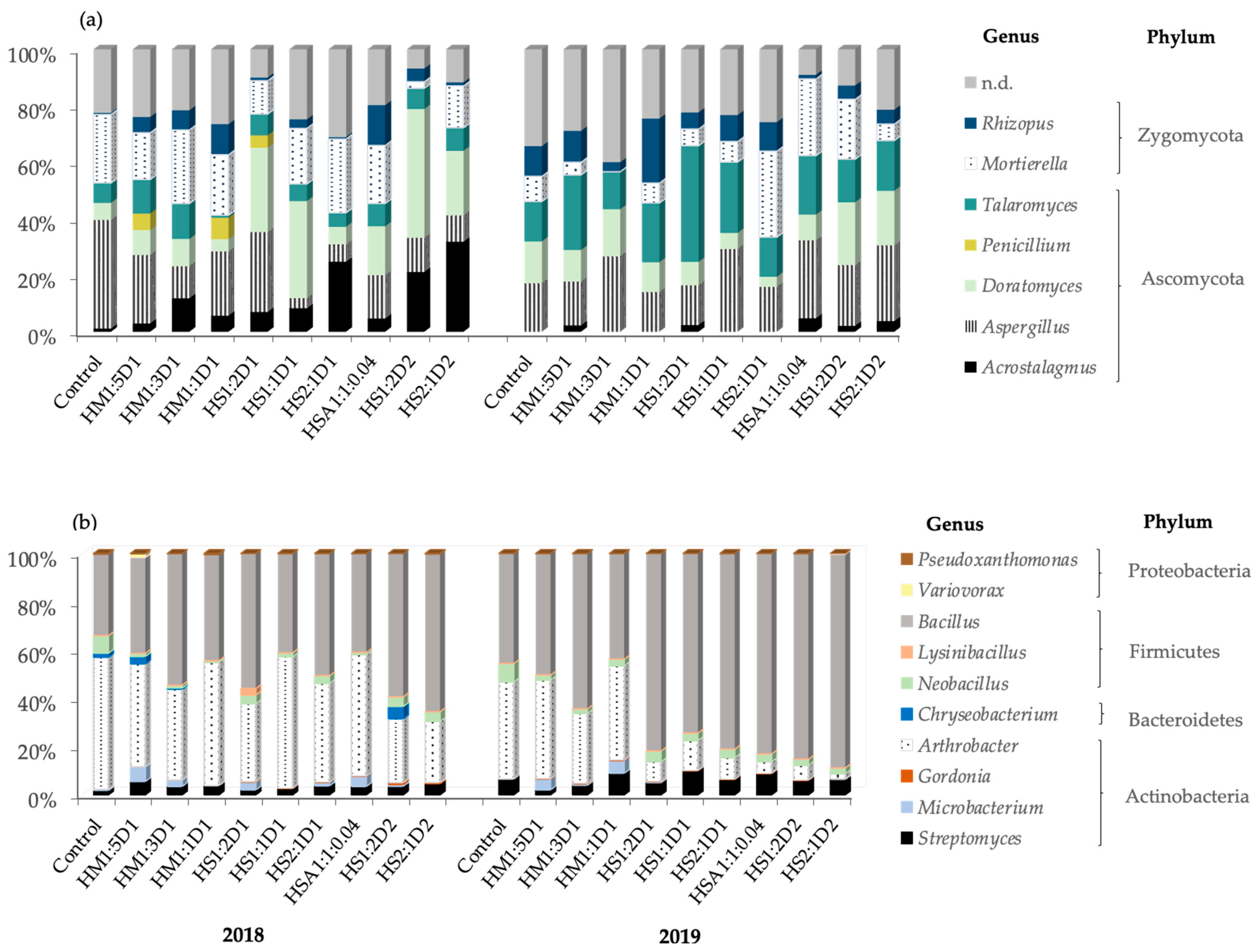
3.2. Microbial Community Composition and Species Identification
3.2.1. Effect of Compost Treatments on Soil Microbial Composition
3.2.2. Correlation Analysis between Microbial Population and Soil Properties
3.2.3. Microbial Species Identification
3.2.4. Correlation Analysis between Microbial Taxonomic Distribution and Soil Nitrogen and Organic Carbon Content
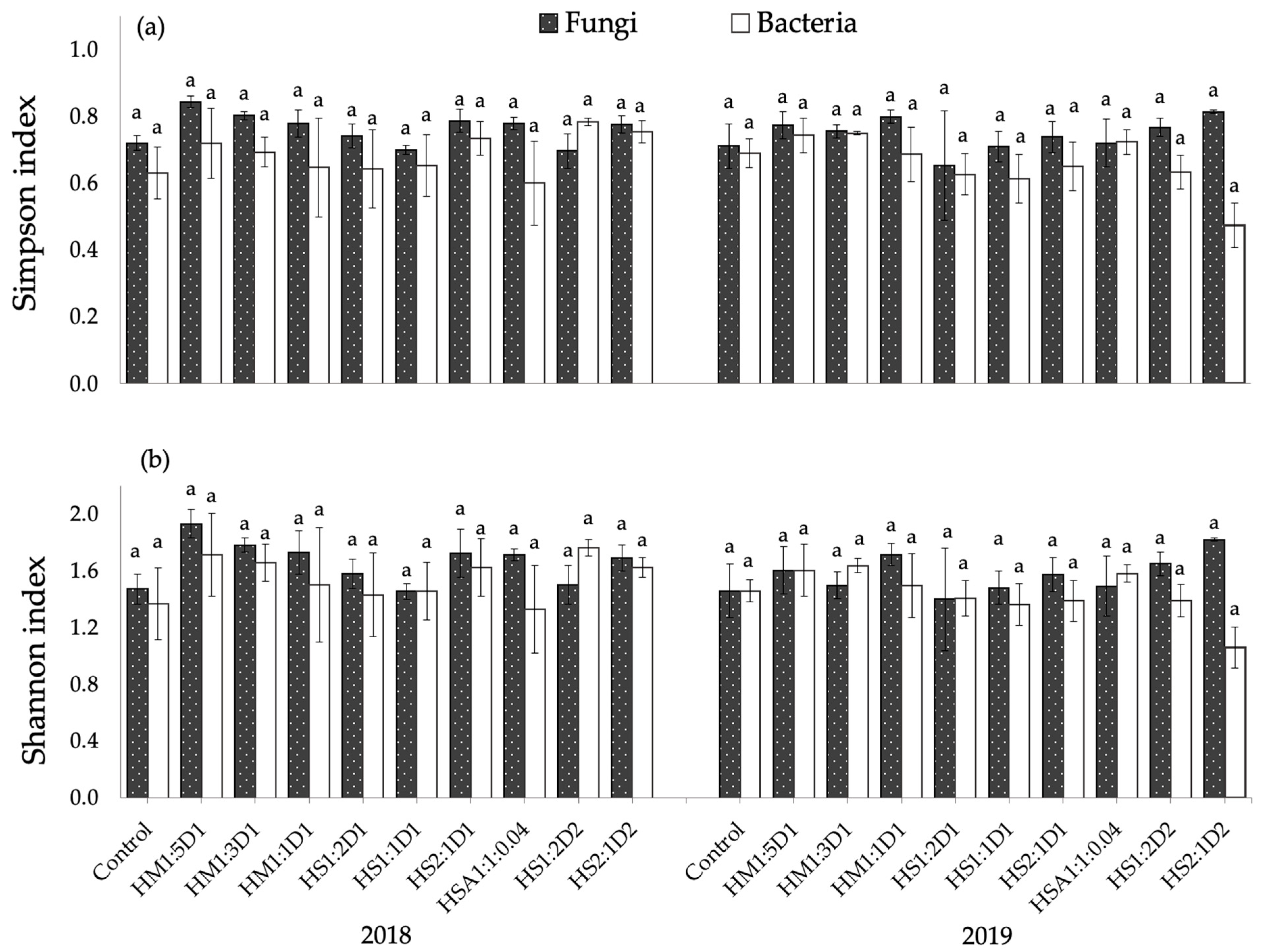
3.2.5. Diversity Indices of Soil Fungi and Bacteria Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Teghtmeyer, S. Hops. J. Agric. Food Inf. 2018, 19, 9–20. [Google Scholar] [CrossRef]
- Small, E. Hop (Humulus lupulus)—A bitter crop with sweet prospects. Biodiversity 2016, 17, 115–127. [Google Scholar] [CrossRef]
- FAOSAT. Production: Crops. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 20 August 2022).
- Rossini, F.; Loreti, P.; Provenzano, M.E.; De Santis, D.; Ruggeri, R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in Central Italy. Ital. J. Agron. 2016, 11, 180–187. [Google Scholar] [CrossRef]
- Mozzon, M.; Foligni, R.; Mannozzi, C. Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content. Foods 2020, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- da Rosa Almeida, A.; Maciel, M.V.d.O.B.; Cardoso Gasparini Gandolpho, B.; Machado, M.H.; Teixeira, G.L.; Bertoldi, F.C.; Noronha, C.M.; Vitali, L.; Block, J.M.; Barreto, P.L.M. Brazilian Grown Cascade Hop (Humulus lupulus L.): LC-ESI-MS-MS and GC-MS Analysis of Chemical Composition and Antioxidant Activity of Extracts and Essential Oils. J. Am. Soc. Brew. Chem. 2020, 79, 156–166. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Pereira, E.L.; Rodrigues, M.Â. Recycling nutrient-rich hop leaves by composting with wheat straw and farmyard manure in suitable mixtures. J. Environ. Manag. 2021, 284, 112105. [Google Scholar] [CrossRef] [PubMed]
- Kanai, N.; Nishimura, K.; Umetani, S.; Saito, Y.; Saito, H.; Oyama, T.; Kawamura, I. Upcycling of Waste Hop Stems into Cellulose Nanofibers: Isolation and Structural Characterization. ACS Agric. Sci. Technol. 2021, 1, 347–354. [Google Scholar] [CrossRef]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Jarosz, R. Biological activity of composts obtained from hop waste generated during the brewing. Biomass Convers. Biorefinery 2020, 12, 1271–1279. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef]
- Pergola, M.; Persiani, A.; Palese, A.M.; Di Meo, V.; Pastore, V.; D’Adamo, C.; Celano, G. Composting: The way for a sustainable agriculture. Appl. Soil Ecol. 2018, 123, 744–750. [Google Scholar] [CrossRef]
- Lal, R. Soils and sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 57–64. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Wang, X.; Selvam, A. Improving compost quality by controlling nitrogen loss during composting. In Current Developments in Biotechnology and Bioengineering; Wong, J.W.C., Tyagi, R.D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–82. [Google Scholar]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Zagal, E.; Muñoz, C.; Quiroz, M.; Córdova, C. Sensitivity of early indicators for evaluating quality changes in soil organic matter. Geoderma 2009, 151, 191–198. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Li, F. Soil enzyme activities and soil fertility dynamics. In Advances in Citrus Nutrition; Spring: Berlin/Heidelberg, Germany, 2012; pp. 143–156. [Google Scholar]
- Raviv, M. Production of high- quality composts for horticultural purposes: A mini-review. HortTechnology 2005, 15, 52–57. [Google Scholar] [CrossRef]
- Gent, D.H.; Barbour, J.D.; Dreves, A.J.; James, D.G.; Parker, R.; Walsh, D.B.; O’Neal, S. Field Guide for Integrated Pest Management in Hops; Oregon State University: Corvallis, OR, USA; University of Idaho: Moscow, ID, USA; USDA-ARS: Washington, DC, USA; Washington State University: Pullman, WA, USA, 2010. [Google Scholar]
- Knapp, B.A.; Ros, M.; Insam, H. Do Composts Affect the Soil Microbial Community? In Microbes at Work: From Wastes to Resources; Insam, H., Franke-Whittle, I., Goberna, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 271–291. [Google Scholar]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J. Differential Nutrient Limitation of Soil Microbial Biomass and Metabolic Quotients (qCO2): Is There a Biological Stoichiometry of Soil Microbes? PLoS ONE 2013, 8, e57127. [Google Scholar] [CrossRef]
- Osman, K.T. Soils: Principles, Properties and Management, 1st ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Maier, R.M. Biogeochemical Cycling. In Environmental Microbiology, 3rd ed.; Pepper, I.L., Gerba, C.P., Gentry, T.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 339–373. [Google Scholar]
- Ma, J.; Nergui, S.; Han, Z.; Huang, G.; Li, H.; Zhang, R.; Zhu, L.; Liao, J. The Variation of the Soil Bacterial and Fungal Community Is Linked to Land Use Types in Northeast China. Sustainability 2019, 11, 3286. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, L.; Kushwaha, S.K.; Ahrén, D.; Hedlund, K. Agricultural land use determines functional genetic diversity of soil microbial communities. Soil Biol. Biochem. 2017, 115, 423–432. [Google Scholar] [CrossRef]
- Navrátilová, D.; Tláskalová, P.; Kohout, P.; Dřevojan, P.; Fajmon, K.; Chytrý, M.; Baldrian, P. Diversity of fungi and bacteria in species-rich grasslands increases with plant diversity in shoots but not in roots and soil. FEMS Microbiol. Ecol. 2019, 95, fiy208. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Cozzolino, V.; Di Meo, V.; Monda, H.; Spaccini, R.; Piccolo, A. The molecular characteristics of compost affect plant growth, arbuscular mycorrhizal fungi, and soil microbial community composition. Biol. Fertil. Soils 2016, 52, 15–29. [Google Scholar] [CrossRef]
- Ding, G.-C.; Bai, M.; Han, H.; Li, H.; Ding, X.; Yang, H.; Xu, T.; Li, J. Microbial taxonomic, nitrogen cycling and phosphorus recycling community composition during long-term organic greenhouse farming. FEMS Microbiol. Ecol. 2019, 95, fiz042. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, L.; Horáková, E.; Fišera, M.; Jerzykiewicz, M.; Menšík, L. Effect of selected organic materials on soil humic acids chemical properties. Environ. Res. 2020, 187, 109663. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Soil and plant analyses to diagnose hop fields irregular growth. J. Soil Sci. Plant Nutr. 2020, 20, 1999–2013. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Witt, C.; Gaunt, J.L.; Galicia, C.C.; Ottow, J.C.G.; Neue, H.U. A rapid chloroform-fumigation extraction method for measuring soil microbial biomass carbon and nitrogen in flooded rice soils. Biol. Fertil. Soils 2000, 30, 510–519. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.; Kragt, J.; Powlson, D.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: The effects of fumigation time and temperature. Soil Biol. Biochem. 1985, 17, 831–835. [Google Scholar] [CrossRef]
- Pereira, E.; Santos, S.; Arrobas, M.; Patrício, M.D.S. Microbial biomass and N mineralization in mixed plantations of broadleaves and nitrogen-fixing species. For. Syst. 2011, 20, 516–524. [Google Scholar] [CrossRef]
- Martins, I.; Martins, F.; Belo, H.; Vaz, M.; Carvalho, M.; Cravador, A.; Choupina, A. Cloning, characterization and in vitro and in planta expression of a glucanase inhibitor protein (GIP) of Phytophthora cinnamomi. Mol. Biol. Rep. 2014, 41, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, F.; Zhou, X.; Fu, X.; Tao, Y.; Xu, W.; Pan, K.; Liu, S. Effects of Intercropping with Potato Onion on the Growth of Tomato and Rhizosphere Alkaline Phosphatase Genes Diversity. Front Plant Sci 2016, 7, 846. [Google Scholar] [CrossRef] [PubMed]
- Sune, D.; Rydberg, H.; Augustinsson, Å.N.; Serrander, L.; Jungeström, M.B. Optimization of 16S rRNA gene analysis for use in the diagnostic clinical microbiology service. J. Microbiol. Methods 2020, 170, 105854. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Anderson, T.-H.; Domsch, K.H. Soil microbial biomass: The eco-physiological approach. Soil Biol. Biochem. 2010, 42, 2039–2043. [Google Scholar] [CrossRef]
- Hu, J.L.; Lin, X.G.; Wang, J.H.; Dai, J.; Chen, R.R.; Zhang, J.B.; Wong, M.H. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soils Sediments 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Jannoura, R.; Joergensen, R.G.; Bruns, C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur. J. Agron. 2014, 52, 259–270. [Google Scholar] [CrossRef]
- Tu, C.; Ristaino, J.B.; Hu, S. Soil microbial biomass and activity in organic tomato farming systems: Effects of organic inputs and straw mulching. Soil Biol. Biochem. 2006, 38, 247–255. [Google Scholar] [CrossRef]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A.; Kumar, P.S. Short-term effects of nutrient management regimes on biochemical and microbial properties in soils under rainfed ginger (Zingiber officinale Rosc.). Geoderma 2012, 173–174, 192–198. [Google Scholar] [CrossRef]
- Prosser, J.I. Nitrogen in soils: Nitrification. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 31–39. [Google Scholar]
- Nemet, F.; Perić, K.; Lončarić, Z. Microbiological activities in the composting process—A review. J. Agric. Environ. Sci. 2021, 8, 41–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Dai, S.; Zhao, J.; Huang, X.; Sun, Y.; Chen, J.; Cai, Z.; Zhang, J. The effect of C:N ratio on heterotrophic nitrification in acidic soils. Soil Biol. Biochem. 2019, 137, 107562. [Google Scholar] [CrossRef]
- Isobe, K.; Koba, K.; Otsuka, S.; Senoo, K. Nitrification and nitrifying microbial communities in forest soils. J. For. Res. 2011, 16, 351–362. [Google Scholar] [CrossRef]
- Ai, Y.-J.; Li, F.-P.; Gu, H.-H.; Chi, X.-J.; Yuan, X.-T.; Han, D.-Y. Combined effects of green manure returning and addition of sewage sludge compost on plant growth and microorganism communities in gold tailings. Environ. Sci. Pollut. Res. 2020, 27, 31686–31698. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota Members Dominate Fungal Communities during Straw Residue Decomposition in Arable Soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.; De Clercq, D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, Y.-Y. Feasibility of Sewage Sludge Leached by Aspergillus Niger in Land Utilization. Pol. J. Environ. Stud. 2016, 25, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, S.K.; Chen, H.; Liu, T.; Zhang, Z.; Zhang, L.; Awasthi, M.K.; Taherzadeh, M.J. Evaluating the impact of bamboo biochar on the fungal community succession during chicken manure composting. Bioresour. Technol. 2019, 272, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yu, Y.; Zhu, Y.; Liu, T.; Miao, R.; Hu, R.; Peng, W.; Chen, J. Impacts of size reduction and alkaline-soaking pretreatments on microbial community and organic matter decomposition during wheat straw composting. Bioresour. Technol. 2022, 360, 127549. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Li, J.; Kumar, S.; Awasthi, S.K.; Wang, Q.; Chen, H.; Wang, M.; Ren, X.; Zhang, Z. Effects of biochar amendment on bacterial and fungal diversity for co-composting of gelatin industry sludge mixed with organic fraction of municipal solid waste. Bioresour. Technol. 2017, 246, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschuk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Sowani, H.; Kulkarni, M.; Zinjarde, S. An insight into the ecology, diversity and adaptations of Gordonia species. Crit. Rev. Microbiol. 2018, 44, 393–413. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, L.; Zhao, B.; Li, Y.; Chen, Y.; Deng, Y.; Wang, Y. Humification process enhancement through relative abundance promotion of Talaromyces and Coprinopsis by inoculated Phanerochaete chrysosporium during the secondary fermentation of composting. Environ. Sci. Pollut. Res. 2023, 30, 9060–9065. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Tayeh, N.A.; Ali, O.M.; Abdel Latef, A.A.H.; Loutfy, N. The Effect of endophytic Talaromyces pinophilus on growth, absorption and accumulation of heavy metals of Triticum aestivum grown on sandy soil amended by sewage sludge. Plants 2021, 10, 2659. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Ticona, A.R.P.; Hamann, P.R.V.; Quirino, B.F.; Noronha, E.F. Deconstruction of Lignin: From Enzymes to Microorganisms. Molecules 2021, 26, 2299. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hammel, K.E.; Hao, J.; Thompson, A.; Timokhin, V.I.; Hall, S.J. Enrichment of Lignin-Derived Carbon in Mineral-Associated Soil Organic Matter. Environ. Sci. Technol. 2019, 53, 7522–7531. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, T.; Müller, B.; Schnürer, A. The microbial community structure in industrial biogas plants influences the degradation rate of straw and cellulose in batch tests. Biotechnol. Biofuels 2016, 9, 128. [Google Scholar] [CrossRef]
- Hernández, T.; Chocano, C.; Moreno, J.-L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Trupiano, D.; Cocozza, C.; Baronti, S.; Amendola, C.; Vaccari, F.P.; Lustrato, G.; Di Lonardo, S.; Fantasma, F.; Tognetti, R.; Scippa, G.S. The effects of biochar and its combination with compost on lettuce (Lactuca sativa L.) growth, soil properties, and soil microbial activity and abundance. Int. J. Agron. 2017, 2017, 3158207. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Yang, H.; Archdeacon, D.; Tippett, O.; Tibi, M.; Whiteley, A.S. Humus-rich compost increases lettuce growth, nutrient uptake, mycorrhizal colonisation, and soil fertility. Pedosphere 2019, 29, 170–179. [Google Scholar] [CrossRef]
| Compost Mixtures | Ratio | C/N | Soil Application Rate | Abbreviation |
|---|---|---|---|---|
| Hop leaves + cow manure (HM) | 1:5 | 11.58 | 20 t ha−1 dry weight (D1) | HM1:5D1 |
| Hop leaves + cow manure (HM) | 1:3 | 12.03 | 20 t ha−1 dry weight (D1) | HM1:3D1 |
| Hop leaves + cow manure (HM) | 1:1 | 10.53 | 20 t ha−1 dry weight (D1) | HM1:1D1 |
| Hop leaves + wheat straw (HS) | 1:2 | 27.75 | 20 t ha−1 dry weight (D1) | HS1:2D1 |
| Hop leaves + wheat straw (HS) | 1:1 | 21.95 | 20 t ha−1 dry weight (D1) | HS1:1D1 |
| Hop leaves + wheat straw + hop stems ash (HSA) | 1:1:0.04 | 25.29 | 20 t ha−1 dry weight (D1) | HSA1:1:0.04D1 |
| Hop leaves + wheat straw (HS) | 1:0.5 | 15.79 | 20 t ha−1 dry weight (D1) | HS1:0.5D1 |
| Hop leaves + wheat straw (HS) | 1:2 | 27.75 | 40 t ha−1 dry weight (D2) | HS1:2D2 |
| Hop leaves + wheat straw (HS) | 1:0.5 | 15.79 | 40 t ha−1 dry weight (D2) | HS1:0.5D2 |
| Total N | TOC | Mic-N | Mic-C | SBR | qCO2 | Mic-C/TOC | APA | |
|---|---|---|---|---|---|---|---|---|
| Treatment | -------- g kg−1 ------- | ------------- mg kg−1 ------------- | mg CO2-C g–1 | mg g−1 | µg PNP g−1 | |||
| 2018 | ||||||||
| Control | 0.94 c | 22.0 e | 15.5 c | 55.1 b | 84.8 a | 92.3 a | 2.5 b | 195.6 bc |
| HM1:5D1 | 1.50 b | 21.9 e | 32.6 bc | 257.8 b | 127.6 a | 23.1 ab | 11.9 b | 193.6 bc |
| HM1:3D1 | 1.32 bc | 23.8 de | 36.7 abc | 98.6 b | 86.8 a | 39.5 ab | 4.1 b | 193.2 bc |
| HM1:1D1 | 1.77 b | 21.0 e | 27.4 bc | 262.5 b | 91.2 a | 14.5 b | 12.9 ab | 199.5 bc |
| HS1:2D1 | 1.30 bc | 26.6 cd | 43.9 abc | 321.7 b | 95.1 a | 22.2 ab | 12.1 ab | 188.4 c |
| HS1:1D1 | 1.47 b | 30.4 b | 40.1 abc | 776.2 a | 92.7 a | 5.1 b | 26.5 a | 198.6 bc |
| HS2:1D1 | 1.68 b | 30.7 b | 67.3 ab | 425.6 ab | 93.6 a | 12.7 b | 13.8 ab | 207.6 bc |
| HSA1:1:0.04D1 | 1.60 b | 29.7 bc | 51.8 abc | 210.5 b | 97.4 a | 21.0 ab | 7.2 b | 217.7 bc |
| HS1:2D2 | 1.56 b | 32.3 ab | 49.0 abc | 280.6 b | 107.5 a | 16.1 b | 8.7 b | 243.6 ab |
| HS2:1D2 | 2.34 a | 35.4 a | 74.8 a | 178.8 b | 108.0 a | 29.4 ab | 5.0 b | 276.1 a |
| Prob. > F | <0.0001 | <0.0001 | 0.0017 | 0.0004 | 0.2713 | 0.0309 | 0.0007 | 0.0125 |
| Standard error | 0.06 | 0.92 | 3.80 | 41.59 | 3.65 | 5.85 | 1.43 | 6.35 |
| 2019 | ||||||||
| Control | 1.31 f | 23.0 d | 24.4 d | 78.3 c | 56.8 b | 117.1 a | 3.4 bc | 173.8 b |
| HM1:5D1 | 1.52 de | 27.6 bc | 49.4 bcd | 113.4 c | 55.7 b | 72.3 a | 4.1 bc | 189.9 b |
| HM1:3D1 | 1.59 cde | 26.9 c | 27.3 cde | 72.4 c | 50.3 b | 114.0 a | 2.7 c | 183.1 b |
| HM1:1D1 | 1.55 e | 27.7 bc | 31.4 cde | 123.9 c | 54.3 b | 65.7 a | 4.5 bd | 195.6 ab |
| HS1:2D1 | 1.52 e | 28.2 bc | 45.4 bcde | 127.1 c | 59.9 b | 68.6 a | 4.5 bc | 199.6 ab |
| HS1:1D1 | 1.64 bcd | 28.3 bc | 37.8 bcde | 135.3 c | 51.2 b | 55.7 a | 4.8 bc | 192.4 b |
| HS2:1D1 | 1.69 bc | 28. bc | 54.3 abc | 120.3 c | 56.0 b | 67.6 a | 4.3 bc | 186.8 b |
| HSA1:1:0.04D1 | 1.48 e | 27.3 c | 44.4 bcde | 137.1 bc | 56.0 b | 66.4 a | 5.0 bc | 189.0 b |
| HS1:2D2 | 1.73 b | 30.1 ab | 60.0 ab | 262.0 a | 121.1 a | 67.3 a | 8.7 a | 244.8 a |
| HS2:1D2 | 2.08 a | 31.9 a | 73.3 a | 204.6 ab | 150.1 a | 106.6 a | 6.4 ab | 186.9 b |
| Prob. > F | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0534 | <0.0001 | 0.0084 |
| Standard error | 0.03 | 0.38 | 2.69 | 9.48 | 19.76 | 5.54 | 0.290 | 4.16 |
| Treatment | Fungi | Bacteria | Actinomycetes | Act./Bacteria | |
|---|---|---|---|---|---|
| ------------------------ [log (cfu g−1)] ------------------------ | (%) | ||||
| 2018 | Control | 4.52 a | 7.04 ab | 6.78 a | 56.06 a |
| HM1:5D1 | 5.01 a | 7.39 ab | 7.13 a | 56.20 a | |
| HM1:3D1 | 5.08 a | 7.00 b | 6.61 a | 43.08 a | |
| HM1:1D1 | 5.00 a | 7.23 ab | 6.92 a | 52.78 a | |
| HS1:2D1 | 4.93 a | 7.14 ab | 6.74 a | 45.80 a | |
| HS1:1D1 | 5.11 a | 7.12 ab | 6.85 a | 54.95 a | |
| HS2:1D1 | 5.03 a | 7.44 a | 7.11 a | 46.85 a | |
| HSA1:1:0.04D1 | 4.90 a | 7.25 ab | 7.02 a | 61.60 a | |
| HS1:2D2 | 5.11 a | 7.21 ab | 6.69 a | 30.45 a | |
| HS2:1D2 | 5.11 a | 7.23 ab | 6.72 a | 30.89 a | |
| Prob. > F | 0.0733 | 0.0353 | 0.0701 | 0.3741 | |
| Standard error | 0.25 | 0.18 | 0.24 | 17.57 | |
| 2019 | Control | 4.44 bcde | 6.63 c | 6.31 a | 48.89 a |
| HM1:5D1 | 4.24 de | 6.88 bc | 6.49 a | 42.39 ab | |
| HM1:3D1 | 4.05 e | 6.77 bc | 6.29 a | 33.77 ab | |
| HM1:1D1 | 4.32 cde | 6.86 bc | 6.47 a | 50.45 a | |
| HS1:2D1 | 4.52 abcde | 6.99 abc | 5.97 a | 13.86 ab | |
| HS1:1D1 | 4.83 abc | 7.06 abc | 6.39 a | 22.96 ab | |
| HS2:1D1 | 4.88 ab | 7.15 ab | 6.26 a | 14.48 ab | |
| HSA1:1:0.04D1 | 4.62 abcd | 7.05 abc | 6.18 a | 13.65 ab | |
| HS1:2D2 | 4.75 abcd | 7.14 ab | 6.24 a | 13.01 ab | |
| HS2:1D2 | 5.00 a | 7.32 a | 6.25 a | 8.96 b | |
| Prob. > F | >0.0001 | 0.0010 | 0.6545 | 0.0029 | |
| Standard error | 0.33 | 0.23 | 0.29 | 19.15 | |
| Total N | TOC | Mic-N | Mic-C | SBR | qCO2 | APA | |
|---|---|---|---|---|---|---|---|
| ---------- g kg−1 -------- | ------------- mg kg−1 ------------ | mg CO2-C g–1 | µg PNP g−1 | ||||
| 2018 | |||||||
| Fungi | 0.344 | 0.264 | 0.118 | 0.346 | 0.058 | −0.345 | 0.174 |
| Bacteria | 0.524 ** | 0.161 | 0.317 | 0.317 | 0.261 | −0.237 | 0.246 |
| Actinomycetes | 0.394 * | −0.090 | 0.225 | 0.303 | 0.183 | −0.207 | 0.106 |
| 2019 | |||||||
| Fungi | 0.494 ** | 0.509 ** | 0.463 * | 0.555 ** | 0.161 | −0.202 | 0.105 |
| Bacteria | 0.534 ** | 0.486 ** | 0.588 ** | 0.574 ** | 0.154 | −0.314 | 0.084 |
| Actinomycetes | 0.159 | −0.064 | −0.158 | −0.153 | −0.056 | 0.086 | −0.113 |
| Phylum | Genus | 2018 | 2019 | ||
|---|---|---|---|---|---|
| Total N | TOC | Total N | TOC | ||
| ---------- g kg−1 -------- | ---------- g kg−1 -------- | ||||
| Fungi | |||||
| Ascomycota | Acrostalagmus | 0.390 * | 0.544 ** | 0.091 | 0.263 |
| Ascomycota | Aspergillus | −0.146 | −0.361 | 0.420 * | 0.460 * |
| Ascomycota | Doratomyces | 0.235 | 0.547 ** | 0.226 | 0.197 |
| Ascomycota | Penicillium | −0.118 | −0.323 | n.d. | n.d. |
| Ascomycota | Talaromyces | 0.110 | 0.206 | 0.413 * | 0.432 * |
| Zygomycota | Mortierella | 0.167 | −0.067 | 0.195 | 0.222 |
| Zygomycota | Rhizopus | 0.126 | −0.175 | 0.249 | 0.184 |
| n.d. | 0.222 | −0.155 | 0.318 | 0.266 | |
| Bacteria | |||||
| Actinobacteria | Arthrobacter | 0.332 | −0.150 | −0.091 | −0.308 |
| Actinobacteria | Gordonia | 0.319 | 0.627 ** | 0.034 | 0.117 |
| Actinobacteria | Microbacterium | −0.147 | −0.085 | 0.093 | 0.185 |
| Actinobacteria | Streptomyces | 0.304 | 0.243 | 0.460 * | 0.478 ** |
| Bacteroidetes | Chryseobacterium | −0.138 | −0.224 | n.d. | n.d. |
| Firmicutes | Bacillus | 0.389 * | 0.308 | 0.541 ** | 0.524 ** |
| Firmicutes | Lysinibacillus | −0.236 | −0.032 | n.d. | n.d. |
| Firmicutes | Neobacillus | 0.136 | 0.339 | −0.071 | 0.017 |
| Proteobacteria | Pseudoxanthomonas | 0.112 | −0.262 | 0.082 | 0.064 |
| Proteobacteria | Variovorax | 0,118 | −0.182 | 0.291 | 0.290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, S.; Pereira, E.L.; Arrobas, M.; Rodrigues, M.Â.; Choupina, A. Composts Obtained by Mixing Hop Leaves with Wheat Straw or Farmyard Manure Improved Soil Properties and Increased Microbial Communities. Horticulturae 2023, 9, 1304. https://doi.org/10.3390/horticulturae9121304
Afonso S, Pereira EL, Arrobas M, Rodrigues MÂ, Choupina A. Composts Obtained by Mixing Hop Leaves with Wheat Straw or Farmyard Manure Improved Soil Properties and Increased Microbial Communities. Horticulturae. 2023; 9(12):1304. https://doi.org/10.3390/horticulturae9121304
Chicago/Turabian StyleAfonso, Sandra, Ermelinda L. Pereira, Margarida Arrobas, M. Ângelo Rodrigues, and Altino Choupina. 2023. "Composts Obtained by Mixing Hop Leaves with Wheat Straw or Farmyard Manure Improved Soil Properties and Increased Microbial Communities" Horticulturae 9, no. 12: 1304. https://doi.org/10.3390/horticulturae9121304
APA StyleAfonso, S., Pereira, E. L., Arrobas, M., Rodrigues, M. Â., & Choupina, A. (2023). Composts Obtained by Mixing Hop Leaves with Wheat Straw or Farmyard Manure Improved Soil Properties and Increased Microbial Communities. Horticulturae, 9(12), 1304. https://doi.org/10.3390/horticulturae9121304








