Abstract
Materials used to replace peat in growing media include wood fibre (WF), often used in combination with composted bark (BC), coir (CR), green compost (GC), and anaerobic digestate fibre (AD). The physical and chemical properties of these materials are relatively well characterised; however, biological properties are less well understood. Biological stability of growing media is an important factor in plant performance. The aim of this research was to identify whether dynamic respirometry methods are suitable for measuring growing media stability and to assess the effect of blending two raw materials in a mix. Raw materials were run for 42 days in aerated conditions at 35 °C. Except for AD, individually run samples were considered stable, with CO2 production over 7 days ranked BC < CR < WF < GC << AD in the early stages of the test. The AD was run at two moisture levels, with greater biological activity at lower moisture content. In the most active mixture, AD and WF, there was an increase of activity when nutrients were added at 28 days, indicating major elements were limiting microbial activity. There were interaction effects in sample mixtures, with the CO2 production of WF + GC, WF + CR greater than the sum of the CO2 production from the separate components.
1. Introduction
Over the past decade there has been a shift in the use of peat within the horticulture industry from a prominent component of growing media blends to being phased out in some European countries [1,2,3]. As a result, a variety of alternative raw materials have been used to replace peat within the industry. The main components of peat-free blends within UK horticulture include coir, wood fibre, bark, anaerobic digestate fibre, and green composts [4]. Wood fibre is increasingly being used as a component of growing media mixes due to its useful physical properties, including water-holding capacity and ability to reduce the hydrophobicity of other mix components [5,6]. Within the literature, a number of materials, often residual materials of other industries, have also been evaluated for their potential use, such as miscanthus and bracken [7,8]. The physical and chemical properties of the main alternative components are generally well understood by the industry and amendments can be made to ensure they are suitable for use within horticulture [3,9,10]. The biological properties of peat-free raw materials are less well understood, though increasing numbers of studies have characterised the microbial populations present within some growing media raw materials [11,12,13].
Biological stability of growing media can be considered the lack of microbial activity. Microbial activity in growing media has the potential to alter the carefully designed physical and chemical properties during storage or within the pot during cultivation, potentially resulting in sub-optimal plant growth performance. This can include changing the physical structure, key to moisture retention and aeration, and chemical properties such as nutrient status, mineralisation, pH, and maintaining ion exchange capacity [14]. Furthermore, this can change conditions for microbial growth over time, resulting in complex interactions.
In use, growing media are expected to be well aerated, with aerobic microbial respiration dominating. Anaerobic activity may be present in micro-sites such as in wet aggregates, but this is not considered here. Aerobic microbial activity may be limited by poor aeration (low oxygen levels), lack of available moisture, lack of key nutrients, or chemical factors including low pH. These could be considered artificial limitations that can cause “false stability” [15], rather than intrinsic to the substrate. Providing optimum, non-limiting conditions should allow determination of substrate quality, especially availability of carbon as the substrate breaks down, in defined conditions. In respirometry, either CO2 production or O2 consumption is used to track respiration.
Common methods for determining the stability or microbial activity of growing media include oxygen uptake rate (OUR) tests, either in vessels with tops that record the pressure changes [16] or as pre- and post-incubation comparison measurements [17]. These tests are useful to give an indication of microbial activity within a strict set of conditions. There are a few limitations with this style of test; for example, the sample size is small (typically 2 g for pressure vessels), which relies on the homogeneity of the test material. Aspray et al. [15] demonstrated that OUR tests can go out of range when the test material is highly active, which could be the case with anaerobic digestate or green compost. The test conditions are not close to the environmental conditions within which a growing medium would be stored or used to grow plants, though various authors have found reasonable correlation between OUR and respirometric tests for compost materials [18,19].
Tests measuring evolved carbon dioxide (CO2) are less commonly used to assess the stability of growing media. Montagne et al. [11] used a modified carbon (C) mineralisation method to calculate the amount of C released as CO2 from coir, peat, and wood fibre samples over 3 months. Vandecasteele [20] used a CO2 respirometry test with daily CO2 measurements, though this was a more similar to a static test, which could lead to O2 becoming limiting.
Established dynamic respiration tests used in the UK composting industry are the four-day dynamic respiration test DR4, designed to monitor composting of waste [21], and ORG0020 [22], used to specify compost quality under the PAS100 scheme [23]. This type of test could be useful for assessing the stability of growing media in conditions similar to those used in glasshouse plant production. These tests are both solid-phase dynamic respiration tests, though the DR4 was designed for more active materials, including compost feedstocks, whereas the ORG is targeted at distinguishing between more stable composted products. Key differences between these tests are shown in Table 1.

Table 1.
Comparison of DR4 and ORG0020 respirometric tests.
These tests are designed to provide robust comparisons between samples, though they use different conditions and are not directly comparable. Neither is specifically optimised for very low-activity (high-stability) growing media components. The recommended aeration rate for DR4 is higher, expected to provide sufficient aeration for relatively high-activity/low-stability samples. The DR4 is considered a “truly dynamic” test [15] as it forces air through the sample mass, while ORG0020 passes air through the chamber headspace only, relying on diffusion to supply oxygen throughout the sample. Aspray et al. [15] found good correlation between these tests for ten composted materials, while static chamber tests, OUR, and self-heating were considered less reliable for these materials.
Moisture is expected to be a key variable, with the optimum value unknown and possibly different between sample types. A fixed gravimetric moisture content as used in the DR4 test takes no account of water-holding properties and is therefore not appropriate to the diverse materials tested here. A “hand squeeze test” approach as used in ORG0020 provides a moisture level closer to conditions used for plant growth. Other moisture conditions have been used, e.g., 75% of water-holding capacity [24], though this has been reported to be less reliable than the hand-squeeze method [22]. Temperature is also expected to be an important variable. Nutrients are added in the DR4 test with the intention that major nutrients are not limiting. This is omitted in ORG0020, apparently assuming composted materials will contain sufficient nutrients. Growing media include components with very high C:N ratio, which may require higher levels of nutrient addition, and may lack trace elements.
A further key difference is the use of an inoculum to supply a microbial population in the DR4 test. In all but recently sterilised media, there will be a microbial population present, adapted to the prevailing conditions and substrate [25]. An inoculum can provide a diverse microbial community, as well as a stable physical structure and chemical buffering, providing a more reliable test [15]. ORG0020 relies on the existing microbial population within the sample, though the initial 3-day equilibration period provides time for the existing microbial population to adapt to test conditions.
ORG0020 does not measure CO2 production during an initial 3-day equilibration period, so that measurements cover the period from start of day 4 to end of day 7. This is explained as an initial flush of activity following disturbance that may not be related to longer-term stability [22]. It seems possible that this initial peak activity may be important, relating for instance to conditions in freshly planted growing media. Using automated data collection over the full period allows a fuller dataset and comparison of different time periods.
Previous studies have either focused on the stability of individual raw materials or of three- or four-component blends [11,20,26], without identifying the interaction effects when just two raw materials are mixed. There is the potential for one raw material with a high or diverse microbial population to act as an inoculum for another raw material with a lower microbial population. Wood fibre, for example, has been described as a stable material with a lower microbial diversity than coir [12], but has the potential to act as a readily available source of carbon when mixed with another raw material with a more diverse microbial population. A DR4-style test could be a useful tool to draw out these interactions between raw materials in a blend of growing media.
The aim of this research was to not only identify whether the dynamic respirometry tests described above (i.e., DR4 and ORG0020) are suitable methods for measuring growing media stability, but also assess the effect of blending two raw materials in a mix. As many of the samples of interest were expected to be very stable, tests were extended to observe longer-term effects that may be relevant to growing media in use.
2. Materials and Methods
2.1. Physico-Chemical Characteristics
Five types of growing media raw materials commonly used in peat-free growing media blends in the UK were tested. These were anaerobic digestate fibre (AD), bark (BC), coir (CR), wood fibre (WF), and green compost (GC). Two samples of WF were obtained from different production batches at the same source; WF2 was the primary sample reported and used in test mixtures. The WF used in the study was produced by steaming, pressure treating, and expanding wood chips. Two samples of AD were obtained, with AD1 reported in detail and used in tests on moisture and nutrients.
Initial characterisation of dry matter (DM), moisture content, and laboratory-compacted bulk density were determined according to BS EN 13040:2007 [27]. Loss on ignition was determined according to BS EN 13039:2012 [28], as a measure of organic matter. The total carbon and nitrogen content of the raw materials was analysed via an Elementar Vario EL Cube elemental analyser (Elementar Analysensysteme GmbH, Langenselbold, Germany). The main physico-chemical characteristics are summarised in Table 2.

Table 2.
Characteristics of test materials used in the study. Means and (standard deviations) of three replicates except where indicated.
2.2. Stability Testing
Test conditions followed the DR4 test [21], extended to 42 days. Each material was run individually, using 100 g dry weight per chamber, without inoculum, as in ORG0020 [22]. In addition, 100 g dry weight of each material was mixed with 100 g dry weight WF per chamber. A full DR4 was conducted on the WF only. In short, 100 g dry matter (DM) of WF was mixed with 100 g DM of GC inoculum. This was tested alongside a blank (100 g DM of GC) and a reference material (100 g DM of GC + 10 g α-cellulose from Sigma-Aldrich, UK).
All samples were incubated at 35 °C throughout the test. All treatments were run in triplicate. Moisture content for each sample was standardised as per the “hand squeeze test” at the start of the experimental run as per ORG0020 [22]. The “hand squeeze test” was performed by the same operative throughout to reduce the potential variability that has been noted with this measure [18]. Nutrients were included in the added water following the DR4 method [21], as NH4Cl and KH2PO4, to provide 0.28 g N, 0.15 g P, and 0.19 g K in each chamber.
To evaluate the effect of moisture on the test, the AD fibre was tested in the ORG style outlined above on an “as received” basis with no additional moisture, other than the initial 10 mL of nutrient solution. The effect of nutrients on this test were determined by running samples of AD and WF with no nutrients added to the test mixture at the start of experiment. These treatments then had the addition of the nutrient solution at 28 days along with all other treatments.
The respirometer setup is shown in Figure 1. The test samples were placed into 4 L respirometer chambers and were incubated at 35 °C, with forced air flows maintained in the range of 250–300 mL/min for 42 days. Inlet air was passed through a condenser at 4 °C to standardise moisture content. Outlet air was also passed through condensers at 4 °C, and further dried before measurement of composition of the dry gas. Inlet flow rates and the CO2 and O2 content of inlet and exhaust gases were recorded at 2 h intervals using on-line analysers (FB8 mass flow meters, MUX3 multiplexers, CAXL CO2 analyser, FCX oxygen analyser, data collection via UI2 interface and ExpeData v.1.8.5 software; Sable Systems International, Las Vegas, NV, USA). Analysers were calibrated using 1% and 2% gas standards in nitrogen for CO2 (Calgaz Ltd., Stoke, UK), ambient outside air for O2, and pure nitrogen as a zero point.
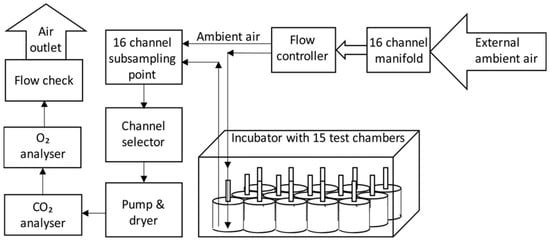
Figure 1.
Schematic diagram of respirometer.
The chambers were weighed and shaken every 7 days to redistribute moisture and nutrients. Further nutrient stock solution, as specified above, was added at 28 days to all of the test chambers during the shaking procedure.
2.3. Data Analysis
Initial data processing was carried out in ExpeData® v.1.8.5, using inbuilt macros. Baseline corrections were applied using ambient outside air at three points in each 2 h run to compensate for drift in the analyser readings. Lags between flow data and CO2 and O2 concentrations were corrected, and the most stable flow, CO2, and O2 signals selected for each channel. Data were exported and further data processing was conducted using an R statistical environment (v.4.2.2). The outlet flow was not measured, and gas composition will have changed in the chamber, changing the mass flow. CO2 production was corrected using O2 composition of the outlet gases using gas-exchange equations [29]. The flow rate was more than adequate to maintain aerobic conditions, with outlet O2 not falling below 18 % at peak O2 demand.
The graphs presented below have been simplified for clarity, with error bars only marked for one point in each two days. Lines have been used rather than individual points, unless otherwise stated.
3. Results
3.1. Microbial Activity of Single Materials
When run as single materials, four out of five substrate types could be considered biologically stable (Table 3; Figure 2). The UK compost quality specification PAS100 [23] defines a threshold for stable compost using ORG0020 as 16 g/kg volatile solids(VS)/day, or 64 g/kgVS over the 4 days of ORG0020 [23]. Llewelyn [22] suggested a single threshold equivalent to 40 g/kgVS/4 days, or in more detail, respiration rate was considered very low below 32 g/kgVS/4 days, low up to 48 g/kgVS/4 days, medium up to 64 g/kgVS/4 days, high up to 80 g/kgVS/4 days, and very high above 80 g/kgVS/4 days. These are based on stability of mature green compost and are broadly related to compost age. The GC sample used here passes the PAS100 stability threshold, though is slightly above Llewelyn’s [22] suggested single threshold (Table 3).

Table 3.
Cumulative values of CO2 production at 4 days (equivalent to DR4), days 3–7 (equivalent to ORG0020), 7 and 28 days, 28-day O2 consumption, and percentage C loss, means and (standard deviations) for three replicates. Samples are in rank order for DR4 results.
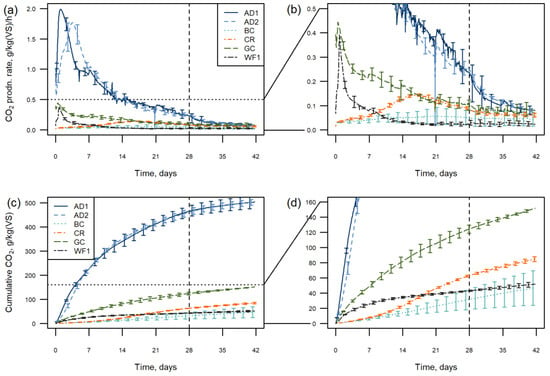
Figure 2.
CO2 production of the single raw materials’ (a) gas production rate; note the area below the horizontal dotted line is expanded in (b) for visibility of the samples with lower CO2 production; (c) cumulative gas production; note the area below the horizontal dotted line is expanded in (d) for visibility of the samples with lower CO2 production. Graphs show means, error bars show ±1 standard deviation and every 24th point is plotted. The vertical dashed line indicates nutrient addition at 28 days.
Most materials showed a distinct initial peak in the first few days of the test, followed by steadily declining CO2 production. This forms a cumulative curve tending to an asymptotic value or linear increase (Figure 2c,d). In contrast, the composted bark did not show any initial peak and had low, though measurable, activity throughout the 42 days. The coir showed an increase in CO2 production after a 7-day lag period, peaking at about 15 days.
The AD was the least stable substrate, with a large initial peak over the first two days, then declining. A comparison of the two AD batches showed a difference in the stability over the first four days (Figure 2a,c) and differing ORG0020 values (99 and 135 g/kgVS/4 days); however, by seven days the cumulative CO2 production was the same. There was an initial lag in the activity of AD2, with peak CO2 production at 3 days, compared with AD1 at 1 day. A similar comparison of the WF batches showed that the biological stability of the two samples over time was almost identical, with all results being within one standard deviation of each other (Table 3).
The WF and GC samples showed a similar initial peak; however, the amount of CO2 produced was much lower in the WF over the first four days (19 and 32 g/kgVS CO2 respectively). The peak in the WF was very sharp and CO2 production then rapidly decreased, whist the GC had more sustained activity over the duration of the whole test.
The BC was the most stable of all the substrate types, with very low CO2 production for the whole time series. There was variability in this data, however, which was attributed to one replicate losing moisture during the test, resulting in higher CO2 production. This suggests the “hand squeeze” test moisture was not optimal for this sample. As a result, CO2 production may have been underestimated by the samples remaining at “hand squeeze” moisture, and stability overestimated in this current test.
The nutrient addition at day 28 appears to have had no obvious effect on the stability of any of the single raw materials, with no pulse in CO2 production seen.
The percentage of carbon loss can be found in Table 3. This shows 30 to 32% carbon loss in the most active samples (AD), with next largest loss from GC at under 8%. Other single materials lost under 5% of carbon in the 42-day test, with WF and BC samples losing least, reflecting the higher stability of these samples over the full period of the test.
3.2. Moisture Effects on Test
The “as received” sample had a dry matter content of 57% compared with 28% when the sample moisture was adjusted to the “hand squeeze test” (Table 1). The moisture content of the AD substrate influenced the CO2 production in the test (Figure 3). The chambers with added moisture had a larger initial peak than those without, with the CO2 production becoming comparable only at the 28-day point. The cumulative CO2 production at 28 days was less than half the “hand squeeze” moisture sample when no moisture was added to the material (216 g/kgVS CO2 “as received”; 495 g/kgVS CO2 adjusted moisture).
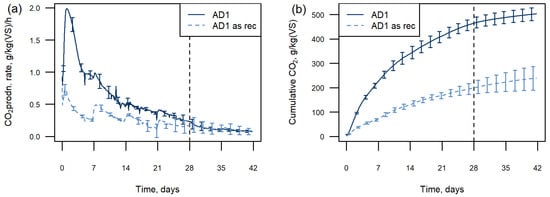
Figure 3.
CO2 production from one AD sample with added moisture and as received: (a) gas production rate, (b) cumulative data. Graphs show means, error bars show ±1 standard deviation and every 12th point is plotted. The vertical dashed line indicates nutrient addition at 28 days.
Pulses in CO2 production were noted in the “as received” sample following each disturbance during the weekly shaking events to redistribute moisture within the chambers (Figure 3a). These pulses can also be seen in the AD sample with adjusted moisture; however, the effect is less pronounced.
3.3. Nutrient Effect on Test
The effects of nutrients on the test were investigated in the AD and WF samples. For both materials, there was a reduction in the CO2 production from the samples when no nutrients were added at the start of the test (Figure 4). The same trend in CO2 production was seen both with and without nutrients, with an initial peak and then decline, but for both substrate types the production was lower in the samples without nutrient addition. The CO2 production at 4 days was 109 g/kgVS CO2 for AD without nutrients compared with 156 g/kgVS CO2 with nutrient addition. For the WF, this was 7.4 g/kgVS CO2 and 18.7 g/kgVS CO2 respectively.
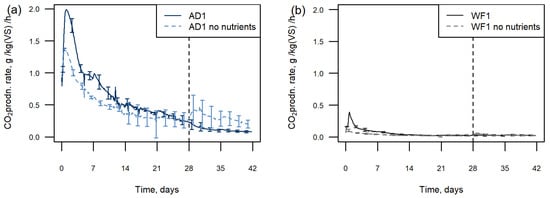
Figure 4.
CO2 production rate from an AD sample (a) and WF sample (b) with and without nutrients added at the start of the test. Graphs show means, error bars show ±1 standard deviation. The vertical dashed line indicates nutrient addition at 28 days.
When nutrients were added at 28 days, there was an initial increase in CO2 production in both substrates, which was sustained in the AD sample. This increase was not seen in the AD fibre that had nutrients from the start (Figure 4a). There was also no nutrient effect seen in any of the single materials when nutrients were added at 28 days (Figure 2). The increase of CO2 production in the WF was short-lived and quickly decreased back to the level seen in the WF with nutrients.
A similar nutrient effect can be seen in the AD + WF mix (Figure 5a), with an increase in CO2 production rate once nutrients were added on day 28. The CO2 production increased up to the end of the test at 42 days, resulting in the curve no longer tending to an asymptotic value.
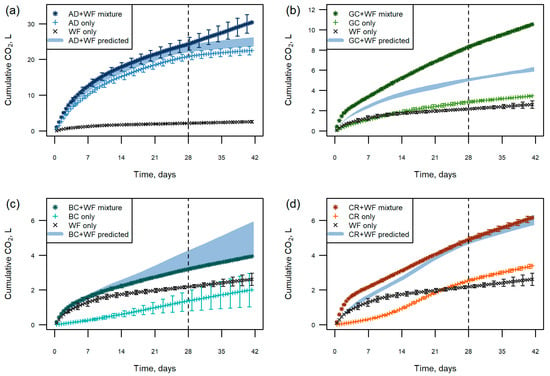
Figure 5.
Raw cumulative CO2 production in litres from 100 g dry matter of the single raw materials and mixtures: (a) AD + WF; (b) GC + WF; (c) BC + WF; (d) CR + WF. The shaded area indicates the range of CO2 production of individual materials summed together. Note Y-axis scale differs between graphs. Graphs show every sixth mean value, and error bars show ±1 standard deviation and every 24th point is plotted. The vertical dashed vertical line indicates nutrient addition at 28 days.
3.4. Interaction Effects
Figure 5 shows the raw CO2 production in litres from mixtures of 100 g dry matter AD, GC, BC, or CR with 100 g dry matter WF, with the results for individual components (100 g dry matter) shown for comparison. In absence of interaction effects, the mixtures may be expected to produce the sum of CO2 of the two individual components. The sum of CO2 production from the individual components provides a predicted value for the mixture as a range from the sum of minimum replicate values to the sum of maximum replicate values for each component (represented by the shaded area on each graph).
The CO2 production of the AD + WF mix was very similar to the prediction based on the single materials, up until the nutrients were added at day 28 (Figure 5a). From this point, there was an increase in the CO2 production in the mixture, with CO2 production of 6.2 L between days 28 and end of test, 3.5 L more than the median predicted value based on CO2 production from the single materials. This was the only mix where the addition of nutrients had an identifiable effect.
The mix containing GC + WF had the most noticeable interaction effect of all of the mixes (Figure 5b). The CO2 production was much higher in the physical mix and was underpredicted by the simple addition of the individual components.
Initially, there was close agreement between the predicted and observed CO2 production curves for the BC + WF mix; however, from 14 days there appears to have been a slight suppression of CO2 production. There is some uncertainty with this as there was variability between the BC replicates in the test. As noted above, the CO2 production of the BC sample may have been underestimated for the wetter replicates, or the stability overestimated. The observed CO2 production of the mixture was at the lower boundary of the predicted range.
The CR + WF mix had higher CO2 production than the sum of the individual materials initially, but by day 21 of the test the predicted gas production matched the actual production.
4. Discussion
4.1. Respirometry
The biological stability of a material is a function of the material and the environmental conditions to which it is exposed. The aim of this study was to create conditions close to real-world use of growing media and assess the biological stability of commonly used materials, using aspects of existing respirometry techniques to standardise those conditions as far as possible. By removing limiting factors, such as oxygen supply, moisture, and nutrients, the CO2 production measured in the study gives a test comparable to the intended use of materials in horticulture, but in standardised, idealised, and replicable conditions. The study was based on conditions of the DR4 test [21], adapted to incorporate single materials without inoculum as used in ORG0020 [22]. Parameters were chosen to be as robust as possible. The test is considered a good proxy for a measure of microbial activity within growing media, limited only by microbial population and substrate quality.
Aeration was not limiting in the experimental set up, with a continuous flow of external ambient air being forced through the test materials and oxygen in the outlet never falling below 17.5% at peak oxygen demand. The flow rate and flow configuration have been found to be important factors within respirometry testing [30,31,32]. Guillen Ferrari et al. [33] found that optimising the aeration within the ORG0020 test improved precision within the setup. An airflow configuration that was purely within the headspace of a chamber, as used in ORG0020, may work reliably especially with more stable materials, and may be considered more realistically representative of the exchange of air over a tray or pot within a glasshouse.
The nutrient supply at the beginning of the test was sufficient not to limit microbial activity across all of the individual test materials and their mixes, except for the most active mix of anaerobic digestate fibre (AD) and wood fibre (WF). Nutrient addition at 28 days did not create a pulse in CO2 production in the individual AD and WF samples that were given nutrients at the beginning; however, pulses in CO2 were seen in the AD and WF samples that were not supplied with nutrients at the start. It is therefore likely that the AD + WF mix had become limited for nutrients at some point during the first 28 days of the test, resulting in an overestimation of the stability of that test mix. This may mean reduced availability of nutrients for plant growth, in competition with microbial activity (N immobilisation) [34].
Moisture was another limiting factor standardised during the test using the “hand squeeze” method. This was chosen as an acceptable level of moisture at which a plant might grow well, simulating the wetting up of a pot media when a plant is first planted. The adjusted moisture for the green compost sample (GC) was within the 40–60% moisture content range within the ORG0020 protocol [22]. The other growing media raw materials required more water to be added to reach the same physical point of water release due to squeezing, resulting in over 70% moisture content for the other four materials. Gurusamy et al. [35] found that moisture had a significant impact on stability in some compost materials during an ORG0020 test. This was seen in the moisture experiment, where the drier “as received” AD sample had higher stability compared with that of the same sample with moisture adjusted to the “hand squeeze” level. It should be noted that the “as received” AD had a moisture content of 43%, which is within the acceptable range for ORG0020 [22]. This was not optimal for this particular material and suggests that the moisture range in the ORG0020 test might not be optimal for materials with different physical properties to green compost. The “hand squeeze” test was not necessarily optimal for all of the materials tested either. The variability within the individual bark sample was identified as being related to one sample that dried out during the test and had elevated CO2 production as a result. This suggests that the moisture within this sample may have been limiting, causing a falsely stable result.
Further investigation is required to determine the optimum water content for this kind of test. Other approaches have been used. The DR4 test specifies 50% gravimetric water content [21], which is likely to be reasonable for compost but is arbitrary. The “hand squeeze” test used by Llewelyn [22] may be considered subjective, and an alternative of 75% of water-holding capacity was tested by Adani [24]. It is likely that matric potential is a key factor [36], making any arbitrary gravimetric or volumetric moisture content questionable. This could also shed light on the likely distribution of moisture between components of a mixture, and availability of water to both microbes and plant roots. This complication can be avoided by using a water-based test such as the OUR, but at the expense of conditions for microbial growth closer to the intended real-world application. OUR tests may also be restricted to short-duration tests by supply of oxygen. This limitation has been addressed in the SOUR test [37] by periodic aeration in aqueous medium. It remains a valid question what microbial communities are supported in each of these environments.
4.2. Stability of Individual Raw Materials
The raw materials tested were a range of the most common peat-free growing media components in UK [4]. All the materials tested, except the AD, were very stable when tests were run individually. Under PAS100, compost is considered sufficiently stable if ORG0020 results are under 16 mg CO2/g VS/d (PAS 100:2011). The wood fibre, coir, and composted bark were all well below this value. Only the testing of the wood fibre was run as a full DR4 [21] including the standard green compost inoculum. This method has no published threshold, though from data in [15], materials under 25 mg CO2/g VS/d may be considered stable. The reference cellulose result demonstrated the test was valid, and the result with GC contribution subtracted can be considered very stable.
Two batches of AD fibre were received and tests were run separately. These were both above the PAS100 threshold, though they differed in activity during the early stages of the test, indicating some initial variability as well as instability. After 7 days in the test conditions, there was no difference between the two samples, suggesting some initial inhibitory effect in one sample, resulting in a short lag in CO2 production. The two batches of WF tested were from the same source and only slightly different in terms of stability. Various authors have noted that the microbial population in composts or wood fibre, for example, is dependent on the production method and source of material [11,38]. For example, Montagne et al. [11] suggests that the physical structure of wood fibre is more important than geographic origin or wood type for determining the microbial population. In this study, the wood fibre was produced by steaming, pressure treating, and expanding wood chips, which could create substrate suitable for a specific microbial community and therefore different levels of stability compared with other methods of production. Testing of batches of raw materials produced by different methods would be necessary to determine the overall variability of substrate types.
An increase in CO2 production was seen in the coir after about 15 days, a similar effect is noted in Montagne et al. [11]. There, coir pith had a lower initial CO2 production rate compared with other materials, such as coir fibre and wood fibre, then after 20 days the rate increased to the same as the coir fibre. The test temperature in the experiment by Montagne et al. [11] was lower than in this study (28 and 35 °C respectively), which may explain the difference in lag time. Lag periods have also been identified as correlating with biochemical composition in coir pith, as opposed to fibre [11]; this could help explain the lag identified here.
The rank order of stability of the single materials tested altered over the testing time period, with the results at 7 days different to those at 28 and 42 days. The biggest changes were that the coir became more active after day 15. At the end of the 42-day test, the order of stability was bark > wood fibre > coir > green compost >> anaerobic digestate fibre. Although not all of the same raw materials were tested, Vandecasteele [20] found a similar pattern in the ranking using respirometric CO2 production in various growing media materials, with wood fibre and composted bark more stable and green compost ranking as one of the least stable materials. The O2 consumption in the OUR test in that study produced a different ranking of stability, with changes in rankings of some of the more active substrate categories.
4.3. Interaction Effects of Mixing Raw Materials on Stability
The wood fibre was chosen as the common material in mixes because it potentially has a low existing microbial population [12] and high carbon content, which could be utilised by the inoculating microbial population from the other materials. As an increasingly large proportion of growing media mixes in the UK [4] include WF, any interactions in terms of biological stability with other raw materials is important to note.
The interaction experiments showed a range of responses to the mixing of individual components. Simply summing the activity of individual materials does not accurately predict the observed responses. Initially, there appears to be an effect of mixing the coir and wood fibre together. The lag that is seen in the coir on its own is no longer present when used as part of a mix. The wood fibre potentially has different forms of carbon, which may be more available than the more recalcitrant forms in the coir [11]. After the initial lag period passed at about 21 days, the predicted and observed CO2 production matched for the rest of the test. This suggests that the overall stability of the mix was the same when compared to the component parts, but that the initial microbial activity was greater.
A large increase in the microbial activity was noted in the GC + WF mix compared with the predicted values, i.e., an overall decrease in the biological stability of the mix. A deviation from predicted microbial activity within any mix could be as a result of physical, chemical, or biological parameters, or potentially a combination of all three. For example, green compost is known to have a diverse microbial population and as such is used as an inoculum in a number of biodegradability tests, such as the DR4 test [21]. Wood fibre has been noted as having a potentially available carbon source [20] and a hydrophilic nature that enhances the moisture distribution in a mix [6], so when added to a diverse microbial population like in the GC, there is the potential for increased microbial activity compared with the raw materials alone.
The interaction effect seen in the AD and WF mix only became apparent when additional nutrients were added part way through the test. As noted above, this suggests that nutrients (most likely N) were limiting during the test. Nitrogen immobilisation (or lock up) is a common effect seen particularly in wood-based materials and can affect plant growth and quality [34].
There is a suggestion of potential suppression of microbial activity in the BC and WF mix, though this is somewhat uncertain due to the variability in the bark test samples. As noted above, this may be due to an effect of sub-optimal moisture conditions within the sample during the test. The observed microbial activity was at the bottom of the range of predicted values for these materials. It is likely that a lower moisture content would be optimal for the bark, and as a result, the microbial activity seen in this test is an overestimation of stability. If this were the case, then the predicted value would be shifted up and a real suppression effect would have been seen. Where the microbial activity is enhanced due to mixing of materials has potential implications for the use of materials as growing media. Blends are carefully constructed by growing media manufacturers to have specific chemical and physical properties when they are produced. The data presented here indicate that there is the potential for large losses of carbon over a 6-week period in optimised conditions, particularly if there is a component with low biological stability in the mix. This carbon loss has the potential to affect the structure of the growing media, particularly if fine fibres are degraded [14]. A low-stability material may not only degrade more rapidly, changing the proportions of the mixture, but also provide a means of degrading more recalcitrant materials through “priming effect” [39].
Microbial activity within growing media raw materials should not necessarily be seen as a negative issue, as there is a wealth of literature showing that microbial genera and species that are known to suppress plant pathogens are present in composted bark, wood fibre, and green composts [12,13,38].
5. Conclusions
Dynamic respirometry is a suitable tool for evaluation of existing and new growing media raw materials and their interactions in mixes. Using a respirometry technique adapted from standard methods, differences in microbial stability between different growing media were successfully identified. Furthermore, by mimicking real-world yet replicable conditions, this technique can produce realistic cultures with potential for additional microbiological or other characterisation. The specific methods DR4 and ORG0020 are not well optimised to this application. Further work is recommended to refine operational parameters in the adapted method, such as moisture status, for a standardised test.
The separate components tested ranked from most to least stable (lowest to highest CO2 production) were BC < CR < WF < GC << AD after 7 days. This order changed through the test as CO2 production from CR peaked in the third week.
Interactions between components were identified in simple two-component mixes. This may be due physical or chemical factors, or cross-inoculation of microbial populations native to each component. Green compost is expected to contain a wide microbial diversity.
Further work is needed to assess variability within and between sample types, and interactions present in horticulture-relevant mixes.
Author Contributions
Conceptualization, S.N., P.A., N.B. and G.H.; Formal analysis, G.H.; Investigation, S.N. and G.H.; Methodology, S.N. and G.H.; Project administration, G.H.; Supervision, S.N.; Validation, G.H.; Visualization, S.N. and G.H.; Writing—original draft, S.N. and G.H.; Writing—review and editing, S.N., P.A., N.B. and G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Bulrush Horticulture Ltd.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors wish to thank the OU technical team, especially Caroline Gurd and Angus McEwen, for technical assistance. SN and GH would like thank NB and PA for their contributions of horticultural expertise.
Conflicts of Interest
This study was designed and carried out by Open University staff. It was exploratory with no prescribed outcome. The funders had no role in the analysis or interpretation of data. The manuscript was written by Open University staff, with expertise on horticultural context provided by NB and PA. SN and GH are grateful for the funders’ permission to publish.
References
- Schmilewski, G. Growing Medium Constituents Used in the EU. Acta Hortic. 2009, 819, 33–46. [Google Scholar] [CrossRef]
- Bragg, N.; Brough, W. The Development of Responsibly Sourced Growing Media Components and Mixes©. Acta Hortic. 2014, 1055, 141–144. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- HTA Growing Media Monitor Report. Trend in the Composition of UK Growing Media Supplied 2011 to 2022. Available online: https://projectbluearchive.blob.core.windows.net/media/Default/Research%20Papers/Horticulture/CP%20203_Final%20report%202011-2022.pdf (accessed on 12 September 2023).
- Gruda, N.; Schnitzler, W.H. Suitability of Wood Fiber Substrate for Production of Vegetable Transplants: I. Physical Properties of Wood Fiber Substrates. Sci. Hortic. 2004, 100, 309–322. [Google Scholar] [CrossRef]
- Durand, S.; Jackson, B.E.; Fonteno, W.C.; Michel, J.-C. The Use of Wood Fiber for Reducing Risks of Hydrophobicity in Peat-Based Substrates. Agronomy 2021, 11, 907. [Google Scholar] [CrossRef]
- Pitman, R.M.; Webber, J. The Character of Composted Bracken (Pteridium Aquilinum L. Kuhn) and Its Potential as a Peat Replacement Medium. Eur. J. Hortic. Sci. 2013, 78, 145–152. [Google Scholar]
- Nguyen, V.T.H.; Kraska, T.; Winkler, W.; Aydinlik, S.; Jackson, B.E.; Pude, R. Primary Mechanical Modification to Improve Performance of Miscanthus as Stand-Alone Growing Substrates. Agronomy 2022, 12, 420. [Google Scholar] [CrossRef]
- Lemaire, F. Physical, Chemical and Biological Properties of Growing Medium. Acta Hortic. 1995, 396, 273–284. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Seiler, J.R. Changes in Chemical and Physical Properties of Pine Tree Substrate and Pine Bark During Long-Term Nursery Crop Production. HortScience 2009, 44, 791–799. [Google Scholar] [CrossRef]
- Montagne, V.; Charpentier, S.; Cannavo, P.; Capiaux, H.; Grosbellet, C.; Lebeau, T. Structure and Activity of Spontaneous Fungal Communities in Organic Substrates Used for Soilless Crops. Sci. Hortic. 2015, 192, 148–157. [Google Scholar] [CrossRef]
- Montagne, V.; Capiaux, H.; Barret, M.; Cannavo, P.; Charpentier, S.; Grosbellet, C.; Lebeau, T. Bacterial and Fungal Communities Vary with the Type of Organic Substrate: Implications for Biocontrol of Soilless Crops. Environ. Chem. Lett. 2017, 15, 537–545. [Google Scholar] [CrossRef]
- Pot, S.; Tender, C.D.; Ommeslag, S.; Delcour, I.; Ceusters, J.; Vandecasteele, B.; Debode, J.; Vancampenhout, K. Elucidating the Microbiome of the Sustainable Peat Replacers Composts and Nature Management Residues. Front. Microbiol. 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Verhagen, H. Stability of Growing Media from a Physical, Chemical and Biological Perspective. Acta Hortic. 2009, 819, 135–142. [Google Scholar] [CrossRef]
- Aspray, T.J.; Dimambro, M.E.; Wallace, P.; Howell, G.; Frederickson, J. Static, Dynamic and Inoculum Augmented Respiration Based Test Assessment for Determining in-Vessel Compost Stability. Waste Manag. 2015, 42, 3–9. [Google Scholar] [CrossRef]
- Grigatti, M.; Cavani, L.; Ciavatta, C. The Evaluation of Stability during the Composting of Different Starting Materials: Comparison of Chemical and Biological Parameters. Chemosphere 2011, 83, 41–48. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; Van Winkel, A. Oxygen Use in Compost Storage as Influenced by Moisture, Temperature and Degradability. Acta Hortic. 2019, 1266, 291–300. [Google Scholar] [CrossRef]
- Adani, F.; Gigliotti, G.; Valentini, F.; Laraia, R. Respiration Index Determination: A Comparative Study of Different Methods. Compost Sci. Util. 2003, 11, 144–151. [Google Scholar] [CrossRef]
- Oviedo-Ocaña, E.R.; Torres-Lozada, P.; Marmolejo-Rebellon, L.F.; Hoyos, L.V.; Gonzales, S.; Barrena, R.; Komilis, D.; Sanchez, A. Stability and Maturity of Biowaste Composts Derived by Small Municipalities: Correlation among Physical, Chemical and Biological Indices. Waste Manag. 2015, 44, 63–71. [Google Scholar] [CrossRef]
- Vandecasteele, B. Oxygen Uptake Rate versus CO2 Based Respiration Rate for Assessment of the Biological Stability of Peat, Plant Fibers and Woody Materials with High C:N Ratio versus Composts. Waste Manag. 2023, 167, 74–80. [Google Scholar] [CrossRef]
- Turrell, J.; Godley, A.; Agbasiere, N.; Lewin, K. Guidance on Monitoring of MBT and Other Treatment Processes for the Landfill Allowances Schemes (LATS and LAS) for England and Wales; Environment Agency: Bristol, UK, 2009.
- Llewelyn, R.H. Development of Standard Laboratory Based Test to Measure Compost Stability. The Waste & Resources Action Programme: The Old Academy. 2005. Available online: https://wrap.org.uk/sites/default/files/2020-10/WRAP-DevLabTestCompostStability.pdf (accessed on 12 September 2023).
- B.S.I. PAS100: 2018 Specification for Composted Materials; BSI Committee: London, UK, 2018. [Google Scholar]
- Adani, F.; Lozzi, P.; Genevini, P. Determination of Biological Stability by Oxygen Uptake on Municipal Solid Waste and Derived Products. Compost Sci. Util. 2001, 9, 163–178. [Google Scholar] [CrossRef]
- Grunert, O.; Reheul, D.; Van Labeke, M.-C.; Perneel, M.; Hernandez-Sanabria, E.; Vlaeminck, S.E.; Boon, N. Growing Media Constituents Determine the Microbial Nitrogen Conversions in Organic Growing Media for Horticulture. Microb. Biotechnol. 2016, 9, 389–399. [Google Scholar] [CrossRef]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.-C.; Reheul, D.; Boon, N. Mineral and Organic Growing Media Have Distinct Community Structure, Stability and Functionality in Soilless Culture Systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef]
- BS EN 13040; Soil Improvers and Growing Media. Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density. CEN: Brussels, Belgium, 2007.
- BS EN 13039; Soil Improvers and Growing Media—Determination of Organic Matter Content and Ash. CEN: Brussels, Belgium, 2012.
- Lighton, J.R.B.; Halsey, L.G. Flow-through Respirometry Applied to Chamber Systems: Pros and Cons, Hints and Tips. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 265–275. [Google Scholar] [CrossRef]
- Adani, F.; Ubbiali, C.; Generini, P. The Determination of Biological Stability of Composts Using the Dynamic Respiration Index: The Results of Experience after Two Years. Waste Manag. 2006, 26, 41–48. [Google Scholar] [CrossRef]
- Komilis, D.; Kanellos, D. A Modified Dynamic Respiration Test to Assess Compost Stability: Effect of Sample Size and Air Flowrate. Bioresour. Technol. 2012, 117, 300–309. [Google Scholar] [CrossRef]
- Almeira, N.; Komilis, D.; Barrena, R.; Gea, T.; Sánchez, A. The Importance of Aeration Mode and Flowrate in the Determination of the Biological Activity and Stability of Organic Wastes by Respiration Indices. Bioresour. Technol. 2015, 196, 256–262. [Google Scholar] [CrossRef]
- Guillen Ferrari, D.; Howell, G.; Aspray, T.J. Improved Precision and Efficiency of a Modified ORG0020 Dynamic Respiration Test Setup for Compost Stability Assessment. Sustainability 2017, 9, 2358. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Pot, S.; Maenhout, K.; Delcour, I.; Vancampenhout, K.; Debode, J. Acidification of Composts versus Woody Management Residues: Optimizing Biological and Chemical Characteristics for a Better Fit in Growing Media. J. Environ. Manag. 2021, 277, 111444. [Google Scholar] [CrossRef]
- Gurusamy, N.N.; Puffer, N.; de Jongh, C.; Rodriguez Gil, C.; Aspray, T.J. Effect of Initial Moisture Content and Sample Storage Duration on Compost Stability Using the ORG0020 Dynamic Respiration Test. Waste Manag. 2021, 125, 215–219. [Google Scholar] [CrossRef]
- Cannavo, P.; Recous, S.; Valé, M.; Bresch, S.; Paillat, L.; Benbrahim, M.; Guénon, R. Organic Fertilization of Growing Media: Response of N Mineralization to Temperature and Moisture. Horticulturae 2022, 8, 152. [Google Scholar] [CrossRef]
- Lasaridi, K.; Stentiford, E. A Simple Respirometric Technique for Assessing Compost Stability. Water Resour. 1998, 32, 3717–3723. [Google Scholar] [CrossRef]
- Hoitink, H.A.J. Status of Compost-Amended Potting Mixes Naturally Suppressive to Soilborne Diseases of Floricultural Crops. Plant Dis. 1991, 75, 869. [Google Scholar] [CrossRef]
- Bréchet, L.M.; Lopez-Sangil, L.; George, C.; Birkett, A.J.; Baxendale, C.; Castro Trujillo, B.; Sayer, E.J. Distinct Responses of Soil Respiration to Experimental Litter Manipulation in Temperate Woodland and Tropical Forest. Ecol. Evol. 2018, 8, 3787–3796. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).