Autochthonous Rose Hybrid Rosa pendulina × spinosissima Overshines Main Genotype Rosa pendulina in the Biochemical Characteristics of Their Hips
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Analysis of Ascorbic Acid
2.3. Extraction and Analysis of Organic Acids
2.4. Extraction and Analysis of Phenolic Compounds
2.5. Statistical Analysis
3. Results
3.1. Asorbic Acid
3.2. Organic Acids
3.3. Phenolic Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Chemical | Supplier | Purity | Calibration Curve Equation (mg/mL) | R2 |
|---|---|---|---|---|
| organic acids | ||||
| citric acid | Sigma-Aldrich | ≥99.5 | 35.196 | 0.9998 |
| malic acid | Sigma-Aldrich | ≥97% | 23.124 | 0.9982 |
| quinic acid | Supelco | ≥98% | 11.868 | 0.9933 |
| shikimic acid | BioChemika | ≥97% | 1666.3 | 0.9994 |
| fumaric acid | Sigma-Aldrich | ≥99% | 3860.3 | 0.9967 |
| ascorbic acid | Sigma-Aldrich | ≥99% | 1964.50 | 0.9974 |
| phenolic compounds | ||||
| cyanidin-3-glucoside | Sigma-Aldrich | ≥95% | 384.06 | 0.9993 |
| kaempferol-3-glucoside | Sigma-Aldrich | ≥98% | 970.75 | 1.00 |
| isorhamnetin-3-glucoside | Extrasynthese | ≥98% | 529.29 | 1.00 |
| quercetin-3-rutinoside | Sigma-Aldrich | ≥95% | 122.28 | 0.98 |
| quercetin-3-galactoside | Sigma-Aldrich | ≥97% | 291.06 | 1.00 |
| quercetin-3-xyloside | Apin Chemicals | ≥98% | 972.86 | 1.00 |
| quercetin-3-rhamnoside | Sigma-Aldrich | ≥95% | 645.63 | 0.97 |
| quercetin-3-arabinofuranoside | Apin Chemicals | ≥98% | 695.8 | 0.9991 |
| quercetin-3-glucoside | Sigma-Aldrich | ≥97% | 349.36 | 0.9998 |
| quercetin-3-arabinopyranoside | Apin Chemicals | ≥98% | 356.97 | 0.996 |
| caffeic acid | Sigma-Aldrich | ≥98% | 1656.8 | 1.00 |
| apigenin-7-glucoside | Sigma-Aldrich | ≥97% | 549.68 | 0.9979 |
| 3-caffeoylquinic acid | Sigma-Aldrich | ≥98% | 1656.8 | 1.00 |
| 4-caffeoylquinic acid | Sigma-Aldrich | ≥98% | 1656.8 | 1.00 |
| chlorogenic acid | Sigma-Aldrich | ≥95% | 466.48 | 1.00 |
| epicatechin | Sigma-Aldrich | ≥97% | 191.97 | 0.9963 |
| catechin | Supelco | ≥99% | 116.26 | 0.9985 |
| p-coumaric acid | Sigma-Aldrich | ≥98% | 1742.52 | 1.00 |
| procyanidin B1 | Supelco | ≥90% | 74.34 | 1.00 |
| ellagic acid | Sigma-Aldrich | ≥95% | 150.06 | 0.99 |
References
- Bavcon, J.; Ravnjak, B.; Vreš, B. Raznolikost Šipkov (Rosa L.) v Sloveniji; Botanični Vrt Univerze v Ljubljani, Biotehniška Fakulteta: Ljubljana, Slovenia, 2018; pp. 1–223. [Google Scholar]
- Koczka, N.; Bányai, E.S.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 73, 10. [Google Scholar]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Aptin, R.; Ghvamaldin, A.; Ahmad, T.; Mariamalsadat, T. Evaluation of biochemical compounds Rosa canina L. in North of Iran (Ramsar and Tonekabon Heights). Acad. J. 2013, 45, 3319–3324. [Google Scholar] [CrossRef]
- Darlington, C.D. Vitamin C and Chromosome Number in Rosa. Nature 1942, 150, 404. [Google Scholar] [CrossRef]
- Uggla, M. Domestication of Wild Roses for Fruit Production. Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2004. [Google Scholar]
- Babis, A.; Kucharska, A.Z. Przydatnosć owocow Rosa spinosissima i Rosa hybrida do produkcji wysokowitaminowych sokow metnych. Biul. Wydz. Farm. Akad. Med. Warszawie 2004, 3, 18–24. [Google Scholar]
- Oprica, L.; Busca, C.; Zamfirache, M.M. Ascorbic Acid Content of Rose Hip Fruit Depending on Altitude. Iran. J. Public Health 2015, 44, 138–139. [Google Scholar]
- Türkben, C.; Uylaşer, V.; İncedayı, B.; Çelikkol, I. Effects of different maturity periods and processes on nutritional components of rose hip (Rosa canina L.). J. Food Agric. Environ. 2010, 8, 26–30. [Google Scholar]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activites of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2013, 57, 126–133. [Google Scholar] [CrossRef]
- Murathan, Z.T.; Zarifkhosroshahmi, M.; Kafkas, E.; Sevindik, E. Characterization of bioactive compounds in rosehip species from east Anatolia region of Turkey. Ital. J. Food Sci. 2016, 28, 314–325. [Google Scholar] [CrossRef]
- Adamczak, A.; Buchwald, W.; Zielinski, J.; Mielcarek, S. Flavonoid and organic acid content in rose hips (Rosa L., SECT. Caninae DC. EM. Christ.). Acta Biol. Crac. Ser. Bot. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Boyd, P.D.A. Past, Present and potential Value of Rosa spinosissima in the Rose Industry. In Proceedings of the 14th WFRS Regional Convention, International Heritage Rose Conference, Copenhagen, Denmark, 18–23 May 2016. [Google Scholar]
- Mikulic-Petkovsek, M.; Slatnar, A.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Chemical profile of black currant fruit modified by different degree of infection with black currant leaf spot. Sci. Hortic. 2013, 150, 399–409. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Rusjan, D. The impact of fruit processing on phenolic content in products made from juneberry (Amelanchier lamarckii) fruits. J. Food Sci. 2020, 85, 386–393. [Google Scholar] [CrossRef]
- Medveckiene, B.; Kulaitiene, J.; Jariene, E.; Vaitkevičiene, N.; Hallman, E. Carotenoids, polyphenols, and ascorbic acid in organic rosehips (Rosa spp.) cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Bayramoğlu, M.; Ekin, S.; Kızıltaş, H.; Oto, G.; Susen, A.E. Antioxidant properties of Rosa pisiformis and its protective effect against isoproterenol-induced oxidative stress in rats. Turk. J. Biochem. 2016, 41, 232–242. [Google Scholar] [CrossRef]
- Javanmard, M.; Asadi-Gharneh, H.A.; Nikneshan, P. Characterization of biochemical traits of dog rose (Rosa canina L.) ecotypes in the central part of Iran. Nat. Prod. Res. 2017, 32, 1738–1743. [Google Scholar] [CrossRef]
- Nojavan, S.; Khaliliana, F.; Kiaiec, F.M.; Rahimic, A.; Arabanianc, A.; Chalavi, S. Extraction and quantitative determination of ascorbic acid during different maturity stages of Rosa canina L. fruit. J. Food Compos. Anal. 2008, 21, 300–305. [Google Scholar] [CrossRef]
- Nybom, H.; Werlemark, G. Realizing the Potential of Health-Promoting Rosehips from Dogroses (Rosa sect. Caninae). Curr. Bioact. Compd. 2017, 13, 3–17. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, A.S.; et al. Polyphenolic, Composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea Extracts and Assessment of Their Antioxidant Activity in Human Endothelial Cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Cunja, V.; Mikulič Petkovšek, M.; Zupan, A.; Štampar, F.; Schmitzer, V. Frost decreases content of sugars, ascorbic acid and some quercetin glycosides but stimulates selected carotenes in Rosa canina hips. J. Plant Physiol. 2015, 178, 55–63. [Google Scholar] [CrossRef]
- Okatan, V.; Çolak, A.M.; Güçlü, S.F.; Korkmaz, N.; Sękara, A. Local genotypes of dog rose from Interior Aegean region of Turkey as a unique source of pro-health compounds. Plant Prot. 2019, 78, 397–408. [Google Scholar] [CrossRef]
- Bozan, B.; Aripov, K.N.; Kozar, M. Comparison of ascorbic and citric acid contents in Rosa canina L. fruit growing in the Central Asian region. Chem. Nat. Compd. 1998, 34, 687–689. [Google Scholar] [CrossRef]
- Gustafsson, A. The constitution of the Rosa canina complex. Hereditas 1944, 30, 405–428. [Google Scholar] [CrossRef]
- Forkmann, G. Flavonoids as flower pigments: The formation of the natural spectrum and its extension by genetic engineering. Plant Breed. 1991, 106, 1–26. [Google Scholar] [CrossRef]

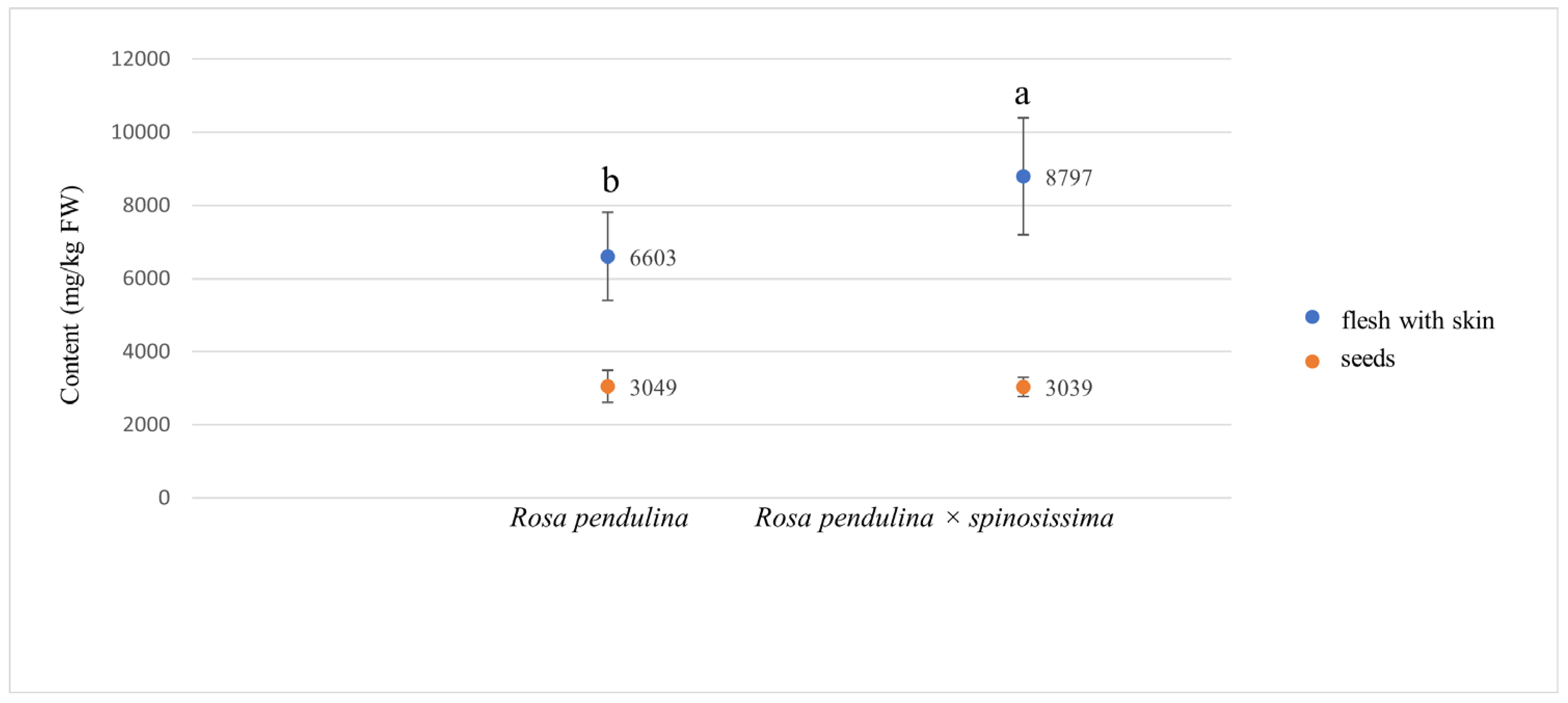

| Compound | Rosa pendulina | Rosa pendulina × spinosissima |
|---|---|---|
| citric acid | 1278.7 ± 316.8 b | 13,611.4 ± 3829.5 a |
| malic acid | 1329.2 ± 541.7 | - |
| quinic acid | 296.6 ± 93.5 b | 48,769.3 ± 28,824.0 a |
| shikimic acid | 8.1 ± 0.6 b | 85.1 ± 4.6 a |
| fumaric acid | 2.6 ± 0.5 b | 365.9 ± 1.8 a |
| TOTAL | 2915.2 ± 953.1 b | 62,831.7 ± 32,659.9 a |
| Phenolic Group | Compound | Rosa pendulina Flesh and Skin | Seeds | Rosa pendulina × spinosissima Flesh and Skin | Seeds |
|---|---|---|---|---|---|
| hydroxybenzoic acid derivatives (HBA) | gallic acid | 32.5 ± 2.7 b | 4.5 ± 0.5 b | 90.8 ± 6.4 a | 51.5 ± 5.7 a |
| galloyl quinic acid | 1055.5 ± 419.4 a | 972.0 ± 207.1 a | 242.0 ± 17.0 b | 137.0 ± 15.4 b | |
| ellagic acid pentoside 1 | 53.0 ± 2.1 a | 2.1 ± 0.2 a | 1.9 ± 0.4 b | 0.4 ± 0 b | |
| ellagic acid pentoside 2 | 8.0 ± 1.6 b | 3.6 ± 0.2 a | 1.8 ± 0.1 a | 0.8 ± 0.1 b | |
| TOTAL | 1151.0 ± 406.7 a | 980.7 ± 210.9 a | 336.5 ± 23.9 b | 189.7 ± 21.2 b | |
| hydroxycinnamic acid derivatives (HCA) | p-coumaric acid hexoside | 0.3 ± 0 b | 0.0 ± 0.0 b | 20.0 ± 2.5 a | 6.3 ± 2.1 a |
| 5-caffeoylquinic acid 1 | 5.5 ± 0.7 b | 0.84 ± 0.0 b | 48.7 ± 2.8 a | 17.5 ± 1.7 a | |
| synapic acid hexoside 1 | 6.7 ± 0.4 b | 0.3 ± 0.03 b | 68.4 ± 3.7 a | 21.1 ± 3.7 a | |
| 5-caffeoylquinic acid 2 | 120.7 ± 85.8 b | 61.1 ± 3.0 b | 768.5 ± 58.0 a | 162.0 ± 21.0 a | |
| 5-p-coumaroylquinic acid 1 | 8.8 ± 1.6 b | 2.7 ± 0.2 a | 30.0 ± 0.9 a | 3.0 ± 0.2 a | |
| p-coumaric acid hexoside 2 | 1.1 ± 0.0 b | 0.0 ± 0.0 b | 2.0 ± 0.2 a | 0.6 ± 0.0 a | |
| 5-p-coumaroylquinic acid 2 | 2.3 ± 0.0 b | 0.1 ± 0.0 b | 15.0 ± 0.3 a | 2.0 ± 0.2 a | |
| synapic acid hexoside | / | / | 24.0 ± 1.8 | 5.0 ± 0.7 | |
| 3-p-coumaroylquinic acid | 0.8 ± 0.0 b | 0.2 ± 0.0 b | 14.0 ± 0.3 a | 5.0 ± 0.2 a | |
| TOTAL | 146.2 ± 88.5 b | 65.2 ± 3.2 b | 990.6 ± 70.5 a | 222.5 ± 29.8 a | |
| gallotannins | methyl gallate rutinoside | 21.0 ± 0.6 b | 3.7 ± 0.9 a | 49.4 ± 4.0 a | 2.9 ± 0.8 b |
| digalloyl hexoside 1 | 28.6 ± 1.9 b | 4.9 ± 1.1 b | 70.9 ± 8.6 a | 19.0 ± 1.2 a | |
| methyl gallate hexoside | 73.4 ± 0.2 b | 22.4 ± 5.7 b | 139.6 ± 8.4 a | 58.6 ± 4.4 a | |
| digalloyl hexoside 2 | 0.7 ± 0.0 b | 0.2 ± 0.0 b | 19.9 ± 1.9 a | 9.6 ± 4.4 a | |
| digalloyl quinic acid | 40.7 ± 4.3 b | 9.3 ± 5.7 b | 90.3 ± 5.7 a | 24.9 ± 7.2 a | |
| digalloylpentoside | 4.7 ± 0.3 b | 1.1 ± 0.0 b | 17.0 ± 0.5 a | 6.5 ± 0.3 a | |
| TOTAL | 169.1 ± 7.3 b | 41.6 ± 13.4 b | 387.1 ± 29.1 a | 121.5 ± 18.3 a | |
| ellagitannins | diHHDP hexoside 1 | 108.6 ± 8.3 b | 31.6 ± 3.3 b | 135.4 ± 12.7 a | 44.7 ± 4.1 a |
| diHHDP hexoside 2 | 9.7 ± 1.4 b | 2.3 ± 0.6 b | 29.0 ± 2.0 a | 6.0 ± 0.5 a | |
| HHDP digalloyl hexoside isomer 1 | 11.0 ± 0.9 b | 3.0 ± 0.0 b | 43.9 ± 3.8 a | 5.9 ± 0.7 a | |
| HHDP galloylhexoside | 29.9 ± 1.9 b | 7.1 ± 0.49 b | 70.0 ± 3.6 a | 6.6 ± 2.1 a | |
| HHDP digalloyl hexoside isomer 3 | 14.0 ± 0.2 b | 0.7 ± 0.2 b | 58.2 ± 6.8 a | 11.8 ± 1.0 a | |
| HHDP digalloyl hexoside isomer 2 | 8.6 ± 0.1 b | 2.7 ± 0.2 b | 46.9 ± 10.7 a | 11.1 ± 2.7 a | |
| galloyl bis HHDP hexoside 1 | 10.5 ± 0.2 a | 0.5 ± 0.2 b | 4.6 ± 0.6 b | 1.4 ± 0.5 a | |
| galloyl bis HHDP hexoside 2 | 89.6 ± 4.8 b | 14.9 ± 0.7 b | 232.8 ± 12.7 a | 75.5 ± 0.7 a | |
| TOTAL | 281.9 ± 17.8 b | 62.8 ± 5.7 b | 620.8 ± 49.3 a | 163.0 ± 12.3 a |
| Phenolic Group | Compound | Rosa pendulina Flesh and Skin | Seeds | Rosa pendulina × spinosissima Flesh and Skin | Seeds |
|---|---|---|---|---|---|
| FLAVANOLS | procyanidin dimer 1 | 102.2 ± 9.2 a | 36.8 ± 5.1 b | 110.0 ± 60.0 a | 50.0 ± 10.0 a |
| procyanidin dimer 2 | 366.4 ± 52.7 b | 86.5 ± 26.1 b | 1169.7 ± 101.2 a | 360.1 ± 40.4 a | |
| catechin | 93.6 ± 5.9 b | 22.4 ± 1.5 b | 538.6 ± 27.9 a | 50.8 ± 16.1 a | |
| procyanidin trimer 1 | 104.6 ± 6.6 b | 24.9 ± 1.7 a | 156.2 ± 8.1 a | 14.8 ± 4.7 b | |
| procyanidin trimer 2 | 75.4 ± 10.2 b | 11.3 ± 2.2 b | 343.1 ± 19.8 a | 123.2 ± 12.2 a | |
| procyanidin dimer 3 | 102.9 ± 11.9 b | 6.1 ± 1.2 b | 1055.5 ± 6.1 a | 416.7 ± 45.8 a | |
| epicatechin | 180.3 ± 9.4 a | 23.2 ± 5.8 a | 53.9 ± 3.1 b | 21.3 ± 2.3 a | |
| procyanidin dimer 4 | 387.0 ± 100.0 b | 57.0 ± 4.0 b | 777.0 ± 370.0 a | 162.0 ± 21.3 a | |
| procyanidin trimer 3 | 281.7 ± 51.9 b | 86.9 ± 6.1 b | 768.6 ± 58.0 a | 611.0 ± 130.0 a | |
| procyanidin dimer 5 | 193.6 ± 3.2 b | 9.3 ± 2.9 b | 803.3 ± 94.4 a | 162.9 ± 13.5 a | |
| PA dimer diglycoside | 121.6 ± 10.5 b | 24.6 ± 6.9 b | 363.0 ± 130.0 a | 354.0 ± 56.0 a | |
| dimer PA monogallate 1 | 14.0 ± 4.0 b | 2.0 ± 1.0 b | 67.6 ± 7.7 a | 23.5 ± 3.5 a | |
| dimer PA monogallate 2 | 7.0 ± 0.1 b | 0.4 ± 0.1 b | 29.1 ± 3.4 a | 5.9 ± 0.5 a | |
| TOTAL | 1860.3 ± 275.6 b | 391.4 ± 64.6 b | 6235.6 ± 889.7 a | 2356.1 ± 356.3 a | |
| FLAVONOLS | taxifolin pentoside 1 | 0.8 ± 0.0 b | 0.1 ± 0.0 b | 6.2 ± 0.9 a | 1.1 ± 0.2. a |
| quercetin galloyl hexoside 1 | 1.5 ± 0.0 b | 0.9 ± 0.0 a | 2.8 ± 0.3 a | 0.4 ± 0.0 a | |
| quercetin-3-rutinoside | 7.0 ± 3.6 b | 0.4 ± 0.2 | 0.3 ± 0.0 a | / | |
| quercetin galloyl hexoside 2 | 5.0 ± 5.0 b | 6.4 ± 0.2 a | 8.4 ± 2.5 a | 1.1 ± 0.4 b | |
| quercetin-3-galactoside | 7.4 ± 0.5 b | 3.8 ± 0.2 b | 15.1 ± 0.9 a | 6.1 ± 0.4 a | |
| quercetin-3-glucoside | 120.2 ± 7.9 a | 7.6 ± 0.2 b | 66.1 ± 3.5 b | 9.4 ± 0.5 a | |
| taxifolin pentoside 2 | / | / | 10.0 ± 0.8 | 3.0 ± 0.4 | |
| kaempferol hexoside | 1.0 ± 0.0 a | 0.1 ± 0.0 b | 1.0 ± 1.0 a | 3.0 ± 0.6 a | |
| taxifolin pentoside 3 | 9.0 ± 0.5 b | 4.0 ± 0.3 b | 30.0 ± 10.0 a | 30.0 ± 1.0 a | |
| phloretin pentosylhexoside | 0.6 ± 0.1 b | 0.1 ± 0.0 b | 1.5 ± 0.3 a | 0.9 ± 0.1 a | |
| quercetin-3-glucuronide | 13.0 ± 1.0 b | 9.6 ± 1.0 a | 66.1 ± 3.5 a | 9.4 ± 0.5 a | |
| quercetin-3-arabinopyranoside | 9.9 ± 0.9 b | 1.5 ± 0.3 a | 14.1 ± 0.7 a | 2.0 ± 1.1 a | |
| quercetin-3-arabinofuranoside | 1.9 ± 0.1 b | 0.5 ± 0.2 b | 4.6 ± 0.4 a | 2.9 ± 0.0 a | |
| isorhamnetin-3-rhamnoside | 1.4 ± 0.1 a | 0.5 ± 0.0 b | 1.7 ± 0.2 a | 1.1 ± 0.0 a | |
| quercetin-3-rhamnoside | 10.7 ± 0.8 a | 3.3 ± 0.7 a | 1.7 ± 0.2 b | 1.1 ± 0.0 b | |
| quercetin-acetylhexoside | 1.7 ± 0.1 a | 0.5 ± 0.1 a | 1.5 ± 0.0 a | 0.4 ± 0.1 a | |
| quercetin galloyl pentoside | 0.3 ± 0.0 b | 0.2 ± 0.0 b | 2.3 ± 0.7 a | 2.3 ± 0.9 a | |
| quercetin-3-xyloside | / | / | 7.0 ± 0.9 | 3.7 ± 0.7 | |
| TOTAL | 191.4 ± 20.6 b | 39.4 ± 3.2 b | 240.4 ± 26.9 a | 77.9 ± 6.9 a | |
| FLAVONES | apigenin derivative 1 | 2.1 ± 1.7 b | 0.2 ± 0.0 b | 6.8 ± 5.0 a | 3.5 ± 0.2 a |
| apigenin derivate 2 | 1.8 ± 0.1 b | 1.1 ± 0.0 b | 5.5 ± 0.4 a | 2.4 ± 0.2 a | |
| TOTAL | 3.9 ± 1.8 b | 1.3 ± 0.0 b | 12.3 ± 5.4 a | 5.9 ± 0.4 a | |
| DIHYDROCHALCONE | phloridzin | 2798.8 ± 195.1 a | 1467.0 ± 96.6 a | 63.4 ± 5.2 b | 23.9 ± 6.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunc, N.; Mikulič-Petkovšek, M.; Hudina, M.; Bavcon, J.; Vreš, B.; Osterc, G.; Ravnjak, B. Autochthonous Rose Hybrid Rosa pendulina × spinosissima Overshines Main Genotype Rosa pendulina in the Biochemical Characteristics of Their Hips. Horticulturae 2022, 8, 669. https://doi.org/10.3390/horticulturae8080669
Kunc N, Mikulič-Petkovšek M, Hudina M, Bavcon J, Vreš B, Osterc G, Ravnjak B. Autochthonous Rose Hybrid Rosa pendulina × spinosissima Overshines Main Genotype Rosa pendulina in the Biochemical Characteristics of Their Hips. Horticulturae. 2022; 8(8):669. https://doi.org/10.3390/horticulturae8080669
Chicago/Turabian StyleKunc, Nina, Maja Mikulič-Petkovšek, Metka Hudina, Jože Bavcon, Branko Vreš, Gregor Osterc, and Blanka Ravnjak. 2022. "Autochthonous Rose Hybrid Rosa pendulina × spinosissima Overshines Main Genotype Rosa pendulina in the Biochemical Characteristics of Their Hips" Horticulturae 8, no. 8: 669. https://doi.org/10.3390/horticulturae8080669
APA StyleKunc, N., Mikulič-Petkovšek, M., Hudina, M., Bavcon, J., Vreš, B., Osterc, G., & Ravnjak, B. (2022). Autochthonous Rose Hybrid Rosa pendulina × spinosissima Overshines Main Genotype Rosa pendulina in the Biochemical Characteristics of Their Hips. Horticulturae, 8(8), 669. https://doi.org/10.3390/horticulturae8080669







