Abstract
Water management is rapidly becoming one of the most pressing issues facing all countries in semi-arid and arid parts of the world. Global water consumption is predicted to increase by 50% in 2030, resulting in an acute water shortage. Presently, the agricultural sector consumes more than 70% of freshwater in most regions of the world, putting more pressure on water scarcity. Hydrogels are superabsorbent polymers that can hold plant nutrients and water when the soil around plant roots starts to dry out. Research evidence has revealed that water stored by hydrogel slowly returns to the soil, thereby increasing the volumetric water content of the soil. Hydrogel increases water use efficiency and irrigation intervals, decreases irrigation costs, and provides plants with the required nutrients and moisture. Numerous properties of hydrogels, including moderate water retention and high swelling, make them ideal as a safe delivery mechanism in agriculture for soil conditioners and agents for the controlled release of fertilizers. Numerous research publications on hydrogel polymer synthesis and its characteristics have been published. However, the current review emphasizes the critical role of superabsorbent hydrogels in an integrated approach for the balanced protection of seeds, plants, and soil to conserve the ecosystem.
1. Introduction
The increasing global demand for water, with the combined effects of climate change, is exerting tremendous strain on water resources, resulting in water shortages in arid, semi-arid, and several other regions of the world. Additionally, there is competition for the limited amount of available water from different sectors, such as urban demands, the industrial sector, and the agricultural sector, which account for more than two-thirds of global freshwater use [1]. Abiotic stresses (temperature, salinity, and drought) are the major factors affecting agriculture production, and these challenges are expected to worsen due to urbanization and land degradation. Similarly, irrigation water is becoming increasingly scarce, and the world is searching for agricultural practices that promote water use efficiency. The agricultural sector accounts for the most significant water consumption in the United States, with more than 85% of overall freshwater consumption in the country expended on agriculture [2]. On a global scale, more than 70% of freshwater is channelled to agriculture; however, by 2050, feeding a population of over nine billion people will necessitate a 50% increase in agricultural productivity and a 15% increase in water demand [1]. Food security is increasingly threatened by rising food consumption and diminishing water supplies, among other factors [3]. Climate change has had profound consequences in recent years, with water scarcity and accompanying desertification showing an evident influence on the agricultural economy. Agriculture remains a significant consumer of water at a time when the availability of water supply is becoming increasingly scarce. One of the most critical limiting elements that impacts crop growth and output is the survival of plants, which can be achieved by irrigation to reduce water stress on the plants. It is now more important than ever to research and create novel materials for water management and long-term viability. Due to water scarcity and the increased focus on environmental preservation, there has been considerable interest in investigating biodegradable hydrogels for use in commercial agricultural applications.
Water-absorbing polymers, often known as hydrogels, were first introduced for agricultural usage in the early 1980s. Hydrogels are crosslinked hydrophilic water-soluble polymers capable of absorbing a large amount of water without dissolving, up to a hundred times the dry weight of the polymer, and desorbing that water when subjected to mechanical stress [4]. Hydrogels can be used to improve the soil’s ability to absorb water in dry areas by dispersing the absorbed water into the soil. As a result, hydrogel is referred to as a superabsorbent polymer (SAP). Several applications of SAP include agriculture, hygienic biosensor products, wound dressing, regenerative medicines, tissue engineering, food additives, drug delivery systems, artificial snow, coal dewatering, sealing, biomedical applications, pharmaceuticals and barrier materials to regulate biological adhesions, and diagnostics separation of biomolecules or cells [4]. Central to these applications of hydrogels are their characteristic water absorbency and water retention capabilities. Thus, SAP can serve several important functions, particularly in agriculture [5].
Proteins, e.g., gelatin and collagen, as well as polysaccharides such as agarose and alginate, are examples of natural polymers that can form hydrogels. Chemical polymerization methods have typically been used to create synthetic polymers to generate hydrogels. A hydrogel is a single polymer molecule comprised of networks of chains linked together on a macroscopic scale to form a single large molecule or a single polymer molecule in the gel. Gels and hydrogels are terms that are frequently used interchangeably. The gel state is a condition that is neither totally solid nor completely liquid in its physical properties. Numerous intriguing relaxation behaviors result from these half-solid, half-liquid qualities that are not present in pure liquid or solid states. The volume of a hydrogel can alter dramatically in response to specific external stimuli, e.g., electric field, pH change, the quality of the solvent, temperature, etc. [6]. However, the vast majority of superabsorbents currently available are acrylate-based products, which means that they are non-biodegradable. Furthermore, there are concerns about toxicity resulting from their agricultural use or applications related to human consumption; therefore, they have been designated as potential soil pollutants [7].
As a result, the growing focus of the public and institutions on environmental conservation has prompted companies producing hydrogel-based products to redirect their efforts towards developing biodegradable superabsorbents as a potential new product. Recent research has concentrated on developing environmentally friendly polymeric absorbent materials based on renewable resources rather than synthetic hydrogels derived from petroleum [8]. Recent developments of cost-effective and eco-friendly materials based on renewable bioresources (derived from industrial and crop residue or modified lignocellulosic materials) have found a wide range of applications because of their biodegradability and long-term sustainability [9]. Most cellulose-based composites have high biodegradability, better absorbency, and a good level of stiffness than acrylic-based composites [10].
As a soil conditioner, hydrogel increases its nutrient and water retention capabilities and acts as an agent for slow-release fertilizers [11]. Applying hydrogels to the soil increases soil density, structure, and permeability, improving water infiltration and evaporation rates while reducing water run-off and erosion, which inversely improve crop productivity and feasible crop yield [12]. When irrigated (artificial irrigation or rainwater) on cultivated land, the hydrogel absorbs and retains water, preventing quick water loss to drainage and evaporation. With the drying of the soil, the stored water is released from the hydrogel in a controlled manner via a diffusion mechanism, which allows the soil or the substrate to remain moist for extended periods. Additionally, mixing a hydrogel with the soil has the advantage of increasing the size of the hydrogel granules (which have comparable dimensions to the substrate granules in the dry form). The advantage of this is an increase in soil porosity, which provides better oxygen circulation to the plant roots.
Sannino et al. [13] reported a patented formulation of unique cellulose-based polyelectrolyte hydrogels that are completely biocompatible and biodegradable with a swelling ability that can be regulated by varying different synthesis parameters. Accordingly, these hydrogels can absorb nearly a litre of water or aqueous solutions for every gram of dry material. Depending on the application, the material can be developed in dry conditions, either as a powder or bulk with a well-defined shape (it is worth noting that the material also has a remarkable memory of its shape after swelling). Furthermore, tiny molecules, such as nutrients, can be loaded into the hydrogel and released in a regulated manner due to the hydrogel’s swelling and deswelling transitions [13,14].
2. Water Scarcity
Water is a significant factor for rainfed or irrigated crop production, with different production systems operating from fully rainfed to purely irrigated. The vast majority of crops are grown using rainwater as a water source; however, agriculture is heavily dependent on irrigation, which accounts for around 40% of total production and is practiced on nearly 20% of cultivated land [15]. Increased agricultural yields and price stability have resulted from the development of irrigated agriculture, which makes it easier to feed the world’s rising population. Increased non-agricultural water demand, global climate change, shifting food tastes, and new biofuel production requirements have further strained water resources, which are already under stress [16]. Wasteful use of existing water supplies, degradation of water-related ecosystems, increasing water pollution, groundwater depletion, soil degradation, and rising costs of establishing new water sources all contribute to the mounting challenges of expanding water resources for agriculture. By 2050, it is predicted that global water use by livestock, industrial, domestic, and irrigation applications will increase by 21% [16]. Regionally, consumption across developing nations is expected to increase by 25%, and consumption in developed regions is predicted to expand by 11%. According to Rosegrant et al. [16], crop water demand, including irrigation and precipitation, is predicted to rise at a rate of 0.7% p.a., from 6400 km3 in 2000 to 8600 km3 by 2025, reaching 9060 km3 by 2050.
Approximately 22% of overall crop water depletion estimated for the year 2000 occurred as a result of irrigation water use. However, it is expected that by the year 2050, this figure will decline by nearly 20% despite higher productivity in irrigated areas compared to rainfed areas [17]. Rockstrom et al. [18] reported that the proportion of irrigation water depletion is projected to decrease in the coming years. During the period 2000–2050, the global irrigated harvested area is predicted to expand by 0.24% p.a., while the rainfed harvested area is projected to increase by 0.13% p.a. After experiencing strong growth between 2000 and 2025, the forecast shows that the total harvested area will experience a decline between 2025 and 2050 as population pressure diminishes throughout this period. In the period between 2000–2050, it is expected that the total harvested irrigated area will increase from 421 million hectares (ha) to 473 million ha, representing a 12% increase from the current level. Globally, Asia, Latin America and the Caribbean, respectively, account for the largest proportion of irrigated land area; meanwhile, irrigation is currently used on only 6% of the land area in sub-Saharan Africa. By 2050, the sub-Saharan African region is expected to have more than doubled its irrigated land area; however, the region will still only account for a very small proportion of the world’s harvested irrigated land area (about 2%) [16]. Growing water and land scarcities are expected to hamper the growth of food production, resulting in negative consequences for food security and human well-being goals. Scarcities of water and land have also been cited as contributing factors to the spikes in food prices that occurred between 2005 and 2007.
3. Climate Change Impacts on Water for Agriculture
Globally productive lands offer humanity a diverse range of resources, and among these are non-replaceable resources such as food, raw materials (wood and textiles) and energy sources [19]. By 2050, most economists predict that increasing human population and economic prosperity will lead to a 70–100% increase in demand for agricultural products [20]. In preparing fifth assessment report (AR5) of the Intergovernmental Panel on Climate Change (IPCC), Stocker et al. [21] stated that anthropogenic activities would likely lead to a 4 °C increase in the temperature of the planet by the end of this century, leading to a severe impact on the human population, especially in vulnerable populations and places [22]. Various factors, including climate change, impact the global hydrological cycle, and these changes have a major effect on agricultural production and food security. Changes in the intensity, volume, and precipitation variability are among the most significant water-related climatic variations due to temperature changes. Drought and more frequent severe flooding are connected to fluctuations in the distribution and timing of rainfall (i.e., rainfall variability) across several places. Regions with a predicted increase in precipitation will experience more severe and frequent floods as well as an increase in reservoir sedimentation and erosion, while areas with precipitation declines will experience more droughts and decreased water availability [23,24]. The forecast for future precipitation is fraught with uncertainty. However, it is generally agreed that precipitation will increase primarily at high latitudes and decrease primarily at lower latitudes and subtropical regions [24,25].
Southeast Asia, Bangladesh and the Mekong Delta, which produce significant amounts of food, could be affected by a sea-level rise. Increased temperature is expected to drive up the evaporation/evapotranspiration rate due to the higher water-holding capacity of the atmosphere, leading to a decrease in the amount of water stored in reservoirs and soils [25]. These negative impacts of global warming on freshwater systems amplify the adverse effects of other stresses, such as urbanization, land-use change, changing economic activity, and population growth, among other things, on freshwater systems [23]. The spatial and temporal distribution of rainfall and water availability significantly impact agricultural output. Fluctuations in rainfall intensity, timing, and volume can negatively impact crop growth, disrupting production and food security. Non-uniformities in climatic factors and an increase in climate extremes, such as floods and droughts, will also negatively impact crop productivity [24]. The results of a simulation study reported by Bates et al. [24] revealed that, while warming in high-latitude areas will boost agricultural productivity, even a minor increase in temperature in low-latitude regions or seasonally dry places would result in a negative impact on crop productivity. Multiple climate change scenarios have been examined, and the results indicate that climate change will likely have a slight to moderate adverse influence on crop productivity [26]. However, crop irrigation requirements will worsen the water stress condition across several regions that rely on irrigation. Given the uncertainty surrounding future water supply and rainfall patterns, agriculture and water management systems face a difficult time as historical rainfall indicators are no longer reliable forecasts of future rainfall patterns [23]. Planning for uncertainties is essential for decision-makers at all levels, from the farm to the policy level. It is important to choose solutions capable of dealing with a variety of different futures. The most viable mitigation strategies for climate change in water-stressed areas appear to be the prevalent use of irrigation (including improved varieties of irrigation), an increase in irrigation efficiency and development, as well as water and soil conservation techniques and technologies [27,28].
4. Mitigation of Water Scarcity through Hydrogel
It has been shown that plants suffer from oxidative stress and increased lipid peroxidation when subjected to drought stress or limited water availability. Among the visible consequences are reduced height, reduced leaf area, foliar matrix damage, etc. Hydrogel has the potential to minimize the impact of drought on plants, resulting in reduced stress and oxygen radical production. This allows for improved growth and yield, even in difficult climates. Hydrogel can increase water use efficiency (WUE), which means the ratio of yield over crop water consumption is improved by reducing water loss in the soil via an increase in the soil’s water-holding capacity, depending on the properties of the hydrogel polymer. As Abd El-Aziz et al. [29] reported, polymers can absorb, hold and release water to meet the plant water requirement. This was demonstrated in an experiment where the water use efficiency of tomatoes was influenced by an eco-friendly hydrogel under various irrigation levels. The use of hydrogel resulted in a reduced amount of water used for tomato irrigation compared to the control treatment (without hydrogel). These results confirmed that hydrogel treatments had a positive influence on the productivity of tomato plants, and consequently, on the values of water use efficiency. Therefore, these natural eco-friendly hydrogels can reduce water loss and improve the water use efficiency, thereby sustaining productivity during drought stress.
5. Characteristics of Superabsorbent Polymers
The terms hydrogel and gel are interchangeable terminologies. The gel state is a condition that is neither totally solid nor entirely liquid in its physical properties. Numerous intriguing relaxation behaviors occur as a result of these half-solid, half-liquid qualities that are unavailable in pure liquids or solids. Hydrogels have been defined in various ways over the years, and the most precise description refers to hydrogels as water-swollen materials with crosslinked polymeric chains. Another description depicts hydrogels as substances having remarkable swelling ability that do not require structural changes or changes to the shape and volume of the material. In some cases, depending on the application, hydrogels can be flexible and easy to form [30]. Hydrogels can be made using synthetic and/or natural polymers, which form as a gel when subjected to various conditions, including temperature, ultraviolet irradiation, ionic strength, and pH. The strong hydrophilicity of polymers leads to the formation of three-dimensional structures [31]. Various environmental factors, including electromagnetic radiation, ionic strength, pH, and temperature, remarkably affect the swelling behavior of hydrogels [32]. Additionally, by altering these parameters, it is possible to modify the properties of hydrogels, such as their form, porosity, opacity, and mechanical flexibility. Generally, hydrogels can be distinguished into two major categories: synthetic-based polymers and natural-based polymers. These two forms of hydrogels have their own set of benefits and drawbacks. However, when considering the medicinal applications of hydrogels, natural-based materials have major advantages because they are environmentally friendly and harmless to the human body, as opposed to synthetic materials, thus making hydrogels from natural-based polymers more desirable than those from synthetic compounds. However, hydrogels made from synthetic materials have higher mechanical properties, which increases their potential for industrial application. Hydrogels can also be classified according to their physical appearance (microsphere, film, matrix), charge (zwitterionic, amphoteric electrolyte, ionic, nonionic), configuration (crystalline, semicrystalline, and amorphous), and the type of crosslinking (physical or chemical crosslinking methods). Hydrogels are soft materials with elastic qualities used in various applications [33].
Depending on the qualities of the polymer used to make the hydrogel and the density of the network, varying levels of water absorption occur when the hydrogel is in its swollen state, and most commonly, the mass of water is more than that of the polymer. Hydrogels can be found in a range of shapes and sizes, including aerogels, cryogels, nanogels, microgels, beads, membranes, and slabs, among others. As a result of their multifunctional qualities, hydrogels are very appealing materials since they may be used in a broad variety of applications in several spheres of life, particularly biomedical science. The ability of these materials to absorb water and dissolve chemicals allow them to be utilized as superabsorbents in water conservation applications. Hydrogels have been dubbed “smart materials” due to their ability to adapt to various environmental conditions, such as osmotic pressure, temperature, and pH. These external factors influence the controlled-release systems, which can benefit the health and agriculture sectors [34]. Agricultural hydrogels are synthetic polymers that are typically derived from petroleum-based materials. Due to their ability to absorb many-fold their weight in water, hydrogels can be spread in dry areas to boost the soil’s ability to absorb available water. Hence, they are often referred to as a superabsorbent polymer (SAP).
Hydrogels can be classified based on:
- 1.
- Size: Microhydrogels and bulk hydrogels are distinct types of hydrogels that can be distinguished by their size. Microhydrogels comprise individual hydrogels that are much smaller than those found in bulk hydrogels. Current research on microhydrogels has concentrated on the nanoscale size since it is well suited to catalysis, magnetism, optics, electricity, and mechanics due to the surface and quantum effects provided by their small size and large specific surface area. On the other hand, bulk hydrogels comprise larger hydrogels with specific sizes and shapes commonly used in food processing and beauty salons. They often take the form of a low-strength jelly that has a particular viscosity and fluidity that be used for antimicrobial coatings, tissue engineering, coatings on inert biosensors, and food processing, depending on their thicknesses and strengths. Although the material has a wide range of applications and good qualities, the moulding and processing of this low-strength and soft watery material remains difficult, and its uses in some specialized industries are limited. Therefore, efforts have been made to improve the strength of bulk hydrogels by introducing a reinforcing phase into the matrix. This provides the hydrogel with new chemical and physical properties, which has improved the strength of bulk hydrogels.
- 2.
- Environmental response: Hydrogels can be categorised as either environmentally unresponsive hydrogels (also known as ordinary hydrogels) or environmentally responsive hydrogels (intelligent hydrogels) based on how well they react to their surrounding environment. Environmentally insensitive hydrogels can maintain their structure as well as their physical and chemical properties under various environmental conditions [35]. For certain specialized applications, this particular type of hydrogel is required. For example, agarose is hydrophilic and biodegradable but almost entirely lacks charged group structures. Thus, this hydrogel is resistant to being desaturated or adsorbed by sensitive biomacromolecules, so it is frequently used as a support matrix in gelation or immunoelectrophoresis experiments. On the other hand, hydrogels that reversibly respond to external stimuli are referred to as intelligent, smart, or environmentally sensitive hydrogels. When a hydrogel is subjected to environmental stimuli such as a magnetic field, stress, light, temperature, electric field, ion strength, pH, etc., the three-dimensional network structure of the hydrogel either changes (shrinking or swelling) or transitions between the dilute phase and the dense phase. As a result, the shape, optical properties, and mechanical properties of the hydrogel are dramatically altered. As soon as the external stimulus is removed, the hydrogel will revert to its original state, which has a lower internal steady-state energy.
- 3.
- Degradability: Hydrogels can be classified as non-biodegradable or biodegradable. Non-biodegradable hydrogels are distinguished by their resistance to the effects of environmental stimuli and their ability to preserve their chemical, physical, and structural properties over an extended period. The vast majority of synthetic hydrogels produced by chemical crosslinking are non-biodegradable. In contrast, the vast majority of natural polymer hydrogels can be classified as biodegradable hydrogels. The three-dimensional structure of these hydrogels is susceptible to degradation due to the actions of enzymes and bacteria when exposed to natural environments. The bonds present both within the molecular chains and between the molecular chains is severed, which results in a decrease in the hydrogel’s overall strength. In due time, the hydrogel will break down into smaller molecules.
- 4.
- Mechanism formation: Physical hydrogels and chemical hydrogels can be differentiated from one another according to the formation method of the three-dimensional network structure. Physical hydrogels are primarily three-dimensional networks formed by secondary bonds, also known as noncovalent bonds (such as hydrophobic interaction, chain entanglement, hydrogen bonding, and electrostatic interaction), between linear molecules to form physical crosslinking joints. Since only a moderate amount of energy is required to disrupt these interactions [36], the sol-gel transformation that occurs in physical hydrogels is typically reversible. No chemical reactions are involved in their creation, and the conditions under which they are prepared are generally mild, so they are well suited to biomedical use [36]. On the other hand, chemical hydrogels are produced by irreversible molecular crosslinking that occurs during their formation. Chemical hydrogels often offer good mechanical properties, tunable structures, and stable properties.
- 5.
- Source: Hydrogels can be divided into two main categories based on their material source: natural and synthetic. Synthetic hydrogels are crosslinked polymers developed in an artificial environment using ring-opening or addition reaction polymerization processes. Synthetic hydrogels are often made with skeletons consisting of polyvinylpyrrolidone, polyvinyl alcohol, polyacrylic acid and its derivatives, and polyethylene glycol and its copolymers as the primary building blocks. Compared to natural polymer hydrogels, synthetic hydrogels have poor biodegradability, bioactivity, and biocompatibility; however, the advantage of synthetic hydrogels include precisely controlled properties, easy chemical modification, and industrial production. Natural polymers, such as cyclodextrin, dextran, chitosan, agarose, sodium alginate, fibrin, hyaluronic acid, gelatin, and collagen, are derived from natural sources and have excellent biodegradability and biocompatibility. Due to their abundance and sensitivity to the surrounding environment, they have emerged as the leading research focus. In general, when choosing a hydrogel as a soil conditioner, three aspects need to be taken into consideration: (1) chemical crosslinking; (2) biodegradability; and (3) superabsorbency [35].
6. Mechanism of Swelling and Water Retention for Hydrogels as Soil Conditioners
Before discussing the function of hydrogels in soil conditioning, it is necessary to understand the process of swelling and the water retention capacity of hydrogels. This process is characterized by the swelling of freely absorbed water, which results in the separation of hydrophilic groups, repulsion among charged groups, and the expansion of polymer coils. Additionally, large amounts of water molecules occupy the free space between network chains, thereby increasing the size of the free space. The swelling capacity and water absorption of hydrogels are important features for their usage as soil conditioners. This is due to their ability to facilitate nutrient transport and supplement irrigation, resulting in gradual nutrient and water release to plants. The water retention ability of hydrogels varies based on the number of hydrophilic groups and the density of crosslinking [36]. The solubility characteristics of water are obtained from the free mobility of water molecules caused by water-water vibrations. When a water molecule attempts to dissolve a hydrogel with strong covalent crosslinking, its vibrational mode is only able to cleave hydrogen bonds. Hydrophilic functional groups in hydrogels such as COONa that come into contact with water molecules convert the kinetic energy from water-water vibrations into water ion vibrations (mobility process), resulting in the dissociation of COONa into COO− and Na+ (dissociation process). The negative charges oppose each other and are neutralized by water molecules, whereas the Na+ ions are hydrated by water molecules (hydrated process). Changes in hydrogen bonding are evident in this phenomenon, which permits the water molecules to become trapped within pores of the specialized structure of the hydrogel (swelling process), as shown in Figure 1. As a result, the existence of osmotically efficient free-moving polar molecules serves as a driving factor for the swelling process [36].
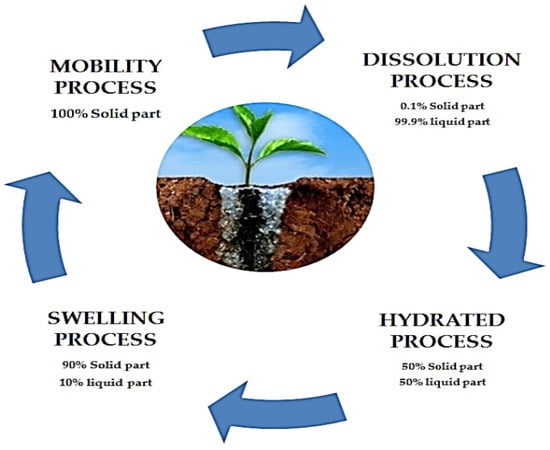
Figure 1.
Hydrogel swelling process.
7. Application of Hydrogels in Agriculture
There are several factors that can adversely affect plant development and agricultural output, such as phytopathogenic attacks, leaching, high transpiration rate, and the low water retention capability of the soil. As a result, the application of hydrogels in agriculture may provide the following benefits: reduce soil erosion (due to surface discharge) and leaching of pesticide/fertilizer into groundwater [37]; enhance nutrient retention and delay fertilizer dissolution due to solute release from hydrogel polymer particles [38]; support plant growth and performance in limited irrigation environments [39]; improve soil drainage [40]; increase water use efficiency and improve water retention by different soil types [41]; increase soil permeability [42]; and decrease soil compaction tendency [43].
7.1. Hydrogel as a “Nano Release” and “Smart Release” Fertilizer
Soil is crucial for plant development due to its role in holding the roots and supplying them with water as well as the required nutrients, including essential minerals, throughout the early stages of growth. Besides that, soil supports plants and acts as a reserve for nutrients that are necessary for plant survival in the natural environment. Furthermore, in order to achieve good soil quality, necessary amounts of aggregate formation are required, which promotes cultivation, aeration and drainage, moisture retention, and prevents soil erosion from oxygen and microbes, among other characteristics. Fertilizers containing nitrogen in the form of nitrate and ammonium (NO3− and NH4+, respectively) encourage the release of N2O into the atmosphere, which contributes to global warming at a faster rate compared to CO2 emissions [44]. Current efforts are directed towards maximizing nitrogen (N) retention while simultaneously reducing methane (CH4) and nitrous oxide (N2O) emissions [45]. Bley et al. [46] reported that a polysaccharide hydrogel used as a soil conditioner could effectively retain most dissolved fertilizer nutrients while decreasing the nutrient requirement of cultivated soils and N leaching.
The nanostructural loading of fertilizers has recently gained wide recognition due to increased carbohydrate content, nitrogen stability, photosynthetic activity, seed germination, and higher crop production when fertilizers are applied in this form [47]. Nanoparticle materials including hydrogels, mesoporous silica, hydroxyapatite nanoparticles, nanoclays, and nanomaterials of carbon and other metal oxides, are often utilized as fertilizer carriers. When a fertilizer and nanomaterials are combined, the resulting nano fertilizers are more readily available to plants than conventional fertilizers, leading to a decrease in the use of conventional fertilizers [48]. In several publications, future directions have been suggested for formulating multifunctional nanomaterials and developing stimuli-responsive agents for carrying nanomaterials. Chitosan nanoparticles are an innovative material used in controlling fertilizer release because of their small size [49]. Soluble nanofertilizers are fertilizers encapsulated by hydrogel nanoparticles, which allow nutrients to be slowly released into the soil. Biodegradable nanofertilized hydrogel undergoes slow diffusion into roots via apoplastic and symplastic pathways and is translocated through the xylem tissue to the upper part of the plant, including the leaves and stems [50]. The pore size of the cell wall (5–20 nm) determines the amounts of nanoparticles that can pass through the wall [51]. Thus, nanoparticles having a diameter smaller than the pore diameter of the plant cell wall might readily diffuse through the wall and into the plasma membrane [52]. As a result, there is a considerable difference in the germination of the lettuce seeds, as described by Khodakovskaya et al. [53]. Excessive quantities of nanofertilizers can cause considerable health and environmental harm, and they are recommended to be used in small amounts to promote plant growth [54]. These biodegradable hydrogel nanoparticles can spread into the entire food chain once it enters the plant via successive organisms. In contrast, other nanomaterial fertilizer carriers, which can be toxic to humans, animals and plants at varying exposure levels, can be used in different applications if they are designed with the critical exposure concentration in mind, as discussed in numerous researches on the utilization of hydrogel nanoparticles as fertilizer carriers. Bortolin et al. [55] utilized a PAAm/MC/MMt nanocomposite hydrogel for delivering urea. According to the researchers, urea release was highly influenced by the pH of the medium, hydrophilicity of the nanocarrier, and a hydrolysis treatment. Ultimately, the hydrolyzed hydrogel released urea slowly, roughly 192 times slower than the control (pure urea). Furthermore, the authors emphasized that their nanocomposites were the first to release 90 grams of urea per gram of dry hydrolyzed hydrogel.
Fertilizer carriers have been formulated to create a critical sensing medium capable of regulated nutrient delivery. Recently, the addition of a smart hydrogel to fertilizer has removed the current constraints of conventional fertilizers [56]. The most modern control-release fertilizer (CRF) technology utilizes polymer-coated fertilizers (PCFs) [57]. However, these materials have been found to be very costly, so it is desirable to wrap fertilizers in a smart biodegradable hydrogel (SBH) to maximize their efficiencies. These fertilizers usually perform better, showing advances in areas such as persisting in an available form for a long period, increasing efficient root uptake by influencing the level of N, K, and P in the soil, and providing easy root access to the fertilizer. The use of a smart biodegradable hydrogel also eliminates compaction and promotes yield increases. Among the issues facing long-term plant development in the future is the synthesis of CRFs based on SBH. Smart hydrogels are effective in releasing fertilizers and meeting plant growth requirements, resulting in a high crop yield while maintaining a safe environment. The release of the fertilizer placed into the polymer can be managed by altering other aspects of the polymer’s performance, such as biological activity, pH, and temperature, as reported by Adams et al. [58] and Meurer et al. [59]. The essential impact of substrate temperature and moisture on micro- and macronutrient release of three PCF types—Osmocote, Nutricote, and Polyon—was investigated by Adams et al. [58]. The results indicated that the influence of temperature on the rate of fertilizer release was more consistent for Nutricote compared to the other fertilizers. Depending on the fertilizer, delay of nutrient release from these three PCF-type fertilizers varied from 20 to 40 days. A steady state of release was reported for temperatures between 20 °C and 30 °C, whereas minimal release occurred between 5 °C and 15 °C. In a series of leaching and elution experiments, Oertli and Lunt [60] evaluated the rate of nutrient release from inorganic fertilizer salts. The researchers discovered that the fertilizer release process was a linear diffusion pathway that was mainly unaffected by the acidity or basicity of the elutant or its environment (soil). However, when the temperature was raised from 10 °C to 20 °C, the rate of emission nearly doubled. In addition, the release of NH4+ and NO3− ions was significantly faster than the release of phosphate and potassium ions. A study on iron-deficient cucumber plants was carried out by Meurer et al. [59] using a biocompatible and pH-sensitive nonphytotoxic poly hydrochloride microgel loaded with Fe3+ ions. The findings revealed that Fe3+ ions could bind strongly to leaf surfaces and enhance the chlorophyll content in leaves, indicating that the ions were delivered efficiently to the leaf surface. Research on CRFs based on SBH is still in its early stages, and further research needs be conducted to advance the field.
Seeds can be coated with protective chemicals (nematicides, insecticides, fungicides, herbicides, and pesticides) and compounds that are meant to directly transport important ingredients (growth regulators, micronutrients, symbiotic microorganisms), and by using this approach, applying fertilizer or spraying is no longer necessary [56]. Furthermore, highly accurate application significantly reduces the doses of nutrients required, which results in very little loss to the environment. In addition, coated seeds can be kept for extended periods without a deterioration in quality. These seeds can be produced by soaking them in solutions containing the necessary components, such as micronutrients, and then drying them or putting them through the process of germination directly [27]. The materials that are utilised for coating make it possible to adhere biological or chemical substances to the surface of the seed. Finely powdered coating substances or liquids that have been dissolved in a solvent is the simplest approach. The carrier can be an adhesive (such as xanthan gum or arabic gum), a filler (such as charcoal, lime, or peat), a polymer (such as derivatives of cellulose), or a polysaccharide (chitosan, alginate). The design of the seed coat must meet certain specifications: (i) an appropriate thickness because the rate of germination and compound release is dependent on it, (ii) biodegradable because the degradation rate is a crucial parameter for the release of certain compounds, and (iii) environmentally friendly and non-toxic to seeds [27]. Because of this, the selection of materials has resulted in the utilization of a wide variety of biopolymers, one of which is based on alginate. Brown algae are the source of the natural polysaccharide known as alginate and it is commonly used to prepare hydrogel formulations with slow or controllable release qualities [27]. The material is simple to create, biodegradable and harmless, and it possesses the capacity to react to the conditions in the surrounding environment (including pH). As a result, it is utilized in various industries, including the food, pharmaceutical, medical, and packaging industries. Alginate has also been used in agriculture, most commonly as a component of slow-release coatings or as transport for fertilizer ingredients. Even though research on the use of this polysaccharide in seed applications is still in its early stages, it is known that seeds can be coated with growth regulators, bacteria (Pseudomonas aeruginosa), and mycoparasites (Trichoderma asperellum) in an alginate coating [27].
7.2. Polysaccharide Hydrogels as Agents for Controlling Plant Diseases
The control of plant diseases is one of the primary foundations of food safety policy, especially for protecting nutrition reservoirs for plant embryos and seed characteristics [61]. In most cases, seed treatment for disease and infection is limited to the use of insecticides and fungicides; nevertheless, new technologies and developments have enabled the use of herbicides in certain situations, subject to certain restrictions [62]. According to Brockwell [63], lime and other organic materials used in seed-coating technology significantly improved nodulation and seedling survival and lowered infection in root hairs. The application of pesticides in a seed-coating treatment can be accomplished by using a pesticide solution, emulsion, or a water-dispersible powder for slurry treatment, among others [64]. The primary mode of action of pesticides used for seed treatment is either systemic or contact mode [65]. For example, thiocarb, carbaryl, and hydramethylnon are the most commonly used contact insecticides, and carbosulfan and benfuracarb are the most commonly used systemic insecticides [65]. The most commonly used types of fungicides are tebuconazole and triadimenol, which have a systemic action, whereas thiram, silthiopham, and fludioxonil have a contact action [65]. However, some pesticides such as carboxin/thiram can be used well for both contact and systemic effects [65].
According to Ismail et al. [66], many pesticides fail to reach their intended target as a result of leaching, degradation, and volatilization effects, which can result in major environmental contamination as well as human, animal, and plant health problems [67]. Similarly, the harmful impact of herbicides and fertilizers on non-targeted plants (plants growing in areas near traditional fields that may be affected by herbicides or fertilizers through spray drift and over-application) can reduce crop output. In order to address the aforementioned issues, controlled release formulations made using hydrogel technology can be employed. This formulation can efficiently deliver the active ingredient in the pesticides, allowing for safe and effective application in agricultural practice. As a result, it has the potential to reduce probable toxicity, pesticide volatilization, soil deterioration, and leaching of chemicals [68]. Most notably, a seed-coating treatment based on a hydrogel enhanced with oregano essential oil exhibited the highest antibacterial efficacy against Pseudomonas syringae pv. phaseolicola and Clavibacter michiganensis [69]. Furthermore, the findings revealed that seed treatment using a hydrogel supplemented with oregano essential oil significantly reduced the incidence of fungal diseases caused by Aspergillus flavus, Penicillium expansum, Rhizoctonia solani, and Fusarium oxysporum in the seeds of Pisum vulgaris under a controlled environment. This study demonstrates the need to adopt hydrogels derived from natural materials in seed-coating formulations to limit the usage of inorganic pesticides, which can pose severe hazards to the environment, human and animal health, among others. Singh et al. [70] conducted an experiment where they investigated the efficacy of a hydrogel-based seed treatment including ammonium persulfate for controlling the release of thiram fungicide, and reported that the efficacy improved with time. Additionally, it was observed that increasing the cross-linker concentration in the polymer matrix resulted in a decrease in the amount of fungicide released.
Mercier et al. [71] researched the activity of laminarin and carrageenan as elicitors (natural elicitors such as chitosan are chemical compounds tasked with defending plants against diseases and pests) in tobacco plant leaves for Phytophthora parasitica. Studies have found that polysaccharides like laminarin and carrageenan may be utilized as elicitors, with chitosan considered a very essential plant defence booster [72]. Polysaccharides (chitine, chitosane, and other similar compounds) have been shown to activate plant defence responses against many phytopathogens, including plant fungi and viruses [73]. Studies have shown that chitosan can prevent viral infections [74] and has been used to reduce the amount of local necroses in beans resulting from alfalfa mosaic virus infection. Chitosan can also inhibit bacteriophage-induced infection. In one study, the potency of the inhibitory effect on bacteriophage infection was directly related to the final concentration of chitosan in the medium [75]. Phage particle inhibition and inactivation of reproductive cells are the two most important mechanisms by which chitosan suppresses phage infection. Chitosan can induce phago-resistance in industrial microorganism cultures to prevent unwanted phagolysis resulting from inoculum contamination by a spontaneous prophage or by induction of virulent bacteriophages in lysogenic cultures. A study conducted by Choudhary et al. [76] used Cu-chitosan nanoparticles to increase the growth and defence responses of maize plants to diseases and found that they were more effective than CuSO4 or chitosan on its own.
7.3. Seed Coating with Hydrogel
To protect seeds from external factors such as drought, salinity, pathogens, and ensure conditions for optimal growth, they can be covered with external materials, and this is considered one of the most common recent and efficient practices for improving germination and plant establishment, particularly in challenging environments [61]. The economic cost of implementing this technology for scientific or private use is estimated to be above one billion USD per annum [61]. Nevertheless, seed coating can be adopted as a viable technology for efficient biological control of numerous plant pathogens, offering a cost-effective strategy for incorporating viable organisms that can be useful as biological control agents [77]. Many beneficial bacteria have been encapsulated with hydrogels or sodium alginate formulations for biological control and the improvement of biodegradation [78]. Seed coating serves as the first line of protection against a variety of external climatic and pathogenic factors, and it has the potential to alter the plant’s metabolism in reaction to unfavorable environmental conditions. Seed coating can be conducted in two ways, as split coats or as melt coats, depending on the application. Unlike a melt coat, which slowly dissolves by becoming wet around the seed, a split coat can hold its wet shape and allow moisture to travel through the pill via capillary action [79]. This method may also aid in germination by reducing the amount of water required for seed germination [80]. When coating seeds, it is important to note that the coating should have neither a negative impact on seed health and germination nor cause secondary dormancy in the seed [80]. Although early reports indicated that seed coating was conducted for the sole purpose of modifying the size and shape of seeds [81], in recent years it has come to include polymer technology, growth regulators, microbial inoculation, micro- and macronutrient applications, as well as systemic and contact pesticide treatment.
Several recent studies have reported that coating materials can be used effectively for a variety of applications, including the transportation of nutrients [82], the transport of biocontrol agents such as mycorrhizal fungi and beneficial microorganisms [83], the improvement of plant growth [81], and the transport of agrochemicals such as insecticides, fungicides, and bactericides. When compared to different traditional uses, the addition of a variety of bio-pesticides, microorganisms, and nutrients to the formulation of seed coats provides a number of advantages without causing any harmful residual effects on the immediate environment [84]. It was noted that a hydrogel seed-coating vastly improved seed aeration and germination for the Caragana korshinskii family of Fabaceae and cushioned the plant against stress under drought conditions [85]. Pathak and Ambrose [86] evaluated the impact of a biodegradable hydrogel used as seed coat on the early germination of corn and found that coated seeds had a higher rate of emergence compared to uncoated seeds under conditions where the water supply was 77 percent of the field capacity. Isabel et al. [87] evaluated the encapsulation of Pseudomonas fluorescens bacteria by sodium alginate as a carrying agent and chitosan as standard carrier materials. It was discovered that there were statistically significant variations in growth parameters between the control plants and their counterparts resulting from encapsulated seeds. The encapsulated treatment was found to be effective in increasing plant growth, yield, and nutrient content. Similarly, the encapsulation of bioagents contributed to the improvement and promotion of crop yield and productivity.
Using alginate to encapsulate meristematic tissues in order to produce artificial seeds has been proposed; however, coating seeds with a hydrogel formulation will pose less danger because the seeds will have greater resistance to degradation than meristematic tissues. Sarrocco et al. [77] investigated the emergence of radish, basil, cabbage, and wheat seeds encapsulated in calcium alginate and treated with antagonistic microorganisms and found that the percentages of seeds that emerged were not significantly different from those of untreated seeds. The results revealed that alginate was capable of regulating nutrient consumption without impairing the emergence of seedlings. As a result, when applied to seeds, this technology represents a promising strategy for increasing the efficacy of antagonistic microorganisms. Aside from these benefits, this review has noted other advantages of seed coating, such as disease control and the prevention of desiccation.
7.4. Mode of Action for Polysaccharide Hydrogels on Microorganisms and Plant Roots
Microorganisms in the soil assist in the adaptation of the ecosystem and are critical to the long-term viability of plants in the natural medium. As a result, soil microbes help to maintain healthy soils and crop productivity. The majority of “aerobic” bacteria prefer well oxygenated soils. For example, the Aerobacter genus commonly occurring in soil and the Actinomycetes bacterium genus Streptomyces are responsible for the pleasant “earthy smell” of soil and are both aerobic bacteria. Polysaccharides are often decomposed into smaller components by soil-associated aerobic bacteria, mainly glucose and sucrose units, and this process occurs rapidly in well-oxygenated soil [88]. Hydric soils have these features and are best suited for plants that have aerenchyma (internal spaces in rhizomes and stems that allow ambient oxygen to be transferred to the root area). Some polysaccharide-like chitins are preserved for longer in the soil by polysaccharides, which are formed as a result of complexes with clays, humic acids, or metals that prevent further decomposition [89]. The relationship between maize yields and soil aggregation is highly essential; for example, Wallace and Garn [90] reported that sugar beet yield was reduced when the soil aeration level was less than 12%. Optimal soil aggregation is mostly due to the high level of polysaccharides that provide a favorable environment for anaerobic microorganisms [91]. Higher cohesive forces in soil leads to poor oxygenation, allowing anaerobic bacteria to flourish in the soil alongside more complex microbes such as fungi.
Anaerobic bacteria thrive in aggregated soils with limited oxygen availability, which are known as hydric soils. Most anaerobic soils contain pathogenic bacteria that can kill aerobic bacteria present in the soil [92]. These anaerobic bacteria have been shown to have adverse impact on plant development and vigor via mechanisms such as phytotoxicity and competition for nutrients, as well as the inhibition of Arbuscular mycorrhizal (AM) fungi. Ruminococcus, Peptostreptococcus, Bifidobacterium, and Eubacterium species have been tested for their capacity to ferment 21 different polysaccharides, including pectin, amylopectin, amylose, polygalacturonate, laminarin, xylan, gum arabic, gum ghatti, locust bean gum, guar gum, and gum tragacanth [93]. In tilled soils, bacteria are the dominant organisms, but they are only 20% to 30 % efficient at recycling carbon (C). Bacteria have a higher nitrogen (N) content (10–30% nitrogen) than most microorganisms because they have a higher carbon to nitrogen ratio (3–10 C:N ratio) [94].
Aside from promoting microbial growth, polysaccharides also increase competition between microorganisms and the roots for nitrogen uptake and other mineral nutrients. As a result of irrigation or precipitation, the pores in the soil are primarily filled with water, which moves further downwards into the soil profile. Soil aeration is beneficial, but the soil is not capable of retaining water. Incorporating a soil conditioner such as a polysaccharide hydrogel can aid in water retention, resulting in more favorable growing conditions for plant roots. The adaptation of plant roots to polysaccharide hydrogels as soil conditioners allows nutrients and ions to be transported rapidly through the soil. The root surface is made up of a plasma membrane that contains steroids, protein, phospholipid molecules, and other materials. Due to dipole-dipole interactions or hydrogen bonds formed between soil particles and roots, a hydrogel improves the interaction between soil particles and roots, thereby aiding the active movement of water and nutrients to plants through negatively charged clay surfaces with anionic functional groups (Ca2+, Fe3+, and Al3+). The application of some absorbent polymers to the soil has been reported to increase the yield of Citrus limon due to an increase in the soil’s water-holding capacity, which allows the soil to retain moisture for a longer period of time, increasing microbial activity and preventing fruit loss [95]. According to Pieve et al. [96], who investigated the influence of polymers on the establishment of coffee plants in the open field, the timely application of a polymer solution at planting can enhance the survival of the coffee plants. Seed coating using highly absorbent polymers has the potential to enhance early germination under dry conditions by improving water availability, hence preventing delays in emergence and crop standing time [27]. Many studies have been carried out to assess the efficacy of the various doses of hydrogels in soil remediation and plant growth improvement. The results indicated that hydrogel application to the soil surface, especially at 0–20 cm depth, has a beneficial impact on soil temperature, plant photosynthetic rate, and yield [97].
8. Conclusions
Globally, new novel solutions for plant protection are being developed to minimize reliance on synthetic and chemical pesticides while also conserving the environment and protecting living organisms. On this note, polysaccharide hydrogels have been developed and used in agriculture for different applications (fertilizer carriers, soil conditioners, and slow pesticide release). A wide range of technological advancements have encouraged the usage of polysaccharide hydrogels due to their numerous advantages. Hydrogels have been effectively employed in soil protection and improvement of plant performance in the face of various harsh environmental conditions. Seed coating is regarded as an intriguing method for protecting seeds against biotic and abiotic factors while simultaneously enhancing yield and plant performance. This review has emphasized the critical nature of integrating hydrogels with soil conservation in order to maximize production efficiency, seed protection, nutrients and water conservation, in addition to protecting the health of living organisms and conserving the environment via sustainable crop production practices. Superabsorbent polymer hydrogels, in particular, have several advantages, but they also have associated downsides. The vast majority of these polymer hydrogels are non-biodegradable, may be toxic, and most are not produced from renewable materials. Despite this, hydrogel-based natural agents, such as growth regulators, moisture attractants, plant essential oils, biopesticides, and microorganisms have demonstrated positive results for seed and plant protection without compromising soil fertility, water consumption, or nutrient loss.
Author Contributions
Y.O., M.Y.R. and F.A. drafted the original manuscript, while the editing, finishing and proofreading were carried out by Y.O., M.Y.R., F.A., S.C.C., T.K.M., M.A.S., I.K.F., S.S. and B.S.H. All named authors contributed to the work by offering recommendations on the original draft manuscript. The paper has been reviewed and approved by all of the authors in its published form. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by a Long-Term Research Grant Scheme (LRGS/1/2019/UKM/01/5/4) from the Malaysia Ministry of Education for food security and sustainable vegetable production technology in urban agriculture.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data to support the finding in this manuscript is presented within.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kolapo, K. Drought resistance in rice from conventional to molecular breeding: A review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcagnile, P.; Sibillano, T.; Giannini, C.; Sannino, A.; Demitri, C. Biodegradable poly (lactic acid)/cellulose-based superabsorbent hydrogel composite material as water and fertilizer reservoir in agricultural applications. J. Appl. Polym. Sci. 2019, 136, 47546. [Google Scholar] [CrossRef]
- Kreye, C.; Bouman, B.A.M.; Castaneda, A.R.; Lampayan, R.M.; Faronilo, J.E.; Lactaoen, A.T.; Fernandez, L. Possible causes of yield failure in tropical aerobic rice. Field Crops Res. 2009, 111, 197–206. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghobashy, M.M. The application of natural polymer-based hydrogels for agriculture. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–356. [Google Scholar]
- Neethu, T.M.; Dubey, P.K.; Kaswala, A.R. Prospects and applications of hydrogel technology in agriculture. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3155–3162. [Google Scholar] [CrossRef]
- Ammar, N.E.B.; Barbouche, M.; Hamzaoui, A.H. Historical view of hydrogel characterization. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 459–479. [Google Scholar]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Dhane, N.S.; Ghorpade, V.S. Citric acid crosslinked carboxymethyl cellulose-based composite hydrogel films for drug delivery. Indian J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Cannazza, G.; Cataldo, A.; de Benedetto, E.; Demitri, C.; Madaghiele, M.; Sannino, A. Experimental assessment of the use of a novel superabsorbent polymer (SAP) for the optimization of Water consumption in agricultural irrigation process. Water. 2014, 6, 2056–2069. [Google Scholar] [CrossRef] [Green Version]
- Nnadi, F.; Brave, C. Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J. Soil Sci. Environ. 2011, 2, 206–211. [Google Scholar]
- Abobatta, W. Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Molden, D. (Ed.) Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Earthscan: London, UK; Colombo, Sri Lanka, 2007. [Google Scholar]
- Rosegrant, M.W.; Ringler, C.; Zhu, T. Water for agriculture: Maintaining food security under growing scarcity. Annu. Rev. Environ. Resour. 2009, 34, 205–222. [Google Scholar] [CrossRef]
- Sulser, T.B.; Ringler, C.; Zhu, T.; Msangi, S.; Bryan, E.; Rosegrant, M. Green and blue water accounting in the Ganges and Nile basins: Implications for food and agricultural policy. J Hydrol. 2010, 384, 276–291. [Google Scholar] [CrossRef]
- Rockstrom, J.; Lannerstad, M.; Falkenmark, M. Assessing the water challenge of a new green revolution in developing countries. Proc. Natl. Acad. Sci. USA 2007, 104, 6253–6260. [Google Scholar] [CrossRef] [Green Version]
- Nhemachena, C.; Nhamo, L.; Matchaya, G.; Nhemachena, C.R.; Muchara, B.; Karuaihe, S.T.; Mpandeli, S. Climate change impacts on water and agriculture sectors in Southern Africa: Threats and opportunities for sustainable development. Water 2020, 12, 2673. [Google Scholar] [CrossRef]
- FAO (Food Agric. Organ. UN). World Agriculture: Towards 2030/2050. Interim Report; FAO: Rome, Italy, 2006. [Google Scholar]
- Stocker, T. (Ed.) Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D. (Eds.) Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Kundzewicz, Z.W.; Mata, L.J.; Arnell, N.W.; Doll, P.; Kabat, P.; Jimenez, B.; Shiklomanov, I. Freshwater resources and their management. In Climate Change 2007: Impacts, Adaptation, and Vulnerability.Working Group II Contribution to the 4th Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 173–210. [Google Scholar]
- Bates, B.; Kundzewicz, Z.; Wu, S. Climate Change and Water; Intergovernmental Panel on Climate Change Secretariat: Geneva, Switzerland, 2008; p. 210. [Google Scholar]
- Arnell, N.W. Climate change and global water resources. Glob. Environ. Change. 1999, 9, S31–S49. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Skrzypczak, D.; Jarzembowski, Ł.; Izydorczyk, G.; Mikula, K.; Hoppe, V.; Mielko, K.A.; Witek-Krowiak, A. Hydrogel Alginate Seed Coating as an Innovative Method for Delivering Nutrients at the Early Stages of Plant Growth. Polymers 2021, 13, 4233. [Google Scholar] [CrossRef]
- Ragab, R.; Prudhomme, C. Climate change and water resources management in arid and semi-arid regions: Prospective and challenges for the 21st century. Biosyst. Eng. 2002, 81, 3–34. [Google Scholar] [CrossRef]
- Abd El-Aziz, G.H.; Ibrahim, A.S.; Fahmy, A.H. Using Environmentally Friendly Hydrogels to Alleviate the Negative Impact of Drought on Plant. Open J. Appl. Sci. 2022, 12, 111–133. [Google Scholar] [CrossRef]
- Liu, Y.X.; Chen, L.; Zhao, Z.G.; Fang, R.C.; Liu, M.J. Design and Synthesis of Bioinspired Multiscale Hydrogels: From Interface to Three-dimensional Network. Acta Polym. Sin. 2018, 9, 1155–1174. [Google Scholar]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciolacu, D.E.; Suflet, D.M. Cellulose-based hydrogels for medical/pharmaceutical applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–439. [Google Scholar]
- Martin, N.; Youssef, G. Dynamic properties of hydrogels and fiber-reinforced hydrogels. J. Mech. Behav. Biomed. Mater. 2018, 85, 194–200. [Google Scholar] [CrossRef]
- Ni, N.; Dumont, M.J. Protein-based hydrogels derived from industrial by-products containing collagen, keratin, zein and soy. Waste Biomass Valori. 2017, 8, 285–300. [Google Scholar] [CrossRef]
- Chen, Y. Properties and development of hydrogels. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–16. [Google Scholar]
- De Kruif, C.K.; Anema, S.G.; Zhu, C.; Havea, P.; Coker, C. Water holding capacity and swelling of casein hydrogels. Food Hydrocoll. 2015, 44, 372–379. [Google Scholar] [CrossRef]
- Sarkar, B.; Basak, B.B.; Sarkar, S.; Mandal, S. Adaptive Soil Management: From Theory to Practices; Springer: Berlin, Germany, 2017. [Google Scholar]
- Wang, W.; Wang, A. Synthesis, swelling behaviors, and slow-release characteristics of a guar gum-g-poly (sodium acrylate)/sodium humate superabsorbent. J. Appl. Polym. Sci. 2009, 112, 2102–2111. [Google Scholar] [CrossRef]
- Koupai, A.J.; Asadkazemi, J. Effects of a hydrophilic polymer on the field performance of an ornamental plant (Cupressus arizonica) under reduced irrigation regimes. Iran. Polym. J. 2006, 15, 715–722. [Google Scholar]
- Akhter, J.; Mahmood, K.; Malik, K.A. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.G.; Yang, P.L.; Luo, Y.P. Porosity change model for watered super absorbent polymer-treated soil. Environ. Earth Sci. 2010, 61, 1197–1205. [Google Scholar] [CrossRef]
- Abd El-Rehim, H.A. Characterization and possible agricultural application of polyacrylamide/sodium alginate crosslinked hydrogels prepared by ionizing radiation. J. Appl. Polym. Sci. 2006, 101, 3572–3580. [Google Scholar] [CrossRef]
- Ekebafe, L.O.; Ogbeifun, D.E.; Okieimen, F.E. Polymer applications in agriculture. Biokemistri 2011, 23, 81–89. [Google Scholar]
- Lenka, S.; Lenka, N.K.; Singh, A.B.; Singh, B.; Raghuwanshi, J. Global warming potential and greenhouse gas emission under different soil nutrient management practices in soybean-wheat system of central India. Environ. Sci. Pollut. Res. 2017, 24, 4603–4612. [Google Scholar] [CrossRef] [Green Version]
- Malla, G.; Bhatia, A.; Pathak, H.; Prasad, S.; Jain, N.; Singh, J. Mitigating nitrous oxide and methane emissions from soil in rice_wheat system of the Indo-Gangetic plain with nitrification and urease inhibitors. Chemosphere 2005, 58, 141–147. [Google Scholar] [CrossRef]
- Bley, H.; Gianello, C.; Santos, L.D.; Selau, L.P.R. Nutrient release, plant nutrition, and potassium leaching from polymer-coated fertilizer. Rev. Bras. Cienc. 2017, 41, e0160142. [Google Scholar] [CrossRef] [Green Version]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 2012, 14, 1294–1308. [Google Scholar] [CrossRef]
- Cui, H.X.; Sun, C.J.; Liu, Q.; Jiang, J.; Gu, W. Applications of nanotechnology in agrochemical formulation, perspectives, challenges and strategies. In Proceedings of the International Conference on Nanoagri, Sao Pedro, Brazil, 20–25 June 2010; pp. 28–33. [Google Scholar]
- Corradini, E.; De Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef]
- Rondeau-Mouro, C.; Defer, D.; Leboeuf, E.; Lahaye, M. Assessment of cell wall porosity in Arabidopsis thaliana by NMR spectroscopy. Int. J. Biol. Macromol. 2008, 42, 83–92. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanbe, F. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano. 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, A.; Aouada, F.A.; Mattoso, L.H.; Ribeiro, C. Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: Evidence of synergistic effects for the slow release of fertilizers. J. Agric. Food Chem. 2013, 61, 7431–7439. [Google Scholar] [CrossRef] [PubMed]
- He, W.A.N.G.; Jing-Jing, L.I.; Hong-liang, W.E.I.; Gang, W.A.N.G.; Hui-Juan, C.H.U.; Jing, Z.H.U. Applied research progress of hydrogels in slow/controlled fertilizer. J. Light Ind. 2017, 32, 43–55. [Google Scholar]
- Feng, C.; Lu, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X. “Smart” fertilizer with temperature- and pH responsive behavior via surface-initiated polymerization for controlled release of nutrients, ACS Sustain. Chem. Eng. 2015, 3, 3157–33166. [Google Scholar]
- Adams, C.; Frantz, J.; Bugbee, B. Macro-and micronutrient-release characteristics of three polymer-coated fertilizers: Theory and measurements. J. Plant Nutr. Soil Sci. 2013, 176, 76–88. [Google Scholar] [CrossRef]
- Meurer, R.A.; Kemper, S.; Knopp, S.; Eichert, T.; Jakob, F.; Goldbach, H.E. Biofunctional microgelbased fertilizers for controlled foliar delivery of nutrients to plants, Angew. Chem. Int. Ed. 2017, 56, 7380–7386. [Google Scholar] [CrossRef]
- Oertli, J.J.; Lunt, O.R. Controlled release of fertilizer minerals by incapsulating membranes: I. Factors influencing the rate of release. Soil Sci. Soc. Am. J. 1962, 26, 579–583. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant. Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Green, J.M.; Beestman, G.B. Recently patented and commercialized formulation and adjuvant technology. Crop Protec. 2007, 26, 320–327. [Google Scholar] [CrossRef]
- Brockwell, J. Studies on seed pelleting as an aid to legume seed inoculation. I. Coating materials, adhesives, and methods of inoculation. Aust. J. Agric. Res. 1962, 13, 638–649. [Google Scholar] [CrossRef]
- Koch, R.; Burkness, E.; Hutchison, W.; Rabaey, T. Efficacy of systemic insecticide seed treatments for protection of early-growthstage snap beans from bean leaf beetle (Coleoptera: Chrysomelidae) foliar feeding. Crop. Prot. 2005, 24, 734–742. [Google Scholar] [CrossRef]
- Nel, L. The Role of Seed Coating in the Establishment and Growth of Medicago sativa L. Cultivars. Master’s Thesis, Faculty of Natural & Agricultural Sciences, University of Pretoria, Pretoria, South Africa, 2013; p. 156. [Google Scholar]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Ravier, I.; Haouisee, E.; Clément, M.; Seux, R.; Briand, O. Field experiments for the evaluation of pesticide spray-drift on arable crops. Pest. Manag. Sci. 2005, 61, 728–736. [Google Scholar] [CrossRef]
- Chevillard, A.; Angellier-Coussy, H.; Guillard, V.; Gontard, N.; Gastaldi, E. Controlling pesticide release via structuring agropolymer and nanoclays based materials. J. Hazard. Mater. 2012, 205–206, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Nuzzaci, M.; Logozzo, G.; Gioia, T.; Camele, I. Biological investigations on the role of hydrogel formulations containing bioactive natural agents against some common phytopathogens of Phaseolus vulgaris L. and seed germination. J. Biol. Res. 2020, 3, 114–122. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.; Gupta, A. In vitro release dynamics of thiram fungicide from starch and poly(methacrylic acid)-based hydrogels. J. Hazard. Mater. 2008, 154, 278–286. [Google Scholar] [CrossRef]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerre’-Tugaye’, M.T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Terry, L.A.; Joyce, D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Technol. 2004, 3, 1–13. [Google Scholar] [CrossRef]
- Pospieszny, H. Antiviroid activity of chitosan. Crop Prot. 1997, 16, 105–106. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of watersoluble N alkylated chitosan. Carbohydr. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Raeta, R.; Vannacci, G. Seeds encapsulation in calcium alginate pellets. Seed Sci. Technol. 2004, 32, 649–661. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Abdallah, A.M. Influence of Hydrogel Type and Concentration, and Water Application Rate on some Hydraulic Properties of a Sandy Soil. Alex. Sci. Exch. J. 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Ehsanfar, S.; Modarres-Sanavy, S.A.M. Crop protection by seed coating. Commun. Agric. Appl. Boil. Sci. 2005, 70, 225–229. [Google Scholar]
- Halmer, P. Method to improve seed performance in the field. In Handbook of Seed Physiology-Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; The Haworth Reference Press: Binghamton, NY, USA, 2004. [Google Scholar]
- Gherardi, M.J.; Rengel, Z. Genotypes of lucerne (Medicago sativa L.) show differential tolerance to manganese deficiency and toxicity when grown in bauxite residue sand. Plant. Soil. 2003, 249, 287–296. [Google Scholar] [CrossRef]
- Wu, N.; Huang, H.; Zhang, S.; Zhu, Y.-G.; Christie, P.; Zhang, Y. Phenanthrene uptake by Medicago sativa L. under the influence of an arbuscular mycorrhizal fungus. Environ. Pollut. 2009, 157, 1613–1618. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Foolad, M. Pre-sowing seed treatment-a shotgun approach to improve germination; plant growth; and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Su, L.-Q.; Li, J.-G.; Xue, H.; Wang, X.-F. Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought. J. Zhejiang Univ. Sci. B 2017, 18, 696–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, V.; Ambrose, K.R.P. Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage. J. Appl. Polym. Sci. 2020, 137, 48523. [Google Scholar] [CrossRef]
- Isabel, J.B.; Devi, P.R.; Balamurugan, A.; Hemananthan, E.; Kumar, V.S.; Suriya, S.B. Encapsulation of Pseudomonas Fluorescens for a Slow Release Biofertilizer. ICAMIB-2019; Sathyabama University: Chennai, India, 2019. [Google Scholar]
- Dungait, J.A.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Change Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Greenland, D.J.; Oades, J.M. Saccharides, Soil Components; Springer: Berlin, Germany, 1975; pp. 213–261. [Google Scholar]
- Wallace, A.; Garn, A.W. Effects of soil conditioners on emergence and growth of tomato, cotton, and lettuce seedlings. Soil Sci. 1986, 141, 313–316. [Google Scholar] [CrossRef]
- Standing, D.; Killham, K. The Soil Environment, Modern Soil Microbiology, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Abingdon, UK, 2006; pp. 1–22. [Google Scholar]
- O’Brien, A.T. Supporting soil fungi to rebuild soils in agriculture. Philos. Act. Nat. 2013, 10, 24–35. [Google Scholar]
- Salyers, A.A.; West, S.E.; Vercellotti, J.R.; Wilkins, T.D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl. Environ. Microbiol. 1977, 34, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.R. Lecture on Soil Physics, Personal Collection of K. Islam; The Ohio State University School of Environment and Natural Resources: Columbus, OH, USA, 2008. [Google Scholar]
- Pattanaaik, S.K.; Singh, B.; Wangchu, L.; Debnath, P.; Hazarika, B.N.; Pandey, A.K. Effect of hydrogel on water and nutrient management of Citrus limon. Int. J. Agric. Innov. Res. 2015, 3, 1656–1659. [Google Scholar]
- Pieve, L.M.; Guimares, R.J.; Assis, G.A.; Amato, G.A.S.; Correa, J.M. Use of water retention polymers during implementation of coffee plantations. Coffee Sci. 2013, 8, 314–323. [Google Scholar]
- Karimi, A.; Noshadi, M.; Ahmadzadeh, M. Effects of super absorbent polymer (igeta) on crop, soil water and irrigation interval. JWSS Isfahan Univ. Technol. 2009, 12, 403–414. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).