Gamma Radiation (60Co) Induces Mutation during In Vitro Multiplication of Vanilla (Vanilla planifolia Jacks. ex Andrews)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Establishment and Multiplication
2.3. Gamma Irradiation of In Vitro Explants
2.4. Data and Molecular Analysis
2.5. DNA Extraction and ISSR Analysis
3. Results
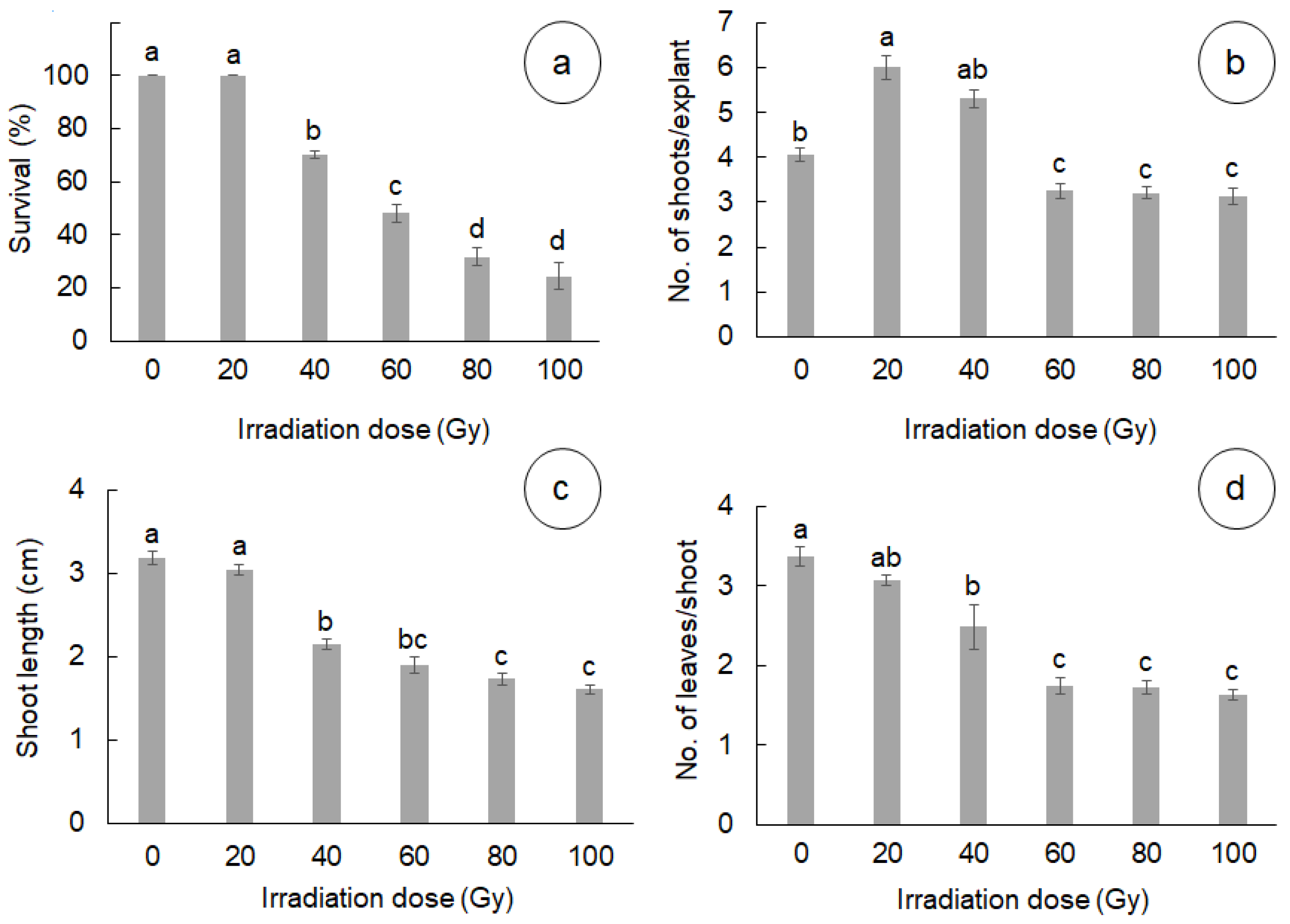
3.1. Effect of Gamma Radiation on In Vitro Survival and Development
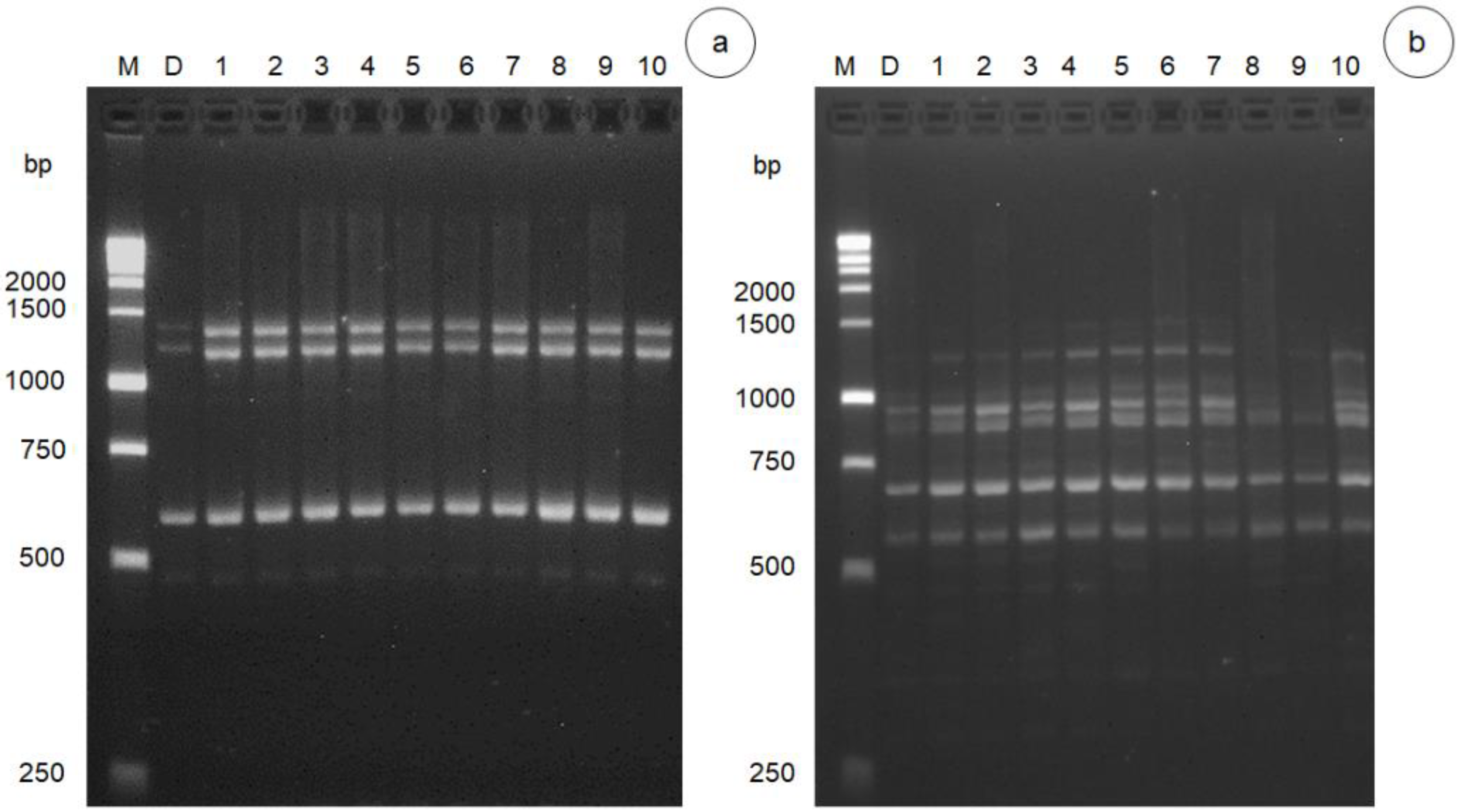
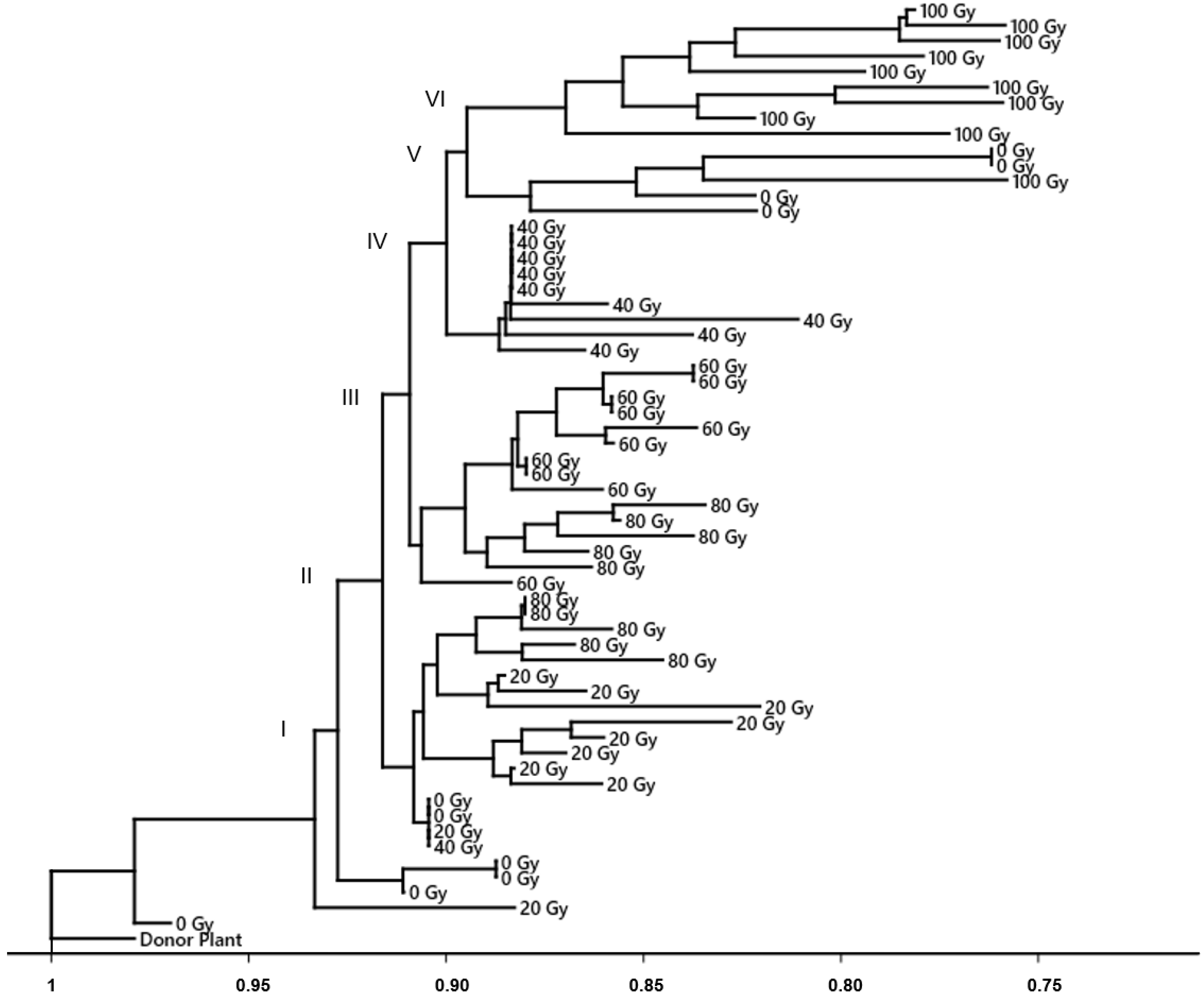
3.2. DNA Polymorphism of Gamma Radiation on Somaclonal Variation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gantait, S.; Kundu, S. In vitro biotechnological approaches on Vanilla planifolia Andrews: Advancements and opportunities. Acta Physiol. Plant. 2017, 39, 196. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Norma Oficial Mexicana (NOM-059-ECOL-2001) de Protección Especial de Especies Nativas de México de Flora y Fauna Silvestres; Diario Oficial de la Federación: Mexico City, Mexico, 2010; pp. 2–56.
- Wan Abdullah, W.M.A.N.; Low, L.; Mumaiyizah, S.B.; Chai, Q.Y.; Loh, J.Y.; Ong-Abdullah, J.; Lai, K.S. Effect of lignosulphonates on Vanilla planifolia shoot multiplication, regeneration and metabolism. Acta Physiol. Plant. 2020, 42, 107. [Google Scholar] [CrossRef]
- Li, J.; Demesyeux, L.; Brym, M.; Chambers, A.H. Development of species-specific molecular markers in Vanilla for seedling selection of hybrids. Mol. Biol. Rep. 2020, 47, 1905–1920. [Google Scholar] [CrossRef]
- Hu, Y.; Resende, M.F.R.; Bombarely, A.; Brym, M.; Bassil, E.; Chambers, A.H. Genomics-based diversity analysis of Vanilla species using a Vanilla planifolia draft genome and Genotyping-By-Sequencing. Sci. Rep. 2019, 9, 3416. [Google Scholar] [CrossRef]
- Li, F.; Shimizu, A.; Nishio, T.; Tsutsumi, N.; Kato, H. Comparison and Characterization of Mutations Induced by Gamma-Ray and Carbon-Ion Irradiation in Rice (Oryza sativa L.) Using Whole-Genome Resequencing. G3 Genes Genom. Genet. 2019, 9, 3743–3751. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Thakur, M.; Tomar, M. In vitro selection of gamma irradiated shoots of ginger (Zingiber officinale Rosc.) against Fusarium oxysporum f.sp. zingiberi and molecular analysis of the resistant plants. Plant Cell Tissue Organ Cult. 2020, 143, 319–330. [Google Scholar] [CrossRef]
- IAEA. Organismo Internacional de Energía Atómica. Inducción de Mutaciones. Available online: https://www.iaea.org/es/temas/induccion-de-mutaciones (accessed on 14 June 2021).
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Waugh, R.; Leader, D.J.; Mc Callum, N.; Cadwell, D. Harvesting the potential of induced biological diversity. Trends Plant Sci. 2006, 11, 71–79. [Google Scholar] [CrossRef]
- Ghani, M.; Sharma, S.K. Induction of powdery mildew resistance in gerbera (Gerbera jamesonii) through gamma irradiation. Physiol. Mol. Biol. Plants 2019, 25, 159–166. [Google Scholar] [CrossRef]
- Salava, H.; Thula, S.; Mohan, V.; Kumar, R.; Maghuly, F. Application of Genome Editing in Tomato Breeding: Mechanisms, Advances, and Prospects. Int. J. Mol. Sci. 2021, 22, 682. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Spinoso-Castillo, J.L.; Escamilla-Prado, E.; Aguilar-Rincón, C.H.; Morales-Ramos, V.; García-de los Santos, G.; Corona-Torres, T. Physiological response of seeds of three coffee varieties to gamma rays (60Co). Rev. Chapingo Ser. Hortic. 2021, 27, 101–112. [Google Scholar] [CrossRef]
- Hernández-Muñoz, S.; Pedraza-Santos, M.E.; López, P.A.; De La Cruz-Torres, E.; Martínez-Palacios, A.; Fernández-Pavía, S.P.; Chávez-Bárcenas, A.T. Estimulación de la germinación y desarrollo in vitro de Laelia autumnalis con rayos gamma. Rev. Fitotec. Mex. 2017, 40, 271–283. [Google Scholar] [CrossRef]
- Abdelnour-Esquivel, A.; Pérez, J.; Rojas, M.; Vargas, W.; Gatica-Arias, A. Use of gamma radiation to induce mutations in rice (Oryza sativa L.) and the selection of lines with tolerance to salinity and drought. Vitr. Cell. Dev. Biol. Plant 2020, 56, 88–97. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Osama, E.; Manal, E.; Samah, A.; Salah, G.; Hazem, K.M.; Nabil, E. Impact of gamma irradiation pretreatment on biochemical and molecular responses of potato growing under salt stress. Chem. Biol. Technol. Agric. 2021, 8, 35. [Google Scholar] [CrossRef]
- Özge, Ç.; Alp, A.; Sinan, M.; Çimen, A. Comparison of tolerance related proteomic profiles of two drought tolerant tomato mutants improved by gamma radiation. J. Biotechnol. 2021, 330, 35–44. [Google Scholar] [CrossRef]
- Pardo, A.; Hernández, A.; Méndez, N.; Alvarado, G. Análisis genético, mediante marcadores RAPD, de microbulbos de ajo conservados e irradiados in vitro. Bioagro 2015, 27, 143–150. [Google Scholar]
- Muñoz-Miranda, L.A.; Rodríguez-Sahagún, A.; Acevedo, H.G.J.; Cruz-Martínez, V.O.; Torres-Morán, M.I.; Lépiz-Ildefonso, R.; Aarland, R.C.; Castellanos-Hernández, O.A. Evaluation of somaclonal and ethyl methane sulfonate-induced genetic variation of Mexican oregano (Lippia graveolens HBK). Agronomy 2019, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Ranghoo-Sanmukhiya, V.M. Somaclonal Variation and Methods Used for its Detection. In Propagation and Genetic Manipulation of Plants; Siddique, I., Ed.; Springer Nature: Singapore, 2021. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome Fingerprinting by Simple Sequence Repeat (SSR)-Anchores Polimerase Chain Reaction Amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Khadivi-Khub, A. Somaclonal variation in callus samples of Plantago major using inter-simple sequence repeat marker. Caryologia 2015, 68, 19–24. [Google Scholar] [CrossRef]
- Martínez-Estrada, E.; Caamal-Velázquez, J.H.; Salinas-Ruiz, J.; Bello-Bello, J.J. Assessment of somaclonal variation during sugarcane micropropagation in temporary immersion bioreactors by intersimple sequence repeat (ISSR) markers. Vitr. Cell. Dev. Biol. Plant 2017, 53, 553–560. [Google Scholar] [CrossRef]
- Kritskaya, T.A.; Kashin, A.S.; Kasatkin, M.Y. Micropropagation and Somaclonal Variation of Tulipa suaveolens (Liliaceae) in vitro. Russ. J. Mar. Biol. 2019, 50, 209–215. [Google Scholar] [CrossRef]
- Vitamvas, J.; Viehmannova, I.; Cepkova, P.H.; Mrhalova, H.; Eliasova, K. Assessment of somaclonal variation in indirect morphogenesis-derived plants of Arracacia xanthorrhiza. Pesqui. Agropecu. Bras. 2019, 54, e00301. [Google Scholar] [CrossRef] [Green Version]
- Ulvrova, T.; Vitamvas, J.; Cepkova, P.H.; Eliasova, K.; Janovska, D.; Bazant, V.; Viehmannova, I. Micropropagation of an ornamental shrub Disanthus cercidifolius Maxim. and assessment of genetic fidelity of regenerants using ISSR and flow cytometry. Plant Cell Tissue Organ Cult. 2021, 144, 555–566. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Spinoso-Castillo, J.L.; Arano-Avalos, S.; Martínez-Estrada, E.; Arellano-García, M.E.; Pestryakov, A.; Toledano-Magaña, Y.; García-Ramos, J.C.; Bogdanchikova, N. Cytotoxic, genotoxic, and polymorphism effects on Vanilla planifolia Jacks ex Andrews after long-term exposure to Argovit® silver nanoparticles. Nanomaterials 2018, 8, 754. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G.; Favián-Vega, E.; Telxeira da Silva, J.A.; Leyva-Ovalle, O.R.; Murguía-González, J. Morphogenetic stability of variegated Vanilla planifolia Jacks. plants micropropagated in a temporary immersion system (TIB®). Rend. Lincei Sci. Fis. Nat. 2019, 30, 603–609. [Google Scholar] [CrossRef]
- Pastelín-Solano, M.C.; Salinas, R.J.; González, A.M.T.; Castañeda, C.O.; Galindo, T.M.E.; Bello-Bello, J.J. Evaluation of in vitro shoot multiplication and ISSR marker based assessment of somaclonal variants at different subcultures of vanilla (Vanilla planifolia Jacks). Physiol. Mol. Biol. Plants 2019, 25, 561–567. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Stewart, C.N.; Via, L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. Biotechniques 1993, 14, 748–750. [Google Scholar] [PubMed]
- Hernández-Muñoz, S.; Pedraza-Santos, M.E.; López, P.A.; Gómez-Sanabria, J.M.; Morales-García, J.L. Mutagenesis in the improvement of ornamental plants. Rev. Chapingo Ser. Hortic. 2019, 25, 151–167. [Google Scholar] [CrossRef]
- Chahal, G.S.; Gosal, S.S. Principles and Procedures of Plant Breeding; Alpha Science International Ltd.: Oxford, UK, 2002; pp. 399–412. [Google Scholar]
- Magdy, A.M.; Fahmy, E.M.; Abd EL-Rahman, M.A.A.; Awad, G. Improvement of 6-gingerol production in ginger rhizomes (Zingiber officinale Roscoe) plants by mutation breeding using gamma irradiation. Appl. Radiat. Isot. 2020, 162, 109193. [Google Scholar] [CrossRef] [PubMed]
- Hasbullah, N.A.; Taha, R.M.; Saleh, A.; Mahmad, N. Irradiation effect on in vitro organogenesis, callus growth and plantlet development of Gerbera jamesonii. Hortic. Bras. 2012, 30, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J. Hormesis: Path and progression to significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef] [Green Version]
- Jalal, A.; Oliveira, J.J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Oliveira, N.M.; de Medeiros, A.D.; de Lima Nogueira, M.; Arthur, V.; de Araújo Mastrangelo, T.; da Silva, C.B. Hormetic effects of low-dose gamma rays in soybean seeds and seedlings: A detection technique using optical sensors. Comput. Electron. Agric. 2021, 187, 106251. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Yang, G.; Yuan, G.; Ma, Y.; Zhang, T. Mechanism of early germination inhibition of fresh walnuts (Juglans regia) with gamma radiation uncovered by transcriptomic profiling of embryos during storage. Postharvest Biol. Technol. 2021, 172, 111380. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E.J. Nanoparticle exposure and hormetic dose–responses: An update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef] [Green Version]
- Beyaz, R.; Kahramanogullari, C.T.; Yildiz, C.; Darcin, E.S.; Yildiz, M. The effect of gamma radiation on seed germination and seedling growth of Lathyrus chrysanthus Boiss. under in vitro conditions. J. Environ. Radioact. 2016, 162, 129–133. [Google Scholar] [CrossRef]
- Kapare, V.; Satdive, R.; Fulzele, D.P.; Malpathak, N. Impact of Gamma Irradiation Induced Variation in Cell Growth and Phytoecdysteroid Production in Sesuvium portulacastrum. J. Plant Growth Regul. 2017, 36, 919–930. [Google Scholar] [CrossRef]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Al Mamun, M.; Halidu, J. DNA fingerprinting, fixation-index (Fst), and admixture mapping of selected Bambara groundnut (Vigna subterranea [L.] Verdc.) accessions using ISSR markers system. Sci. Rep. 2021, 11, 14527. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia Jacks. Plant Cell Tissue Organ Cult. 2015, 123, 657–664. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Venkatachalam, L.; Bhagyalakshmi, N. Genetic fidelity of long-term micropropagated shoot cultures of vanilla (Vanilla planifolia Andrews) as assessed by molecular markers. Biotechnol. J. 2007, 2, 1007–1013. [Google Scholar] [CrossRef]
- Gantait, S.; Mandal, N.; Bhattacharyya, S.; Das, P.K.; Nandy, S. Mass multiplication of Vanilla planifolia with pure genetic identity confirmed by ISSR. Int J. Plant Dev. Biol 2009, 3, 18–23. [Google Scholar]
- Manokari, M.; Priyadharshini, S.; Jogam, P.; Dey, A.; Shekhawat, M.S. Meta-topolin and liquid medium mediated enhanced micropropagation via ex vitro rooting in Vanilla planifolia Jacks. ex Andrews. Plant Cell Tissue Organ Cult. 2021, 146, 69–82. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Variación somaclonal en plantas: Causas y métodos de detección. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Yang, G.; Luo, W.; Zhang, J.; Yan, X.; Du, Y.; Zhou, L.; Li, W.; Wand, H.; Chen, Z.; Guo, T. Genome-wide comparisons of mutations induced by carbon-ion beam and gamma-rays irradiation in rice via resequencing multiple mutants. Front. Plant Sci. 2019, 10, 1514. [Google Scholar] [CrossRef] [Green Version]
- Holme, I.B.; Gregersen, P.L.; Brinch-Pedersen, H. Induced genetic variation in crop plants by random or targeted mutagenesis: Convergence and differences. Front. Plant Sci. 2019, 10, 1468. [Google Scholar] [CrossRef]
- Jain, S.M. Tissue culture-derived variation in crop improvement. Euphytica 2001, 118, 153–166. [Google Scholar] [CrossRef]
- Nair, R.R.; Ravindran, P.N. Somatic association of chromosomes and other mitotic abnormalities in Vanilla planifolia (Andrews). Caryologia 1994, 47, 65–73. [Google Scholar] [CrossRef]
- Bory, S.; Catrice, O.; Brown, S.; Leitch, I.J.; Gigant, R.; Chiroleu, F.; Grisoni, M.; Duval, M.F.; Besse, P. Natural polyploidy in Vanilla planifolia (Orchidaceae). Genome 2008, 51, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Predieri, S. Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Organ Cult. 2001, 64, 185–210. [Google Scholar] [CrossRef]
- Villanueva-Viramontes, S.; Hernández-Apolinar, M.; Fernández-Concha, G.C.; Dorantes-Euán, A.; Dzib, G.R.; Martínez-Castillo, J. Wild Vanilla planifolia and its relatives in the Mexican Yucatan Peninsula: Systematic analyses with ISSR and ITS. Bot. Sci. 2017, 95, 169–187. [Google Scholar] [CrossRef] [Green Version]

| Primers | Sequence (5′–3′) | Annealing Temperature (°C) | Bands | Range (bp) | Polymorphism (%) |
|---|---|---|---|---|---|
| UBC-809 | AGAGAGAGAGAGAGAGG | 45 | 7 | 250–1500 | 4 |
| T06 | AGAGAGAGAGAGAGAGT | 50 | 4 | 400–750 | 3 |
| UBC-840 | GAGAGAGAGAGAGAGAYT | 52 | 6 | 500–1500 | 28.7 |
| UBC-836 | AGAGAGAGAGAGAGAGYA | 50 | 6 | 400–1500 | 34.7 |
| UBC-812 | GAGAGAGAGAGAGAGAA | 50 | 4 | 500–1500 | 0 |
| UBC-825 | ACACACACACACACACT | 50 | 6 | 500–2000 | 14.9 |
| UBC-808 | AGAGAGAGAGAGAGAGC | 53 | 9 | 400–1000 | 42.6 |
| T05 | CGTTGTGTGTGTGTGTGT | 53 | 1 | 750 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Fuentes, M.K.; Gómez-Merino, F.C.; Cruz-Izquierdo, S.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Gamma Radiation (60Co) Induces Mutation during In Vitro Multiplication of Vanilla (Vanilla planifolia Jacks. ex Andrews). Horticulturae 2022, 8, 503. https://doi.org/10.3390/horticulturae8060503
Serrano-Fuentes MK, Gómez-Merino FC, Cruz-Izquierdo S, Spinoso-Castillo JL, Bello-Bello JJ. Gamma Radiation (60Co) Induces Mutation during In Vitro Multiplication of Vanilla (Vanilla planifolia Jacks. ex Andrews). Horticulturae. 2022; 8(6):503. https://doi.org/10.3390/horticulturae8060503
Chicago/Turabian StyleSerrano-Fuentes, María Karen, Fernando Carlos Gómez-Merino, Serafín Cruz-Izquierdo, José Luis Spinoso-Castillo, and Jericó Jabín Bello-Bello. 2022. "Gamma Radiation (60Co) Induces Mutation during In Vitro Multiplication of Vanilla (Vanilla planifolia Jacks. ex Andrews)" Horticulturae 8, no. 6: 503. https://doi.org/10.3390/horticulturae8060503
APA StyleSerrano-Fuentes, M. K., Gómez-Merino, F. C., Cruz-Izquierdo, S., Spinoso-Castillo, J. L., & Bello-Bello, J. J. (2022). Gamma Radiation (60Co) Induces Mutation during In Vitro Multiplication of Vanilla (Vanilla planifolia Jacks. ex Andrews). Horticulturae, 8(6), 503. https://doi.org/10.3390/horticulturae8060503








