Indirect Quantitative Analysis of Biochemical Parameters in Banana Using Spectral Reflectance Indices Combined with Machine Learning Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Physical Parameters
2.2.1. Respiration Rate
2.2.2. Firmness
2.3. Chemical Parameters
2.3.1. Determination of Chlorophyll a & b
2.3.2. Total Soluble Solids (TSS)
2.4. Spectral Reflectance Measurements
2.5. Selection of SRIs of Banana Fruits
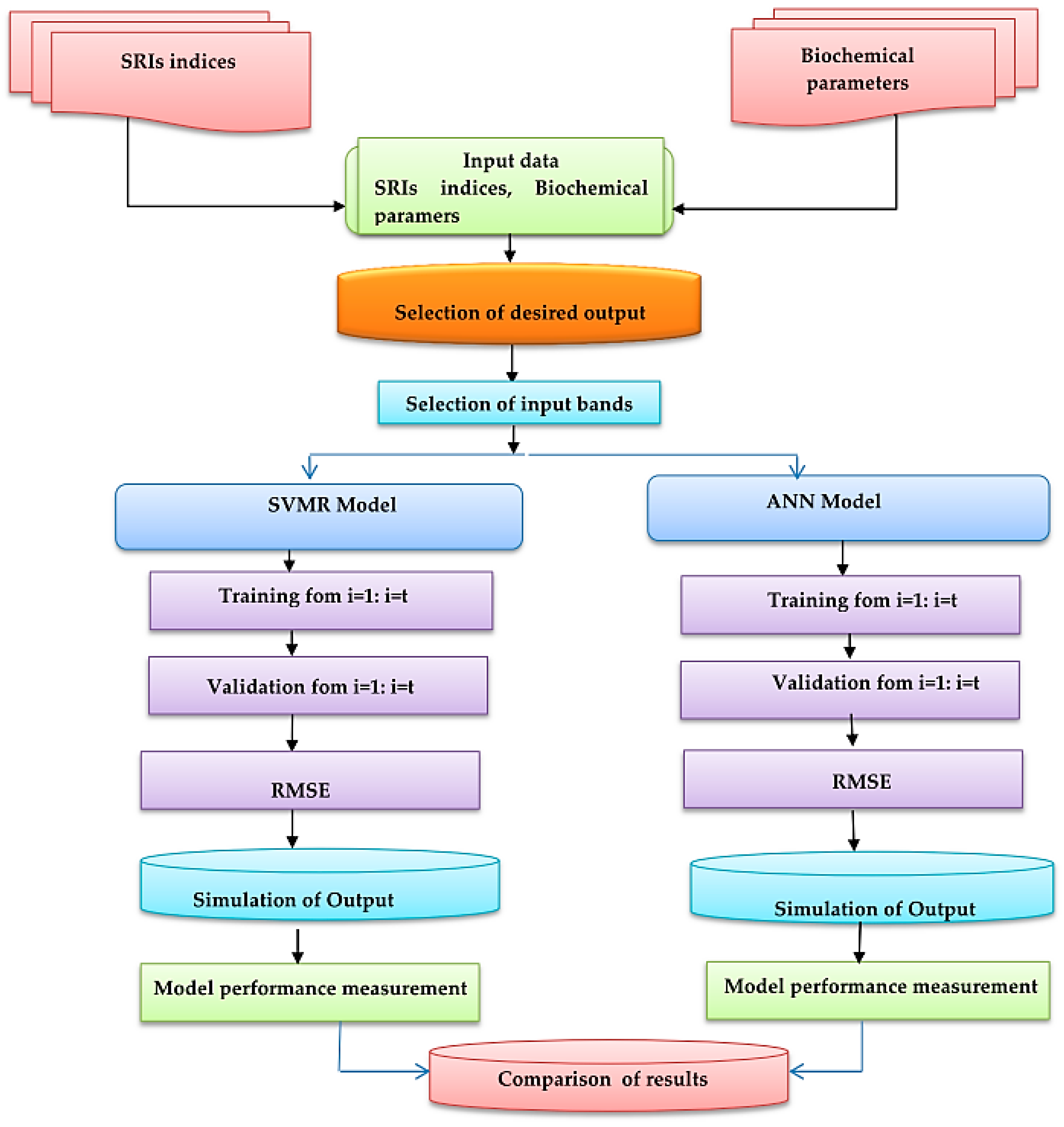
2.6. Back-Propagation Neural Network (BPNN)
2.7. SVMR Model
2.8. Model Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Variation of Biochemical Parameters under Different Ripening Degrees and Correlation Analysis for Banana Fruits
3.2. Variation of SRIs at Different Ripening Degrees for Banana Fruits
3.3. Evaluation of Spectral Reflectance Indices (SRIs) to Assess the Biochemical Parameters
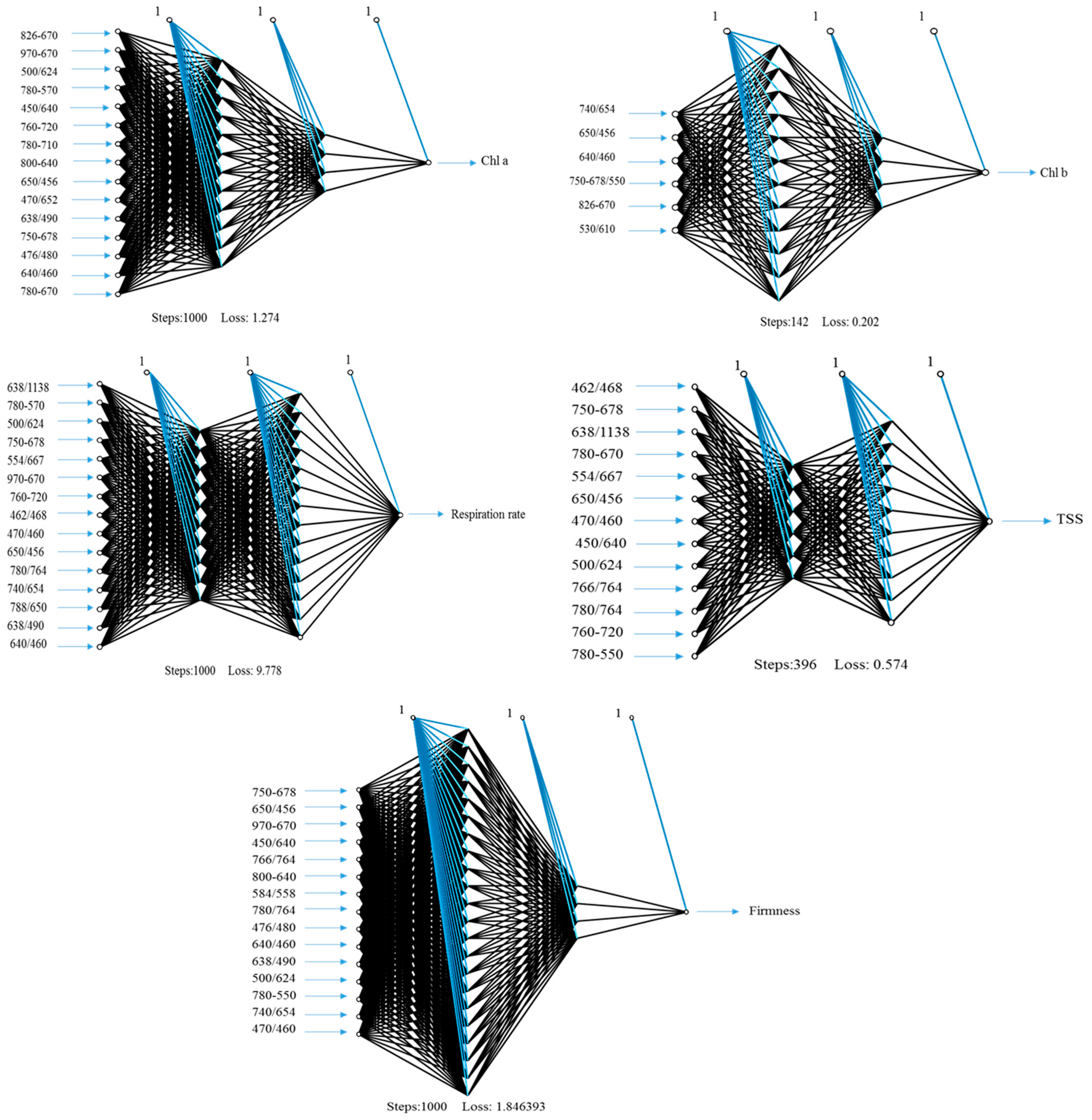
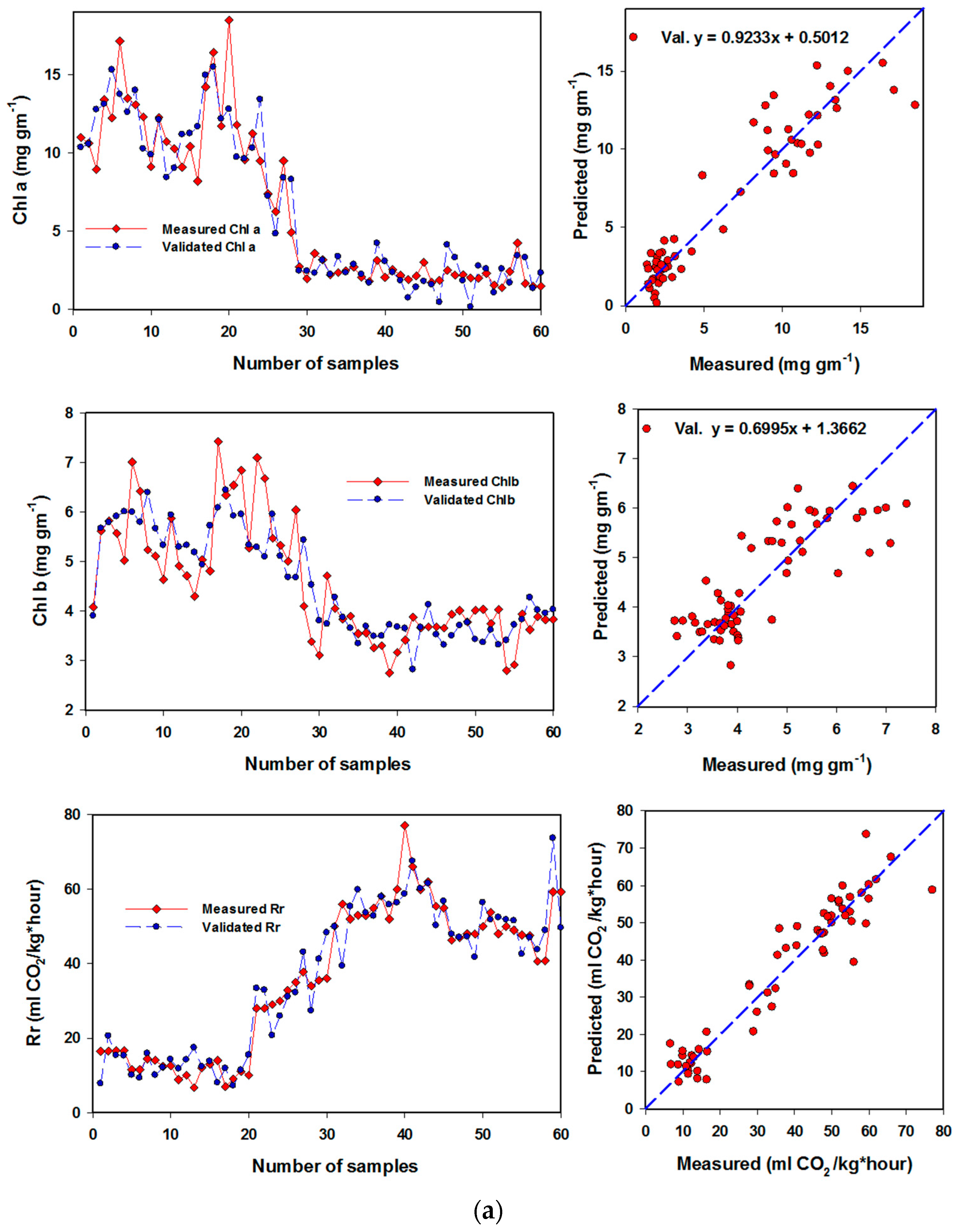
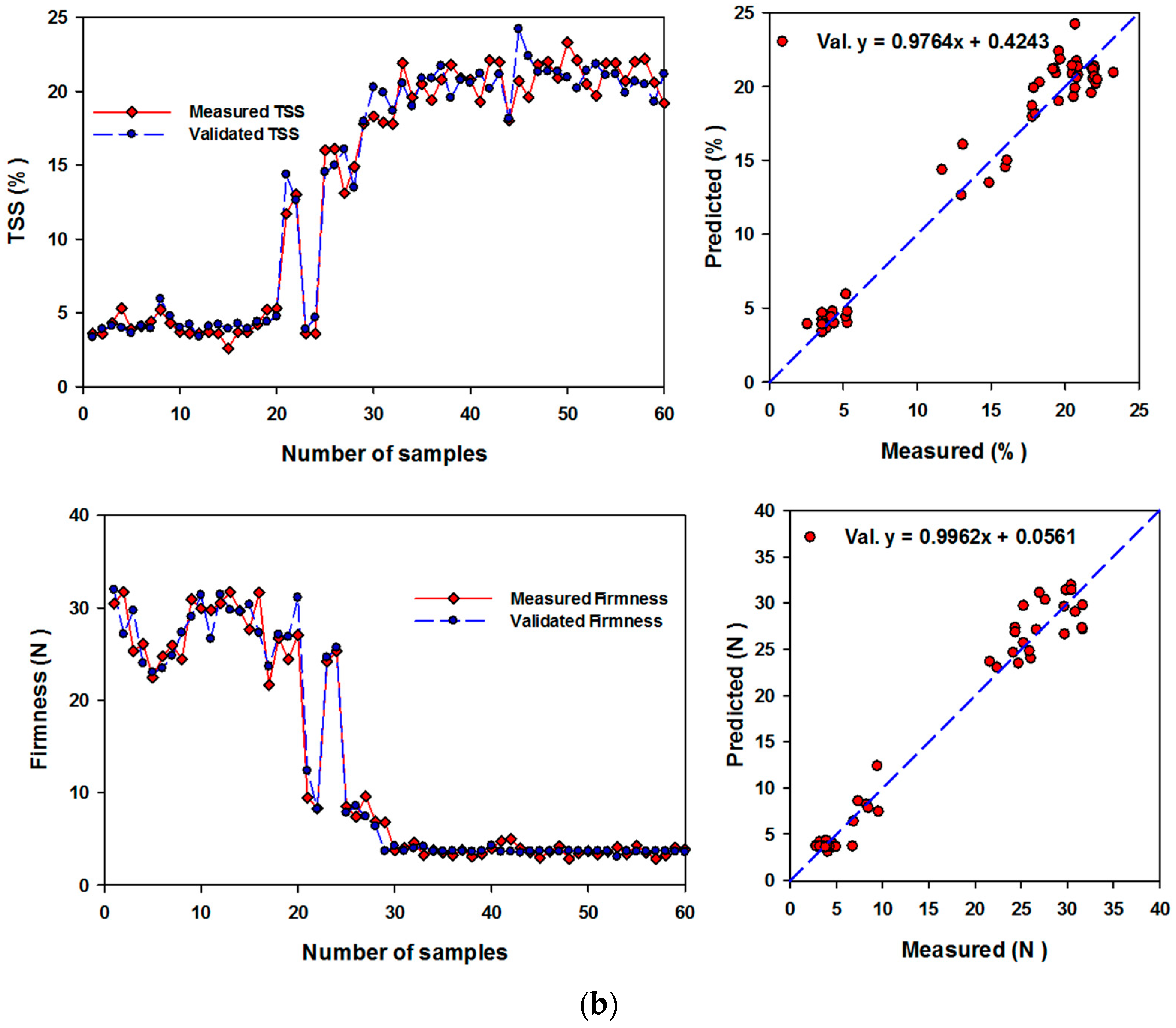
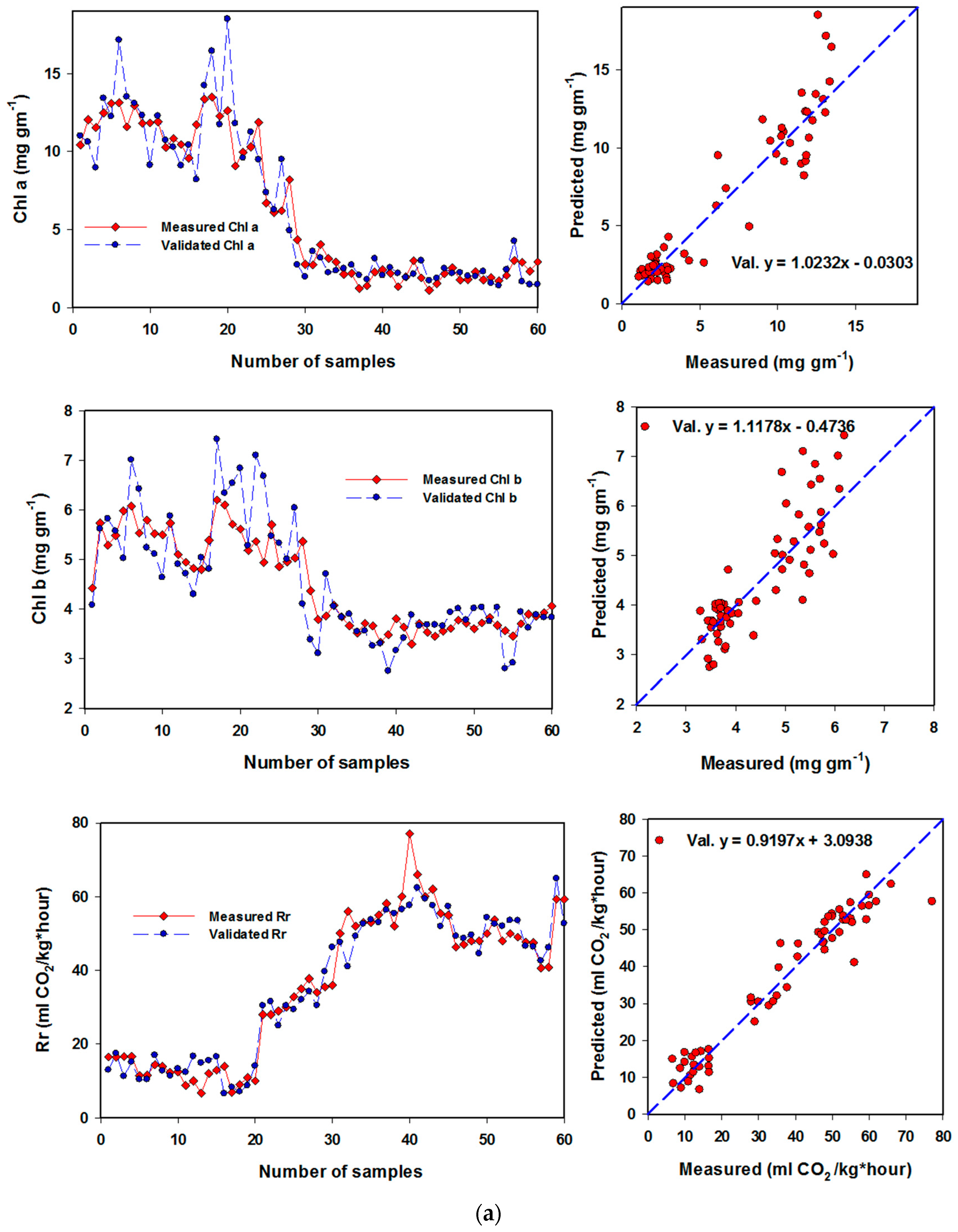
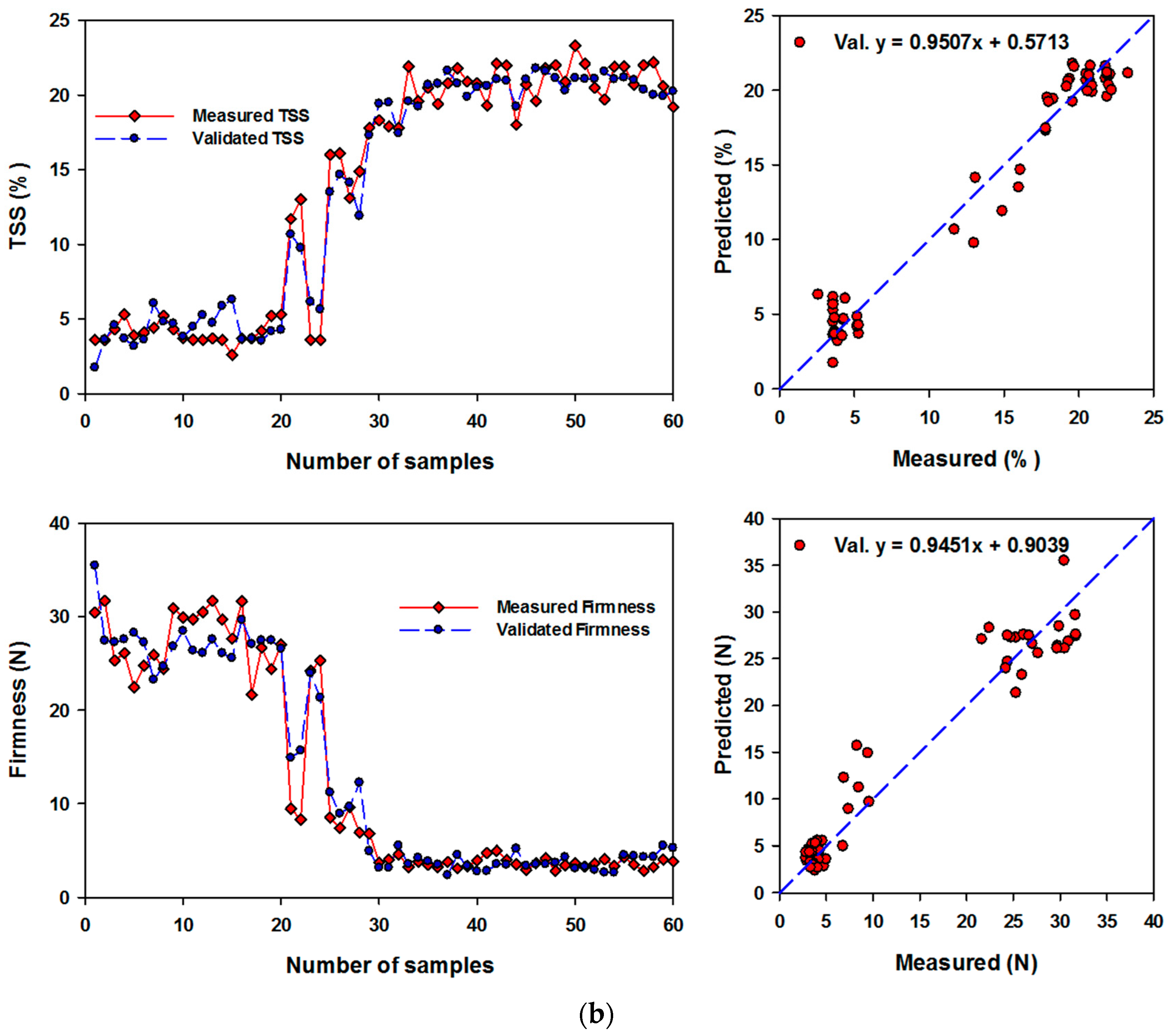
3.4. Performance of Artificial Neural Networks and Support Vector Machine Regression Based on SRIs to Assess Biochemical Parameters of Banana Fruits
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Food and Agricultural Organization of the United Nations. Available online: http://faostat.fao.org (accessed on 30 March 2002).
- Egyptian Ministry of Agriculture and Land Reclamation. Bulletin of the Agricultural Statistics Part (2); Summer & Nile Crops: Cairo, Egypt, 2019.
- Zhu, X.; Luo, J.; Li, Q.; Li, J.; Liu, T.; Wang, R.; Chen, W.; Li, X. Low temperature storage reduces aroma-related volatiles production during shelf-life of banana fruit mainly by regulating key genes involved in volatile biosynthetic pathways. Postharvest Biol. Technol. 2018, 146, 68–78. [Google Scholar]
- Moreno, J.L.; Tran, T.; Cantero-Tubilla, B.; López-López, K.; Lavalle, L.A.B.L.; Dufour, D. Physicochemical and physiological changes during the ripening of Banana ( Musaceae) fruit grown in Colombia. Int. J. Food Sci. Technol. 2021, 56, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Maduwanthi, S.D.T.; Marapana, R. Biochemical changes during ripening of banana: A review. Int. J. Food Sci. Nutr. 2017, 2, 166–169. [Google Scholar]
- Ahmed, Z.F.R.; Taha, E.M.A.; Abdelkareem, N.A.A.; Mohamed, W.M. Postharvest properties of unripe bananas and the potential of producing economic nutritious. Int. J. Fruit Sci. Prod. 2020, 20, 995–1014. [Google Scholar] [CrossRef]
- Pissard, A.; Baeten, V.; Dardenne, P.; Dupont, P.; Lateur, M. Use of NIR spectroscopy on fresh apples to determine the phenolic compounds and dry matter content in peel and flesh. Biotechnol. Agron. Soc. Environ. 2018, 22, 3–12. [Google Scholar] [CrossRef]
- Elsayed, S.; Galal, H.; Allam, A.P.; Schmidhalter, U. Passive reflectance sensing and digital image analysis for assessing quality parameters of mango fruits. Sci. Hortic. 2016, 212, 136–147. [Google Scholar] [CrossRef]
- Tamburini, E.; Costa, S.; Rugiero, I.; Pedrini, P.; Marchetti, M.G. Quantification of lycopene, β-carotene, and total soluble solids in intact red-flesh watermelon (Citrullus lanatus) using on-line near-infrared spectroscopy. Sensors 2017, 17, 746. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit quality evaluation using spectroscopy technology: A Review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, S.; El-Gozayer, K.; Allam, A.; Schmidhalter, U. Passive reflectance sensing using regression and multivariate analysis to estimate biochemical parameters of different fruits kinds. Sci. Hortic. 2019, 243, 21–33. [Google Scholar] [CrossRef]
- Pourdarbani, R.; Sabzi, S.; Hernández-Hernández, M.; Hernández-Hernández, J.L.; Gallardo-Bernal, I.; Herrera-Miranda, I. Non-destructive estimation of total chlorophyll content of apple fruit based on color feature, spectral data and the most effective wavelengths using hybrid artificial neural network-imperialist competitive algorithm. Plants 2020, 9, 1547. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, R.; Hao, Y.; Sun, X.; Ouyang, A. Improvement of near-infrared spectral calibration models for brix prediction in ‘gannan’ navel oranges by a portable near-infrared device. Food Bioprocess. Technol. 2012, 5, 1106–1112. [Google Scholar] [CrossRef]
- Nagy, A.; Riczu, P.; Tamás, J. Spectral evaluation of apple fruit ripening and pigment content alteration. Sci. Hortic. 2016, 201, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lv, W.; Zhao, R.; Guo, H.; Liu, J.; Han, D. Non-destructive assessment of quality parameters in ‘Foriar’ plums during low temperature storage using visible/near infrared spectroscopy. Food Control. 2017, 73, 1334–1341. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Narsaiah, K.; Gupta, M.; Bhardwaj, R.; Singh, A.K. Non-destructive prediction of sweetness of intact mango using near infrared spectroscopy. Sci. Hortic. 2012, 138, 171–175. [Google Scholar] [CrossRef]
- Sirisomboon, P. NIR Spectroscopy for Quality Evaluation of Fruits and Vegetable. Mater. Today Proc. 2018, 5, 22481–22486. [Google Scholar] [CrossRef]
- McClure, W.F. Near-infrared spectroscopy—the giant is running strong. Anal. Chem. 1994, 66, 43–53. [Google Scholar] [CrossRef]
- Acharya, U.K.; Subedi, P.P.; Walsh, K.B.; McGlasson, W.B. Estimation of fruit maturation and ripening using spectral indices. Acta Hortic. 2016, 1119, 265–272. [Google Scholar] [CrossRef]
- Rutkowski, K.P.; Michalczuk, B.; Konopacki, P. Nondestructive determination of ‘golden delicious’ apple quality and harvest maturity. J. Fruit Ornam. Plant. Res. 2008, 16, 39–52. [Google Scholar]
- Zude, M.; Herold, B.; Roger, J.M.; Bellon-Maurel, V.; Landahl, S. Non-destructive tests on the prediction of apple fruit flesh firmness and soluble solids content on tree and in shelf life. J. Food Eng. 2006, 77, 254–260. [Google Scholar] [CrossRef]
- Slaughter, D.C.; Crisosto, C.H.; Tiwari, G. Nondestructive determination of flesh color in clingstone peaches. J. Food Eng. 2013, 116, 920–925. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Assessment of tomato soluble solids content and pH by spatially-resolved and conventional Vis/NIR spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Sabzi, S.; Hernández-Hernández, M.; Hernández-Hernández, J.L.; Azadshahraki, F. Nondestructive estimation of the chlorophyll b of apple fruit by color and spectral features using different methods of hybrid artificial neural network. Agronomy 2019, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, S.; El-Hendawy, S.; Khadr, M.; Elsherbiny, O.; Al-Suhaibani, N.; Alotaibi, M.; Tahir, M.U.; Darwish, W. Combining thermal and RGB imaging indices with multivariate and data-driven modeling to estimate the growth, water status, and yield of potato under different drip irrigation regimes. Remote Sens. 2021, 13, 1679. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Zhou, L.; Feng, L.; Qiu, Z. Integration of visible and thermal imagery with an artificial neural network approach for robust forecasting of canopy water content in rice. Remote Sens. 2021, 13, 1785. [Google Scholar] [CrossRef]
- Elmetwalli, A.H.; Mazrou, Y.S.A.; Tyler, A.N.; Hunter, P.D.; Elsherbiny, O.; Yaseen, Z.M.; Elsayed, S. Assessing the efficiency of remote sensing and machine learning algorithms to quantify wheat characteristics in the Nile Delta region of Egypt. Agric. 2022, 12, 332. [Google Scholar] [CrossRef]
- Thawornwong, S.; Enke, D. The adaptive selection of financial and economic variables for use with artificial neural networks. Neurocomputing 2004, 56, 205–232. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Ouyang, A. Non-destructive measurement of soluble solid content in gannan navel oranges by visible/near-infrared spectroscopy. Acta Opt. Sin. 2008, 28, 478–481. [Google Scholar]
- Zhang, S.; Wang, F.; Zhang, H.; Zhao, C.; Yang, G. Detection of the fresh jujube varieties and ssc by nir spectroscopy. Trans. CSAM 2009, 40, 139–142. [Google Scholar]
- Zheng, H.; Lu, H. A least-squares support vector machine (LS-SVM) based on fractal analysis and CIELab parameters for the detection of browning degree on mango (Mangifera indica L.). Comput. Electron. Agric. 2012, 83, 47–51. [Google Scholar] [CrossRef]
- Marković, M.S.; Pavlović, D.V.; Tošić, S.M.; Stankov-Jovanović, V.P.; Krstić, N.S.; Stamenković, S.M.; Mitrović, T.L.J.; Matković, V.L.J. Chloroplast pigments in post-fire grown cryptophytes on Vidlič Mountain (Southeastern Serbia). Arch. Biol. Sci. 2012, 64, 531–538. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Hendawy, S.; Dewir, Y.H.; Schmidhalter, U.; Ibrahim, H.H.; Ibrahim, M.M.; Elsherbiny, O.; Farouk, M. Estimating the leaf water status and grain yield of wheat under different irrigation regimes using optimized two- and three-band hyperspectral indices and multivariate regression models. Water 2021, 13, 2666. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement of Retro Gradation of Natural Vegetation; Type III. Final Report; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 1974.
- Gutierrez, M.; Reynolds, M.P.; Raun, W.R.; Stone, M.L.; Klatt, A.R. Spectral water indices for assessing yield in elite bread wheat genotypes in well irrigated, water stressed, and high temperature conditions. Crop. Sci. 2010, 50, 197–214. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Lukina, E.V.; Thomason, W.E.; Schepers, J.S. In-season prediction of potential grain yield in winter wheat using canopy reflectance. Agron. J. 2001, 93, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Carrow, R.N. Broadband spectral reflectance models of turfgrass species and cultivars to drought stress. Crop. Sci. 2007, 47, 1611–1618. [Google Scholar] [CrossRef]
- Schalkoff, J. Artificial Neural Networks; McGraw-Hill Companies Inc.: New York, NY, USA, 1997. [Google Scholar]
- Haykin, S. Neural Networks: A Comprehensive Foundation, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Glorfeld, L.W. A methodology for simplification and interpretation of backpropagation-based neural network models. Expert Syst. Appl. 1996, 10, 37–54. [Google Scholar] [CrossRef]
- Malone, B.P.; Styc, Q.; Minasny, B.; McBratney, A.B. Digital soil mapping of soil carbon at the farm scale: A spatial downscaling approach in consideration of measured and uncertain data. Geoderma 2017, 290, 91–99. [Google Scholar] [CrossRef]
- Saggi, M.K.; Jain, S. Reference evapotranspiration estimation and modeling of the Punjab Northern India using deep learning. Comput. Electron. Agr. 2019, 156, 387–398. [Google Scholar] [CrossRef]
- Subagio, A.; Morita, N.; Sawada, S. Carotenoids and their fatty-acid esters in banana peel. J. Nutr. Sci. Vitaminol. 1996, 42, 553–566. [Google Scholar] [CrossRef] [Green Version]
- Ringer, T.; Blanke, M. Non-invasive, real time in-situ techniques to determine the ripening stage of banana. J. Food Meas. Charact. 2021, 15, 4426–4437. [Google Scholar] [CrossRef]
- Marriott, J.; Palmer, J.K. Bananas-physiology and biochemistry of storage and ripening for optimum quality. Crit. Rev. Food Sci. Nutr. 1980, 13, 41–88. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Thompson, A.K.; John, P. Inhibition of degreening in the peel of bananas ripened at tropical temperatures. Ann. Appl. Biol. 1987, 110, 145–151. [Google Scholar] [CrossRef]
- Reddy, C.K.; Haripriya, S.; Vidya, P.V. Morphology, physicochemical and functional characteristics of starches from different banana cultivars. J. Food Sci. Technol. 2015, 52, 7289–7296. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant. Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Maduwanthi, S.D.T.; Marapana, R.A.U.J. Induced ripening agents and their effect on fruit quality of banana. Int. J. Food Sci. 2019, 2019, 2520179. [Google Scholar] [CrossRef]
- Williams, M.H.; Vesk, M.; Mullins, M.G. A scanning electron microscope study of the formation and surface characteristics of the peel of the banana fruit during its development. Bot. Gaz. 1989, 150, 30–40. [Google Scholar] [CrossRef]
- Costa, G.; Fiori, G.; Noferini, M.; Ziosi, V. Internal fruit quality: How to influence it, how to de fine it. Acta Hortic. 2006, 2, 339–346. [Google Scholar] [CrossRef]
- Ruiz-Altisent, M.; Lleó, L.; Riquelme, F. Instrumental quality assessment of peaches: Fusion of optical and mechanical parameters. J. Food Eng. 2006, 74, 490–499. [Google Scholar] [CrossRef]
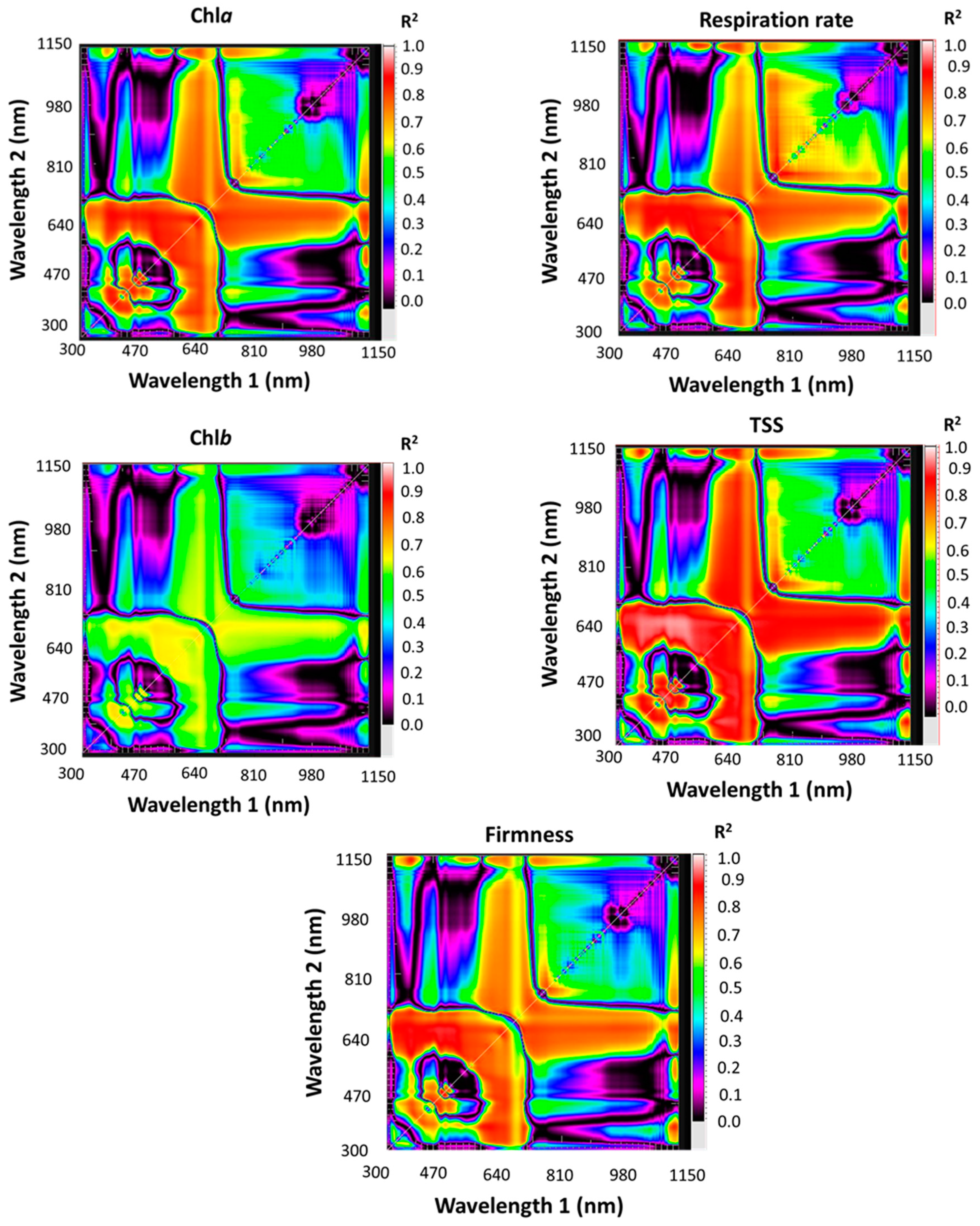
| SRIs | Formula | References |
|---|---|---|
| Greenness index (GI) | R554/R677 | [20] |
| Pigment sensitive Ripening Monitoring Index (PRMI) | (R750 − R678)/R550 | [15] |
| Normalized chlorophyll index (NCI) | (R750 − R678)/(R750 + R678) | [15] |
| Anthocyanin index (NAI) | (R760 − R720)/(R760 + R720) | [35] |
| Normalized difference index (NDI) | ||
| NDI780,550 | (R780 − R550)/(R780 + R550) | [36] |
| NDI780,570 | (R780 − R570)/(R780 + R570) | [21] |
| NDI780,670 | (R780 − R670)/(R780 + R670) | [37] |
| NDI780,710 | (R780 − R710)/(R780 + R710) | [38] |
| NDI800,640 | (R800 − R640)/(R800 + R640) | [12] |
| NDI826,670 | (R826 − R670)/(R826 + R670) | [12] |
| NDI970,670 | (R970 − R670)/(R970 + R670) | [12] |
| Ratio spectral index | ||
| RSI450,640 | R450/R640 | Present study |
| RSI462,468 | R462/R468 | |
| RSI470,460 | R470/R460 | |
| RSI470,652 | R470/R652 | |
| RSI476,480 | R476/R480 | |
| RSI500,624 | R500/R624 | |
| RSI530,610 | R530/R610 | |
| RSI584,558 | R584/R558 | |
| RSI638,490 | R638/R490 | |
| RSI638,1138 | R638/R1138 | |
| RSI640,460 | R640/R460 | |
| RSI650,456 | R650/R456 | |
| RSI740,654 | R740/R654 | |
| RSI766,764 | R766/R764 | |
| RSI780,764 | R780/R764 | |
| RSI780,650 | R780/R650 |
| Mature | Semi-Ripening | Ripening | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| Chl a (mg g−1) | 8.199 | 18.500 | 12.160 a | 4.927 | 11.794 | 8.667 b | 1.397 | 4.247 | 2.282 c |
| Chl b (mg g−1) | 4.079 | 7.421 | 5.654 a | 4.099 | 7.099 | 5.559 a | 2.747 | 4.710 | 3.651 b |
| Respiration rate (mL CO2/kg*hour) | 6.705 | 30.00 | 13.046 c | 27.998 | 37.779 | 32.089 b | 35.505 | 77.084 | 52.230 a |
| TSS (%) | 2.600 | 5.300 | 4.057 c | 3.600 | 16.100 | 12.629 b | 17.800 | 23.300 | 20.563 a |
| Firmness (N) | 21.650 | 31.700 | 27.630 a | 6.900 | 24.200 | 10.62 b | 2.838 | 6.800 | 3.800 c |
| Chl a | Chl b | Respiration Rate | TSS | Firmness | |
|---|---|---|---|---|---|
| Chl a | 1.00 ** | ||||
| Chl b | 0.87 ** | 1.00 ** | |||
| Respiration rate | −0.89 ** | −0.75 ** | 1.00 ** | ||
| TSS | −0.91 ** | −0.76 ** | 0.92 ** | 1.00 ** | |
| Firmness | 0.86 ** | 0.67 ** | −0.90 ** | −0.97 ** | 1.00 ** |
| Mature | Semi-Ripening | Ripening | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| GI | 2.177 | 2.806 | 2.481 a | 2.154 | 2.540 | 2.372 a | 0.535 | 1.928 | 0.919 b |
| PRMI | 1.663 | 2.268 | 1.984 a | 1.601 | 1.877 | 1.773 b | 0.400 | 1.497 | 0.742 c |
| NCI | 0.643 | 0.752 | 0.713 a | 0.649 | 0.693 | 0.676 a | 0.118 | 0.588 | 0.242 b |
| NAI | 0.085 | 0.135 | 0.113 a | 0.086 | 0.106 | 0.099 b | 0.038 | 0.069 | 0.051 c |
| NDI780,550 | 0.011 | 0.021 | 0.015 c | 0.019 | 0.028 | 0.022 b | 0.022 | 0.046 | 0.031 a |
| NDI780,570 | 0.385 | 0.493 | 0.444 a | 0.369 | 0.425 | 0.404 b | 0.268 | 0.431 | 0.318 c |
| NDI780,670 | 0.653 | 0.774 | 0.725 a | 0.649 | 0.700 | 0.681 a | 0.162 | 0.588 | 0.278 b |
| NDI780,710 | 0.155 | 0.241 | 0.201 a | 0.160 | 0.188 | 0.177 b | 0.068 | 0.128 | 0.088 c |
| NDI800,640 | 0.502 | 0.650 | 0.584 a | 0.473 | 0.529 | 0.512 b | 0.199 | 0.411 | 0.264 c |
| NDI826,670 | 0.662 | 0.781 | 0.732 a | 0.658 | 0.712 | 0.692 a | 0.189 | 0.601 | 0.307 b |
| NDI970,670 | 0.545 | 0.686 | 0.630 a | 0.543 | 0.607 | 0.579 a | 0.023 | 0.478 | 0.173 b |
| RSI450,640 | 0.441 | 0.551 | 0.472 a | 0.348 | 0.461 | 0.386 b | 0.219 | 0.302 | 0.263 c |
| RSI462,468 | 0.986 | 0.995 | 0.992 a | 0.977 | 0.987 | 0.982 b | 0.958 | 0.972 | 0.966 c |
| RSI470,460 | 1.013 | 1.028 | 1.0174 c | 1.026 | 1.040 | 1.034 b | 1.047 | 1.071 | 1.057 a |
| RSI470,652 | 0.601 | 0.719 | 0.632 a | 0.451 | 0.607 | 0.504 b | 0.237 | 0.371 | 0.288 c |
| RSI476,480 | 0.985 | 0.991 | 0.987 c | 0.989 | 0.995 | 0.992 b | 0.995 | 1.006 | 1.003 a |
| RSI500,624 | 0.604 | 0.685 | 0.638 a | 0.499 | 0.637 | 0.547 b | 0.328 | 0.447 | 0.380 c |
| RSI530,610 | 1.085 | 1.260 | 1.174 a | 0.998 | 1.095 | 1.047 b | 0.639 | 0.913 | 0.766 c |
| RSI584,558 | 0.816 | 0.896 | 0.853 c | 0.883 | 0.925 | 0.903 b | 0.962 | 1.140 | 1.062 a |
| RSI638,490 | 1.577 | 1.883 | 1.779 c | 1.787 | 2.362 | 2.154 b | 2.729 | 3.861 | 3.266 a |
| RSI638,1138 | 0.639 | 0.822 | 0.704 c | 0.749 | 0.813 | 0.779 b | 0.852 | 1.232 | 1.080 a |
| RSI640,460 | 1.669 | 2.046 | 1.933 c | 1.965 | 2.632 | 2.389 b | 3.076 | 4.393 | 3.671 a |
| RSI650,456 | 1.484 | 1.786 | 1.698 c | 1.783 | 2.439 | 2.190 b | 2.954 | 4.576 | 3.786 a |
| RSI740,654 | 3.338 | 5.411 | 4.408 a | 3.012 | 3.499 | 3.333 b | 1.300 | 2.454 | 1.535 c |
| RSI766,764 | 0.993 | 0.999 | 0.995 c | 0.997 | 0.999 | 0.998 b | 0.999 | 1.006 | 1.002 a |
| RSI780,764 | 0.991 | 1.015 | 0.996 c | 1.008 | 1.015 | 1.011 b | 1.012 | 1.046 | 1.027 a |
| RSI780,650 | 3.352 | 5.481 | 4.437 a | 3.068 | 3.576 | 3.424 b | 1.434 | 2.529 | 1.686 c |
| SRIs | Chl a | Chl b | Respiration Rate | TSS | Firmness |
|---|---|---|---|---|---|
| GI | 0.79 *** | 0.64 *** | 0.78 *** | 0.83 *** | 0.71 *** |
| PRMI | 0.75 *** | 0.57 *** | 0.74 *** | 0.81 *** | 0.71 *** |
| NCI | 0.74 *** | 0.58 *** | 0.74 *** | 0.80 *** | 0.68 *** |
| NAI | 0.76 *** | 0.59 *** | 0.73 *** | 0.84 *** | 0.75 *** |
| NDI780,550 | 0.69 *** | 0.47 *** | 0.81 *** | 0.77 *** | 0.73 *** |
| NDI780,570 | 0.66 *** | 0.50 *** | 0.61 *** | 0.74 *** | 0.66 *** |
| NDI780,670 | 0.76 *** | 0.59 *** | 0.75 *** | 0.81 *** | 0.70 *** |
| NDI780,710 | 0.76 *** | 0.59 *** | 0.74 *** | 0.84 *** | 0.75 *** |
| NDI800,640 | 0.80 *** | 0.62 *** | 0.79 *** | 0.87 *** | 0.78 *** |
| NDI826,670 | 0.75 *** | 0.59 *** | 0.74 *** | 0.81 *** | 0.69 *** |
| NDI970,670 | 0.75 *** | 0.59 *** | 0.72 *** | 0.81 *** | 0.70 *** |
| RSI450,640 | 0.83 *** | 0.58 *** | 0.87 *** | 0.95 *** | 0.90 *** |
| RSI462,468 | 0.87 *** | 0.66 *** | 0.84 *** | 0.93 *** | 0.86 *** |
| RSI470,460 | 0.86 *** | 0.65 *** | 0.84 *** | 0.92 *** | 0.85 *** |
| RSI470,652 | 0.85 *** | 0.61 *** | 0.88 *** | 0.95 *** | 0.89 *** |
| RSI476,480 | 0.85 *** | 0.65 *** | 0.76 *** | 0.89 *** | 0.79 *** |
| RSI500,624 | 0.84 *** | 0.60 *** | 0.87 *** | 0.95 *** | 0.88 *** |
| RSI530,610 | 0.85 *** | 0.64 *** | 0.86 *** | 0.91 *** | 0.82 *** |
| RSI584,558 | 0.83 *** | 0.50 *** | 0.85 *** | 0.88 *** | 0.79 *** |
| RSI638,490 | 0.80 *** | 0.60 *** | 0.85 *** | 0.89 *** | 0.81 *** |
| RSI638,1138 | 0.79 *** | 0.61 *** | 0.81 *** | 0.85 *** | 0.75 *** |
| RSI640,460 | 0.81 *** | 0.60 *** | 0.86 *** | 0.90 *** | 0.82 *** |
| RSI650,456 | 0.82 *** | 0.61 *** | 0.86 *** | 0.90 *** | 0.81 *** |
| RSI740,654 | 0.82 *** | 0.62 *** | 0.82 *** | 0.89 *** | 0.81 *** |
| RSI766,764 | 0.75 *** | 0.54 *** | 0.89 *** | 0.79 *** | 0.75 *** |
| RSI780,764 | 0.76 *** | 0.53 *** | 0.89 *** | 0.82 *** | 0.79 *** |
| RSI780,650 | 0.81 *** | 0.61 *** | 0.80 *** | 0.88 *** | 0.80 *** |
| Variable | Parameters | Best Indices | Calibration | Validation | ||
|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | |||
| Chl a | (12,4) & identity | NDI826,670; NDI970-670; RSI500,624; NDI780-570; RSI450,640; NDI760-720; NDI780-710; NDI800,640; RSI650,456; RSI470,652; RSI638,490; NDI750,678; RSI476,480; RSI640,460; NDI780,670 | 0.93 *** | 1.287 | 0.89 *** | 1.162 |
| Chl b | (12,4) & identity | RSI740,654; RSI650,456; RSI640,460; PRMI, RSI826,670; RSI530,610 | 0.71 *** | 0.635 | 0.63 *** | 0.525 |
| Respiration rate | (10,14) & identity | RSI638,1138; NDI780-570; RSI500,624; NDI750,678; RSI554,667; NDI970-670; NDI760-720; RSI462,468; RSI470,460; RSI650,456; RSI780,764; RSI740,654; RSI788,650; RSI638,490; RSI38,460 | 0.95 *** | 4.253 | 0.91 *** | 4.212 |
| TSS | (6,10) & relu | RSI462,468; NDI750-678; RSI638,1138; NDI780,670; RSI554,667; RSI650,456; RSI470,460; RSI450,640; RSI500,624; RSI766,764; RSI780,764; NDI760-720; NDI780-570 | 1.00 *** | 0.539 | 0.97 *** | 1.034 |
| Firmness | (22,4) & logistic | NDI750-678; RSI650,456; NDI970-670; RSI450,640; RSI766,764; NDI800,640; RSI584,558; RSI780,764; RSI476,480; RSI640,490; RSI638,490; RSI500,624; NDI780-550; RSI740,654; RSI470,460 | 1.00 *** | 0.417 | 0.98 *** | 1.161 |
| Parameters | Calibration | Validation | ||
|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | |
| Chl a | 0.90 *** | 1.60 | 0.86 *** | 1.87 |
| Chl b | 0.74 *** | 0.62 | 0.60 *** | 0.75 |
| Respiration rate | 0.94 *** | 4.82 | 0.91 *** | 5.66 |
| TSS | 0.97 *** | 1.43 | 0.95 *** | 1.83 |
| Firmness | 0.95 *** | 2.57 | 0.91 *** | 3.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galal, H.; Elsayed, S.; Allam, A.; Farouk, M. Indirect Quantitative Analysis of Biochemical Parameters in Banana Using Spectral Reflectance Indices Combined with Machine Learning Modeling. Horticulturae 2022, 8, 438. https://doi.org/10.3390/horticulturae8050438
Galal H, Elsayed S, Allam A, Farouk M. Indirect Quantitative Analysis of Biochemical Parameters in Banana Using Spectral Reflectance Indices Combined with Machine Learning Modeling. Horticulturae. 2022; 8(5):438. https://doi.org/10.3390/horticulturae8050438
Chicago/Turabian StyleGalal, Hoda, Salah Elsayed, Aida Allam, and Mohamed Farouk. 2022. "Indirect Quantitative Analysis of Biochemical Parameters in Banana Using Spectral Reflectance Indices Combined with Machine Learning Modeling" Horticulturae 8, no. 5: 438. https://doi.org/10.3390/horticulturae8050438
APA StyleGalal, H., Elsayed, S., Allam, A., & Farouk, M. (2022). Indirect Quantitative Analysis of Biochemical Parameters in Banana Using Spectral Reflectance Indices Combined with Machine Learning Modeling. Horticulturae, 8(5), 438. https://doi.org/10.3390/horticulturae8050438








