Abstract
White lupine (Lupinus albus L.) is an annual legume that is grown for both seeds and green biomass, but several agronomic aspects of this crop, including response to weed competition, have not been studied extensively. Field experiments over two growing seasons (2012 and 2016) were carried out in Orestiada, Greece, to study the growth and development of spring-sown white lupine under season-long weed competition from natural weed flora compared with its growth without weed competition. Treatments were arranged in a randomized complete block design with four replications and included (i) a non-treated (weedy) control, where weeds (Chenopodium album and Sorghum halepense) remained in the plots throughout the experiments and (ii) a weed-free control, where weeds were removed upon crop emergence and the plots were kept free of weeds throughout the experiments by hand removal. The presence of Chenopodium album and Sorghum halepense reduced the aboveground dry matter accumulation of white lupine ‘Multitalia’ at 7 weeks after crop emergence by 18.0% in the first growing season and 29.5% in the second growing season, while the corresponding decrease in the aboveground dry matter accumulation at 9 weeks after crop emergence was 25.3 and 33.4%. However, the reduction in dry matter accumulation was limited to lower levels after flowering (9.9% in the first and 12.8% in the second growing season). In both growing seasons, values of the ability to withstand competition (AWC) index were lower at 7 and 9 weeks from crop emergence than at maturity. Seed yields were 1.58 Mg per ha under weedy conditions and 2.20 Mg per ha under weed free conditions in the first growing season, and 1.59 and 2.32 Mg per ha, respectively, in the second growing season. The values of the relative yield loss (RYL) index for seed yield were 28.2% in the first growing season and 31.5% in the second growing season. Overall, white lupine growth and seed yield was significantly affected by the occurrence of weeds mostly at the early vegetative stages, resulting in the potential yield not being achieved due to weed competition. Future research on weed competition across several sites and years would be useful to define more clearly the critical period of weed control in white lupine.
1. Introduction
White lupine (Lupinus albus L.), a member of the genus Lupinus in the Fabaceae family, is an annual legume traditionally cultivated in the Mediterranean region [1,2]. It has been reported as the most adapted legume species to the Mediterranean conditions of southern Spain [3] and it is mainly produced by smallholder farmers in Ethiopia and other African countries due to its multipurpose functions [4]. Most of the available information for crop management, however, comes from Australia, which is the largest producer and exporter of lupine globally [5]. White lupine is cultivated mainly for ruminant feed, but it is also grown for green manure as a method of soil amendment and often for human nutrition, because of the high protein and oil content in seeds [1,3]. White lupine seeds may contain 33–47% protein, depending on genotype and location [6]. Thus, the white lupine seeds are a good source of quality protein for humans and exogenous amino acids in chicken broilers [7]. Nevertheless, white lupine has a minor position among other grain legumes, and therefore research on this crop is largely limited compared with other legume crops. Additionally, white lupine is not consumed as commonly as other legumes, probably because many consumers are not familiar with this food, are not aware of its nutritional value, or are not accustomed to consuming it [8].
Competition between crops and weeds for the acquisition of growth resources (light, water, and nutrients) is abundant in the agricultural ecosystems, where large amounts of resources are added to maximize crop productivity [9]. In cropping systems, competition refers to a struggle of plants for acquiring resources under limited supply in the presence of neighbors. In the crop–weed competition situations, competition relates to the negative effect of resource capture by weeds on crop growth and productivity. Competition for limited resources (nutrients, water, and light) will lead to reduced crop yield and often to products of lower quality [10]. Yield losses due to crop–weed competition can range from 10 to 100%, depending on crop and related weed flora [11]. The extent of yield losses may vary largely depending on the relative competitive ability between crops and weeds. Agroecologists have studied inter-species (crop–weed) competition extensively. However, due to the complex relationships in crop–weed competition, developing general trends of crop yield reduction has been difficult. Moreover, information on crop–weed competition based on single weeds is of limited importance because weed infestations normally include multi-species weed communities.
Lupinus albus and other Lupinus species are reported to be poor weed competitors especially during early establishment [12]. The crop is slow in developing a full plant canopy, a fact that facilitates light penetration and weed seed germination, providing sufficient time for several weeds to emerge, grow rapidly, and compete with lupine plants [13]. Lupines reach the maximum levels of vegetative growth and competitiveness during flowering [14]. Several crop management practices, such as early sowing, appropriate population densities, and selection of robust varieties may give lupine cultivars a competitive advantage, but experimental evidence on this topic is scarce in the literature. In any case, effective weed control, especially during lupine crop establishment, is necessary for the success of the crop [14]. However, chemical weed control is a problem, due to the phytotoxicity of most herbicides in lupine plants [15]. In addition, hand pulling and tillage are effective for controlling established weeds, but the soil disturbance from these methods may promote recruitment of new weeds. Weed control must be implemented considering the significant losses caused by multiple weeds, if left uncontrolled in the growing season. Thus, it is a common case that the competitive effect of a mixed infestation by multiple species must be estimated. Apart from accuracy problems in yield loss predictions, quantitative data concerning the impact of weeds on certain crops are sparse. For example, quantitative information on the impact of weed competition on white lupine growth and yield does not exist.
The purpose of this research was to study the effects of season-long competition by the natural weed flora on the growth and productivity of white lupine. The specific objective was to quantify the effect of weed competition on white lupine growth and productivity in terms of biomass and yield losses.
2. Materials and Methods
2.1. Crop Management
Field experiments were conducted in 2012 and 2016 at the farm of Democritus University of Thrace in Orestiada (41° 33′ latitude, 26° 31′ longitude, 22 m above sea level), northern Greece. The experiments were established on a silty clay loam soil (silt 52.4%, clay 39.6%, 8.0% sand), with organic matter 1.01% and pH (1:1 H2O) 6.7. The previous crop in the field was sunflower (Helianthus annuus L.). Soil preparation was performed in autumn of each year, consisting of conventional tillage practices (i.e., moldboard plowing and chisel plowing). One pass with a cultivator was undertaken in early spring a week before sowing.
Sowing was completed manually on 6 March in the first growing season and 18 March in the second growing season. The commercially available Italian cultivar ‘Multitalia’ was used. ‘Multitalia’ is an early-maturing cultivar of white lupine, with high productivity and resistance to cold, water stress, and lodging. The row spacing was 25 cm. A larger amount of seed (120 kg per ha) than that required was used at sowing and then hand thinning was applied upon emergence by removing plants in overcrowded areas on the rows, resulting eventually in an inter-row spacing of 5–6 cm and in a population density of 800,000 plants per ha. The sowing depth was 3–4 cm. The experimental field was fertilized with 60 kg/ha P2O5 applied as basic fertilization before sowing in the form of triple superphosphate fertilizer (0-46-0), following fertilization recommendations in the area. The amount of fertilizer was evenly dispersed on the soil surface and then properly incorporated into the soil using a rake. No nitrogen fertilization was applied because lupine is a legume crop and can easily grow even in nitrogen-poor soils without the need for additional nitrogen fertilizer. Due to previous cultivation of white lupine in the area, there was an indigenous rhizobia population in the soil, resulting in an adequate number of nodules in plant roots. Therefore, inoculation was not considered necessary. The crop remained without irrigation (dryland cropping) throughout the duration of the biological cycle (from sowing to harvest). The average temperature and the total rainfall in each month during the experiments (from sowing to harvest) were provided from the weather station of the Greek Sugar Industry in Orestiada, which is located approximately 1.5 km from Democritus University farm.
2.2. Experimental Design and Treatments
The experiments were set in a randomized complete block design (RCBD) with two treatments replicated four times in two growing seasons. Plot size was 3.0 by 2.5 m, comprising 10 rows of the crop. Treatments included (i) a non-treated (weedy) control, where weeds (Chenopodium album and Sorghum halepense) remained in the plots throughout the experiments and (ii) a weed-free control, where weeds were removed upon crop emergence and the plots were kept free of weeds by hand removal throughout the experiments. The experimental field was naturally infested with several weed species.
2.3. Sampling and Measurements
At 5, 7, 9, and 14 weeks after crop emergence, white lupine plants from 1 m of crop row from each plot were cut at the ground level. These sampling periods followed the growth cycle of the crop combined with weed occurrence and covered two stages of the vegetative development, the flowering stage, and close to the physiological maturity stage of the crop. At the same periods, weed samples from the 1 m2 area between two crop rows were hand harvested. All samples were placed in separate paper bags and immediately transferred to the laboratory where they were weighed to determine fresh weight. The samples were then placed in an oven at 70 °C until constant weight to determine dry weight. Similarly, at maturity, plants from 1 m of crop row were cut at the ground level, when the pods turned yellow and seed moisture reached 14%. After harvest, the plants remained in the field to dry completely. Pods were removed manually and then threshed with an experimental threshing machine. Threshed seeds from each plot were weighed and data of seed weight were expressed in kg per ha. Nitrogen concentration of the seeds was determined by the Kjeldahl method [16] and seed protein content was estimated as N × 6.25.
Competition indices can quantify and express composite ideas through a simple primary measure that can be used by different researchers to compare the results from different studies [17]. It is possible to use several indices in a study, particularly when different attributes of competition are being investigated. Nevertheless, recommendation of the ‘best’ indices is unlikely to find universal agreement, because no index is without some limitation. In the current study, the Relative Competition Intensity (RCI) index, the Ability to Withstand Competition (AWC) index, the Ability to Compete (AC) index, and the Relative Yield Loss (RYL) index were calculated according to the available data collected, as described below. In the index where weed performance was used, the weed performance refers to the aboveground dry weight of the dominant weeds (Chenopodium album and Sorghum halepense).
The Relative Competition Intensity (RCI) index was determined according to Equation (1) [18], where Pweed-free = crop performance in weed-free condition and Pweedy = crop performance in weedy condition. RCI indicates the strength of competition as it compares crop performance in the absence and presence of plant neighbors. When RCI = 0, the interspecific competition is equal to the intraspecific competition; when RCI > 0, the interspecific competition is greater than the intraspecific competition; when RCI < 0, the intraspecific competition is greater than the interspecific competition.
Relative Competition Intensity (RCI) = (Pweed-free − Pweedy)/Pweed-free
The Ability to Withstand Competition (AWC) index was calculated according to Equation (2) [19], where Pweed-free = crop performance in weed-free condition and Pweedy = crop performance in weedy condition. High values of this index indicate high ability of the crop to withstand the presence of weeds without yield loss.
Ability to Withstand Competition (AWC) = (Pweedy/Pweed-free) × 100
The Ability to Compete (AC) index was calculated according to Equation (3) [20], where Bweed = weed performance and Btotal = crop and weed performance. High values of this index indicate great ability of the crop to suppress weed growth.
Ability to Compete (AC) = 100 − [(Bweed/Btotal) × 100]
The Relative Yield Loss (RYL) index was calculated according to Equation (4) [21], where Pweed-free = crop performance in weed-free condition and Pweedy = crop performance in weedy condition.
Relative Yield Loss (RYL) = 100 * (Pweed-free − Pweedy)/Pweed-free
2.4. Data Analysis
The two-year data were subjected to analysis of variance (ANOVA) to compare the effect of weeds on white lupine growth and yield in the weedy plots relative to the weed-free plots. Data analysis of white lupine dry weight for each sampling and yield components followed a two-factor factorial approach, where the two growing seasons were considered the first factor and the treatments (weedy and weed-free controls) were considered the second factor. Data analysis for competition indices followed a two-factor factorial approach, where the two growing seasons were considered the first factor and assessment timing was considered the second factor. Treatment means were compared using Fischer’s protected least significant difference (LSD) at the 5% level of significance. All analyses were performed with the statistical software MSTAT-C [22].
3. Results
3.1. Weather Parameters
The overall mean temperature was almost similar in the two growing seasons (18.8 °C in the first growing season vs. 19.9 °C in the second growing season), while the overall total rainfall was slightly higher in the second growing season (166 mm), with increased rain events in late June, compared with the first growing season (146 mm) (Table 1).

Table 1.
Mean monthly temperature and total monthly rainfall in each growing season.
3.2. Weed Flora
The dominant weeds in both growing seasons were common lambsquarters (Chenopodium album) and johnsongrass (Sorghum halepense), together accounting for over 95% of the weed flora of the field. Most common lambsquarters plants emerged within 1–2 weeks after crop emergence, while most johnsongrass plants emerged within 4–5 weeks after crop emergence. On average, there were 8–10 Chenopodium album plants per m2 and 2–4 Sorghum halepense plants per m2. Both weed species emerged earlier in the second growing season. Other weeds in the experimental field in descending order of dominance were volunteer plants of sunflower (previous crop), black bindweed (Bilderdykia convolvulus), barnyardgrass (Echinochloa crus-galli), field bindweed (Convolvulus arvensis), and European field pansy (Viola arvensis). Because it was estimated that the uneven distribution of these species in the field (non-presence in all plots) would not allow an objective evaluation of their competitive effects, these weeds were removed from the weedy treatment upon crop emergence and thus their effect on white lupine growth was not assessed.
3.3. White Lupine Dry Weight
The main effects and their interactions between growing season and treatment for the dry weight of white lupine plants are illustrated in Table 2.

Table 2.
Analysis of variance (mean squares) for the dry weight of white lupine plants at each sampling.
Due to significant main effects and interactions between growing season and treatment for some variables, crop dry weight data are presented separately for each growing season (Figure 1). At all samplings, dry weight of white lupine plants was consistently higher in the weed-free treatment than in the weedy treatment in both growing seasons.
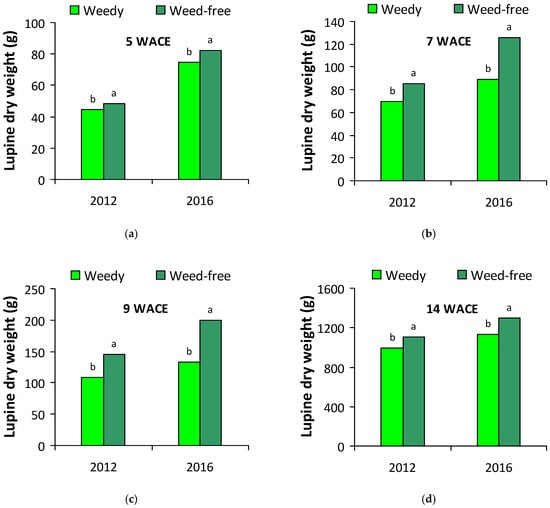
Figure 1.
Dry weight of white lupine plants in weedy and weed-free plots (a) 5 weeks after crop emergence (WACE); (b) 7 WACE; (c) 9 WACE; (d) 14 WACE in the first and second growing season. Different letters in each growing season within each sub-figure indicate statistically significant differences at p < 0.05.
3.4. White Lupine Yield Components
The main effects and interactions between growing season and treatment for the yield and yield components of white lupine plants are illustrated in Table 3.

Table 3.
Analysis of variance (mean squares) for the seed yield, yield components, and seed protein content of white lupine plants.
Due to significant main effects and interactions between growing season and treatment for some variables, yield components data are presented separately for each growing season (Figure 2). The presence of weeds reduced the number of pods per plant only in the second growing season and the number of seeds per plant in both years (Figure 2). The reduction in the number of seeds per plant was 15.6% in the first growing season (Figure 2a) and 24.6% in the second growing season (Figure 2b). The weight of 1000 seeds was also reduced in the weedy plots in both growing seasons (Figure 3) and was on average 338 vs. 371 g for the weedy and the weed-free treatment, respectively. Protein concentration in the seeds was on average 33.4% in plants without weed competition and 32.8% in plants under weed competition.
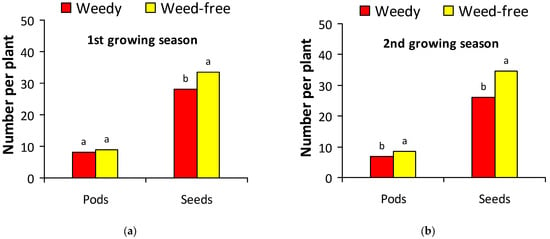
Figure 2.
Number of white lupine pods and seeds per plant in weedy and weed-free treatments (a) in the first growing season; (b) in the second growing season. Different letters in each variable indicate statistically significant differences at p < 0.05.
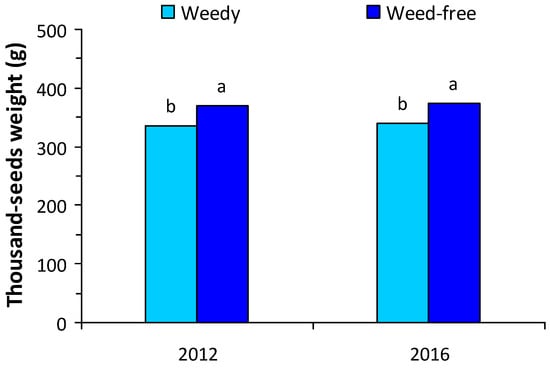
Figure 3.
Thousand-seeds weight of white lupine in weedy and weed-free treatments in the first and second growing seasons. Different letters in each growing season indicate statistically significant differences at p < 0.05.
Seed yield of white lupine without weed competition was 2.20 Mg per ha in the first growing season and 2.32 Mg per ha in the second growing season (Figure 4).
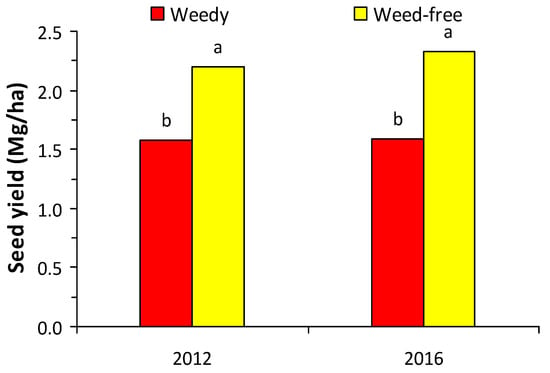
Figure 4.
Seed yield of white lupine in weedy and weed-free treatments in the first and second growing seasons. Different letters in each growing season indicate statistically significant differences at p < 0.05.
The high levels of seed yield in both years revealed the high adaptability of the crop to the growing area, which were also supported by the high fertility of the experimental soil. However, seed yield in both years was significantly reduced by the presence of weeds (Figure 4), resulting in the potential yield not being achieved due to weed competition.
3.5. Competition Indices
Crop competitiveness can be assessed in several ways. In this study, to characterize the weed competition effects on growth and yield of spring-sown white lupine, competition indices were calculated based on biomass ratios. The main effects and interactions between growing season and treatment for the competition indices are illustrated in Table 4. Due to significant main effects and interactions between growing season and treatment for some indices, competition indices data are presented separately for each growing season.

Table 4.
Analysis of variance (mean squares) for the calculated competition indices.
Values of the relative competition intensity (RCI) index were above 0, indicating that the interspecific competition was greater than the intraspecific competition (Figure 5). Moreover, values of the relative RCI index showed that the intensity of competition increased during the late vegetative phase of white lupine (7 and 9 weeks after crop emergence) in both growing seasons and was generally more intense in the second growing season of the experimentation.
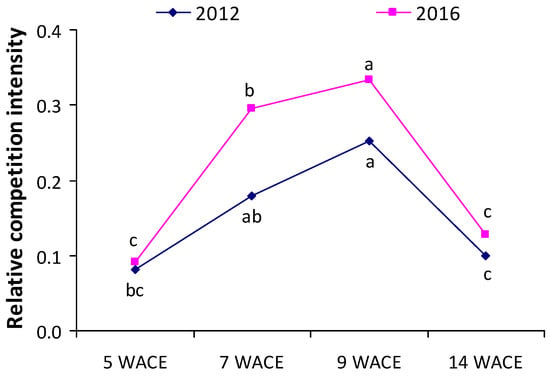
Figure 5.
Relative competition intensity (RCI) index at various weeks after crop emergence (WACE) in the first and second growing seasons. Different letters in each growing season indicate statistically significant differences at p < 0.05.
Values of the ability to withstand competition (AWC) index were lower at 7 and 9 weeks after crop emergence in both years (Figure 6). Furthermore, the ability of white lupine to tolerate weed competition was generally lower in the second year of the experimentation due to the earlier emergence and the higher growth rates of the weeds.
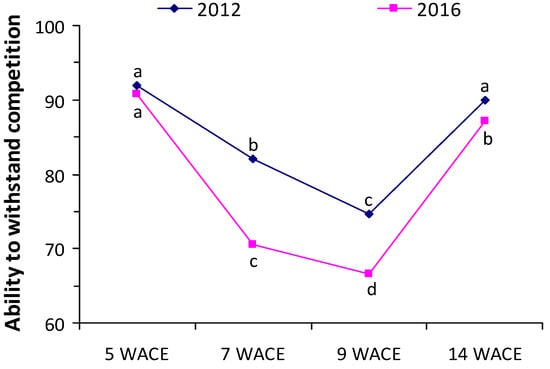
Figure 6.
Ability to withstand competition (AWC) index at various weeks after crop emergence (WACE) in the first and second growing seasons. Different letters in each growing season indicate statistically significant differences at p < 0.05.
Similar to the values of the AWC index, values of the ability to compete (AC) index were lower at 7 and 9 weeks after crop emergence, indicating poor competitive ability of white lupine at the vegetative stage (Figure 7). Furthermore, the ability of white lupine to compete with weeds was generally lower in the second year of the experimentation.
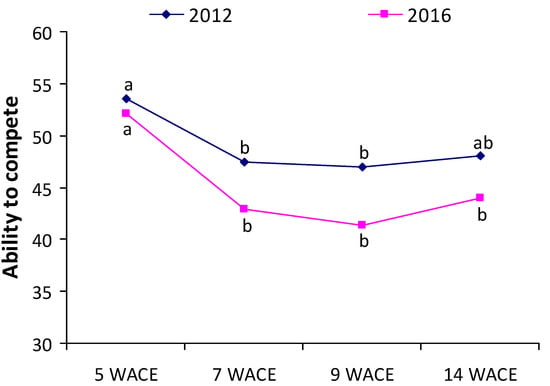
Figure 7.
Ability to compete (AC) index at various weeks after crop emergence (WACE) in the first and second growing seasons. Different letters in each growing season indicate statistically significant differences at p < 0.05.
The relative yield losses in terms of dry matter accumulation were 18.0 and 25.3% in the first growing season, and 29.5 and 33.4% in the second growing season in the vegetative stage (7 and 9 weeks from crop emergence, respectively) (Figure 8). However, the reduction in the aboveground dry matter accumulation after flowering (14 weeks after crop emergence) was limited to lower levels (9.9% and 12.8% in the first and the second growing season, respectively), indicating that the crop was more susceptible during the early vegetative stages than in the reproductive stage.
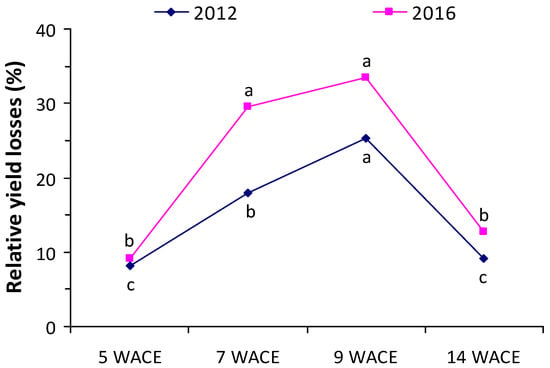
Figure 8.
Relative yield loss (RYL) index at various weeks after crop emergence (WACE) in the first and second growing seasons. Different letters in each growing season indicate statistically significant differences at p < 0.05.
4. Discussion
The present work provides an assessment of the season-long weed competition effects by natural weed flora on spring-sown white lupine growth and productivity for two growing seasons. In particular, the collected evidence illustrates the intensity of competition of white lupine with Chenopodium album and Sorghum halepense during the growth cycle and the impact of competition on dry matter accumulation and seed yield of the crop. Similar studies do not exist in the literature for white lupine. According to findings, the presence of Chenopodium album and Sorghum halepense reduced aboveground dry matter accumulation of white lupine ‘Multitalia’ by 18.0 and 25.3% in the first growing season and by 29.5 and 33.4% in the second growing season in the vegetative stage (7 and 9 weeks from crop emergence, respectively). However, the reduction in the aboveground dry matter accumulation was lower after flowering (9.9 and 12.8% in the first and the second growing season, respectively), indicating susceptibility of the crop to weed competition, especially in the early vegetative stages of growth. Although this finding seems obvious, it clearly distinguishes between early and late competition during the growing season, where the growth habit of the crop plays a key role. Similarly, lower values of the AWC index and the AC index were identified at 7 and 9 weeks from crop emergence in both years compared with maturity, confirming the susceptibility of white lupine to weed competition. In particular, the lower values of those indices at the vegetative stage highlight the susceptibility of white lupine to tolerate weed competition and its poor competitive ability with weeds at this stage. Findings of the current study are in line with pertinent literature that reports Lupinus species as poor weed competitors, especially during early establishment [11]. This response could be attributed to the slow development of a full plant canopy by the crop in the early stages of growth, a fact that facilitates light penetration, weed seed germination, and rapid weed growth at those early growth stages [12]. Lupines reach the maximum levels of vegetative growth and competitiveness during flowering [13] probably due to full canopy development and rapid dry matter accumulation compared with early stages. Therefore, early sowing, appropriate population densities, and selection of robust varieties could give lupine cultivars a competitive advantage.
Each crop has a period in its life cycle when it is most sensitive to occurrence of weeds, known as critical period of competition [23]. The critical period defines the most vulnerable stages of crop growth to weed competition. It is defined as the time (e.g., weeks after crop emergence) for which a crop should remain free of weeds to keep yield losses below acceptable levels [24,25]. According to findings of the current study, the presence of Chenopodium album and Sorghum halepense reduced the aboveground dry matter accumulation of white lupine ‘Multitalia’, with the reduction being more apparent in the vegetative stage, as indicated by all indices used for the assessment. From this point of view, the crop must be kept weed-free preferably in the early growth stage, because a weed infestation at this stage showed greater competition intensity and, furthermore, the crop showed low ability to tolerate competition and low ability to compete with the weeds. Therefore, it is reasonable to assume that yield loss might be avoided if weed control is performed at any point in the early growth stages. Experimental data in soybean (Glycine max L.) showed that weeds should be removed before plants reach 18 cm (V2 to V3 growth stages) to avoid a 5% loss in seed yield, so that early-season control of weeds maintains yield potential [26]. In addition, the seed yield of the legume cowpea (Vignia unguiculata L.) largely increased with maintaining weed-free conditions up to 60 days after sowing (DAS), which was similar with the 80 DAS and season-long weed-free situation [27]. Nevertheless, findings of the current study cannot define the critical period of weed competition, because the study did not assess separate seed yields after a specific growth period of weed competition but yield only after season-long competition. This limitation should be addressed in future studies, so that a critical period of competition can be clearly determined.
The density of weeds required to cause a significant reduction in crop yield is difficult to define because of the capacity of weeds for continuous emergence throughout the growing season or due to their appearance in cohorts. For practical reasons, weed competition in the present study was limited to Chenopodium album and Sorghum halepense, which were the dominant weeds in the study area with a relatively uniform density of populations, while other weeds were removed. In particular, there were, on average, 8–10 plants per m2 for Chenopodium album and 2–4 plants per m2 for Sorghum halepense. However, weed density is not the only factor affecting crop yield; the relative time of weed emergence also plays a key role in competition with crops, allowing weeds to capture the space earlier [28]. Therefore, the time of weed emergence relative to the crop, along with densities, may be responsible for the variation in crop–weed interference relationships among locations and years [29]. In the current study, most common lambsquarters plants emerged within 1–2 weeks after crop emergence, while the majority of johnsongrass plants emerged within 4–5 weeks following crop emergence. Both weeds emerged earlier in the second growing season, which probably explains the greater competition intensity and yield losses in that growing season. The importance of the timing of weed emergence in defining the critical period of weed control is underlined by Knezevic et al. [30], who reported that earlier weed emergence could cause earlier beginning of the critical period. Moreover, the pattern of weed emergence varies with weed species and it is greatly affected by the microclimate of the area [31].
Many research studies have tried to address the issue of defining harmful weed densities to help in the establishment of some thresholds, but usually results reflect specific crop management practices, specific weeds, and specific growth conditions, all of which normally vary, making findings difficult to generalize. Therefore, establishing consistent thresholds for specific yield reduction has been difficult. For example, different findings concerning the impact of crop–weed competition should not be considered surprising but could be due to different environmental conditions that affect plant growth. In light of the above, research on competition across several sites and years is needed to establish or refine thresholds. Nevertheless, proposing general threshold guidelines for crop losses would be possible, even with some degree of risk, when likely responses occur, as in the current study that reflects almost similar growth conditions with the same weeds in two growing seasons.
Crops vary greatly in their competitive ability against weeds; for example, certain crops are susceptible to weed competition while others are strong competitors. However, crop competitive ability is a relative issue. Crop–weed competition studies offer useful information concerning the necessity of weed control, and if so, about the most appropriate timing for weed control [32]. Moreover, such studies can be used to predict yield losses from weed competition and define optimum timing of weed control. From this point of view, data of this study provide useful information on the response of white lupine to weed competition, for which no information exists in the literature. Nevertheless, weed–crop competition is a complex phenomenon that is influenced by various biological and environmental factors. Therefore, findings provide an assessment of weed competition in white lupine under the specific circumstances of the study area. Crop–weed competition studies in the field are largely affected by the plant density and the spatial arrangement of each species, the soil type, and the environment [32]. Consequently, more studies should be conducted for several cropping seasons at different locations to ensure applicability in different environments.
5. Conclusions
The present work assessed the effects of season-long weed competition by natural weed flora on the growth and productivity of spring-sown white lupine over two growing seasons. Competition by Chenopodium album and Sorghum halepense reduced the aboveground dry matter accumulation of white lupine in the vegetative stage by 18.0 and 25.3% in the first growing season and by 29.5 and 33.4% in the second growing season 7 and 9 weeks from crop emergence, respectively. The values of the relative yield loss (RYL) index for seed yield were 28.2% in the first growing season and 31.5% in the second growing season. Findings offer novel information in the area of legume competition with weeds because similar studies do not exist in the literature for white lupine. Given the lack of herbicides for weed control in this crop, the results can be useful for decision making about weed management in this crop, with respect to the proper timing of weed control, e.g., for the time of weed removal or for improving the effectiveness of weed management by implementing crop management practices that enhance crop competitiveness. With this in mind, emphasis should be placed on developing appropriate cropping methods (e.g., competitive cultivars, early sowing, narrow crop spacing, weed removal soon after crop emergence) to increase the competitive ability of white lupine crop over weeds. Future research on weed competition across several sites and years would be useful to define more clearly the critical period of weed control in white lupine.
Author Contributions
Conceptualization, C.A.D.; methodology, C.A.D.; validation, C.A.D. and S.D.K.; data curation, C.A.D. and S.D.K.; writing—original draft preparation, C.A.D.; writing—review and editing, C.A.D. and S.D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huyghe, C. White lupin (Lupinus albus L.). Field Crops Res. 1997, 53, 147–160. [Google Scholar] [CrossRef]
- Prusinski, J. White lupin (Lupinus albus L.)—Nutritional and health values in human nutrition—A review. Czech J. Food Sci. 2017, 35, 95–105. [Google Scholar]
- López-Bellido, L.; Fuentes, M. Growth, yield and yield components of lupin cultivars. Agron. J. 1990, 82, 1050–1056. [Google Scholar] [CrossRef]
- Nigussie, Z. Contribution of white lupin (Lupinus albus L.) for food security in north-western Ethiopia: A review. Asian J. Plant Sci. 2012, 11, 200–205. [Google Scholar] [CrossRef]
- FAO. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 March 2022).
- Erbas, M.; Certel, M.; Uslu, M.K. Some chemical properties of white lupin seeds (Lupinus albus L.). Food Chem. 2005, 89, 341–345. [Google Scholar] [CrossRef]
- Mierlita, D.; Simeanu, D.; Pop, I.M.; Criste, F.; Pop, C. Chemical composition and nutritional evaluation of the lupine seeds (Lupinus albus L.) from low-alkaloid varieties. Rev. Chim. 2018, 69, 453–458. [Google Scholar] [CrossRef]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Aldrich, R. Predicting crop yield reductions from weeds. Weed Technol. 1987, 1, 199–206. [Google Scholar] [CrossRef]
- Van Heemst, H.D.J. The influence of weed competition on crop yield. Agric. Syst. 1985, 18, 81–93. [Google Scholar] [CrossRef]
- Folgart, A.; Price, A.J.; Van Santen, E.; Wehtje, G.R. Organic weed control in white lupin (Lupinus albus L.). Renew. Agric. Food Syst. 2011, 26, 193–199. [Google Scholar] [CrossRef][Green Version]
- Seiffert, M.; Makowski, N.; Moll, A.; Naumann, S.; Oehme, I.L.; Schulz, H.; Wicke, H.-J.; Virsing, F. Drusch- und Hackfruchtproduktion; VEB Deutscher Landwirtschaftsverlag: Berlin, Germany, 1981; 399p. [Google Scholar]
- Putnam, D.H.; Wright, J.; Field, L.A.; Ayisi, K.K. Seed yield and water-use efficiency of white lupin as influenced by irrigation, row spacing, and weeds. Agron. J. 1992, 84, 557–563. [Google Scholar] [CrossRef]
- Dewitte, K.; Latré, J.; Haesaert, G. Possibilities of chemical weed control in Lupinus albus and Lupinus luteus-screening of herbicides. Commun. Agric. Appl. Biol. Sci. 2006, 71, 743–751. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Weigelt, A.; Jolliffe, P. Indices of plant competition. J. Ecol. 2003, 91, 707–720. [Google Scholar] [CrossRef]
- Grace, J.B. On the measurement of plant competition intensity. Ecology 1995, 76, 305–308. [Google Scholar] [CrossRef]
- Watson, P.R.; Derksen, D.A.; Van Acker, R.C. The ability of 29 barley cultivars to compete and withstand competition. Weed Sci. 2006, 54, 783–792. [Google Scholar] [CrossRef]
- Szumigalski, A.; Van Acker, R. Weed suppression and crop production in annual intercrops. Weed Sci. 2005, 53, 813–825. [Google Scholar] [CrossRef]
- Saito, K.; Azoma, K.; Rodenburg, J. Plant characteristics associated with weed competitiveness of rice under upland and lowland conditions in West Africa. Field Crops Res. 2010, 116, 308–317. [Google Scholar] [CrossRef]
- Russel, D.F.; Eisensmith, S.P. MSTAT-C; Department of Crop and Soil Sciences, Michigan State University: East Lansing, MI, USA, 1983. [Google Scholar]
- Mubeen, K.; Tanveer, A.; Nadeem, M.A.; Sarwar, N.; Shahzad, M. Critical period of weed-crop competition in fennel (Foeniculum vulgare Mill.). Pakistan J. Weed Sci. Res. 2009, 15, 171–181. [Google Scholar]
- Hall, M.R.; Swanton, C.J.; Anderson, G.W. The critical period of weed control in grain corn (Zea mays). Weed Sci. 1992, 40, 441–447. [Google Scholar] [CrossRef]
- Knezevic, Z.S.; Weise, S.F.; Swanton, C.J. Interference of redrood pigweed in corn. Weed Sci. 1994, 42, 568–573. [Google Scholar] [CrossRef]
- Harre, N.T.; Young, B.G. Early-season nutrient competition between weeds and soybean. J. Plant Nutr. 2020, 43, 1887–1906. [Google Scholar] [CrossRef]
- Yadav, T.; Chopra, N.K.; Kumar, R.; Soni, P.G. Assessment of critical period of crop-weed competition in forage cowpea (Vigna unguiculata) and its effect on seed yield and quality. Indian J. Agron. 2018, 63, 124–127. [Google Scholar]
- Knezevic, S.Z.; Evans, S.P.; Mainz, M. Row spacing influences the critical timing for weed removal in soybean (Glycine max). Weed Technol. 2003, 17, 666–673. [Google Scholar] [CrossRef]
- Lindquist, J.L.; Mortensen, D.A.; Westra, P.; Lambert, W.J.; Bauman, T.T.; Fausey, J.C.; Kells, J.J.; Langton, S.J.; Harvey, R.G.; Bussler, B.H.; et al. Stability of corn (Zea mays)–foxtail (Setaria spp.) interference relationships. Weed Sci. 1999, 47, 195–200. [Google Scholar] [CrossRef][Green Version]
- Knezevic, S.Z.; Evans, S.P.; Blankenship, E.E.; Van Acker, R.C.; Lindquist, J.L. Critical period for weed control: The concept and data analysis. Weed Sci. 2002, 50, 773–786. [Google Scholar] [CrossRef]
- Forcella, F.; Wilson, R.G.; Dekker, J.; Kremer, R.J.; Cardina, J.; Anderson, R.L.; Buhler, D.D. Weed seed bank emergence across the Corn Belt. Weed Sci. 1997, 45, 67–76. [Google Scholar] [CrossRef]
- Swanton, C.J.; Nkoa, R.; Blackshaw, R.E. Experimental methods for crop-weed competition studies. Weed Sci. 2015, 63, 2–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).