Essential Oils in Citrus Fruit Ripening and Postharvest Quality
Abstract
1. Introduction
2. Essential Oils of Citrus Fruits
3. Main Changes in the Essential Oils Content during Fruit Maturation
4. Biological Properties as Related to Protection against Postharvest Deterioration
5. The Role of Endophytic Fungi
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghada, B.; Amel, O.; Aymen, M.; Aymen, A.; Amel, S.H. Phylogenetic patterns and molecular evolution among ‘True citrus fruit trees’ group (Rutaceae family and Aurantioideae subfamily). Sci. Hortic. 2019, 253, 87–98. [Google Scholar] [CrossRef]
- Zhong, G.; Nicolosi, E. Citrus origin, diffusion, and economic importance. In The Citrus Genome; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-10799-4. [Google Scholar]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- BS EN ISO 9235:2021; BSI Standards Publication Aromatic Natural Raw Materials—Vocabulary. ISO: Geneve, Switzerland, 2021.
- Malik, S. Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-030-16545-1. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosyntheic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-0-470-74168-9. [Google Scholar]
- Simas, D.L.R.; de Amorim, S.H.B.M.; Goulart, F.R.V.; Alviano, C.S.; Alviano, D.S.; da Silva, A.J.R. Citrus species essential oils and their components can inhibit or stimulate fungal growth in fruit. Ind. Crops Prod. 2017, 98, 108–115. [Google Scholar] [CrossRef]
- Pavela, R.; Stepanycheva, E.; Shchenikova, A.; Chermenskaya, T.; Petrova, M. Essential oils as prospective fumigants against Tetranychus urticae Koch. Ind. Crops Prod. 2016, 94, 755–761. [Google Scholar] [CrossRef]
- Taghadomi-Saberi, S.; Garcia, S.M.; Masoumi, A.A.; Sadeghi, M.; Marco, S. Classification of bitter orange essential oils according to fruit ripening stage by untargeted chemical profiling and machine learning. Sensors 2018, 18, 1922. [Google Scholar] [CrossRef]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Tounsi, M.S. Changes of peel essential oil composition of four tunisian citrus during fruit maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Aouadi, K.; Snoussi, M.; Noumi, E.; Kadri, A. GC-MS profile, α-glucosidase inhibition potential, antibacterial and antioxidant evaluation of peels Citrus aurantium (L.), essential oil. J. Pharm. Res. Int. 2021, 33, 1580–1591. [Google Scholar] [CrossRef]
- Navarra, M.; Ferlazzo, N.; Cirmi, S.; Trapasso, E.; Bramanti, P.; Lombardo, G.E.; Minciullo, P.L.; Calapai, G.; Gangemi, S. Effects of bergamot essential oil and its extractive fractions on SH-SY5Y human neuroblastoma cell growth. J. Pharm. Pharmacol. 2015, 67, 1042–1053. [Google Scholar] [CrossRef]
- Furneri, P.M.; Mondello, L.; Mandalari, G.; Paolino, D.; Dugo, P.; Garozzo, A.; Bisignano, G. In vitro antimycoplasmal activity of Citrus bergamia essential oil and its major components. Eur. J. Med. Chem. 2012, 52, 66–69. [Google Scholar] [CrossRef]
- Sawamura, M.; Onishi, Y.; Ikemoto, J.; Tu, N.T.M.; Phi, N.T.L. Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Fragr. J. 2006, 21, 609–615. [Google Scholar] [CrossRef]
- Rana, V.S.; Blazquez, M.A. Compositions of the volatile oils of Citrus macroptera and C. maxima. Nat. Prod. Commun. 2012, 7, 1371–1372. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. J. Agric. Food Chem. 2005, 53, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.; Ha, P.T.H.; Phong, H.X.; Hien, T.T.; Ngan, T.T.K. Extraction of essential oils and volatile compounds of Kaffir lime (Citrus hystrix D.C) by hydrodistillation method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012024. [Google Scholar] [CrossRef]
- Colecio-Juárez, M.C.; Rubio-Núñez, R.E.; Botello-Álvarez, J.E.; Martínez-González, G.M.; Navarrete-Bolaños, J.L.; Jiménez-Islas, H. Characterization of volatile compounds in the essential oil of sweet lime (Citrus limetta Risso). Chil. J. Agric. Res. 2012, 72, 275–280. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mohanty, S.; Pal, A.; Chanotiya, C.S.; Bawankule, D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: In-vitro and in-vivo study. J. Ethnopharmacol. 2018, 212, 86–94. [Google Scholar] [CrossRef]
- Paw, M.; Begum, T.; Gogoi, R.; Pandey, S.K.; Lal, M. Chemical composition of Citrus limon L. Burmf peel essential oil from North East India. J. Essent. Oil-Bearing Plants 2020, 23, 337–344. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Chhikara, N.; Kour, R.; Jaglan, S.; Gupta, P.; Gat, Y.; Panghal, A. Citrus medica: Nutritional, phytochemical composition and health benefits–a review. Food Funct. 2018, 9, 1978–1992. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Chutia, M.; Deka Bhuyan, P.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT–Food Sci. Technol. 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Mungai, H.N.; Koaze, H.; Phi, N.T.L.; Sawamura, M. Volatile constituents of mandarin (Citrus reticulata Blanco) peel oil from Burundi. J. Essent. Oil Res. 2006, 18, 659–662. [Google Scholar] [CrossRef]
- Ferrer, V.; Paymal, N.; Quinton, C.; Costantino, G.; Paoli, M.; Froelicher, Y.; Ollitrault, P.; Tomi, F.; Luro, F. Influence of the rootstock and the ploidy level of the scion and the rootstock on sweet orange (Citrus sinensis) peel essential oil yield, composition and aromatic properties. Agriculture 2022, 12, 214. [Google Scholar] [CrossRef]
- Choi, H.S.; Sawamura, M. Composition of essential oil of Citrus tamurana Hort. ex Tanaka (Hyuganatsu). J. Agric. Food Chem. 2002, 66, 4868–4873. [Google Scholar] [CrossRef]
- Choi, H.S.; Sawamura, M. Effects of storage conditions on the composition of Citrus tamurana hort. ex Tanaka (hyuganatsu) essential oil. Biosci. Biotechnol. Biochem. 2002, 66, 439–443. [Google Scholar] [CrossRef]
- Yang, X.N.; Kang, S.C. Chemical composition, antioxidant and antibacterial activities of essential oil from Korean Citrus unshiu peel. J. Agric. Chem. Environ. 2013, 2, 42–49. [Google Scholar] [CrossRef]
- Bermejo, A.; Cano, A. Analysis of nutritional constituents in twenty citrus cultivars from the Mediterranean area at different stages of ripening. Food Nutr. Sci. 2012, 3, 639–650. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Costell, E.; Carbonell, I.; Rojas, C.; Peña, L. Impact of D-limonene synthase up- or down-regulation on sweet orange fruit and juice odor perception. Food Chem. 2017, 217, 139–150. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Juárez, M.L.; Alzogaray, R.A.; Arrighi, F.; Arroyo, L.; Gastaminza, G.; Willink, E.; Bardón, A.D.V.; Vera, T. Toxic effect of citrus peel constituents on Anastrepha fraterculus Wiedemann and Ceratitis capitata Wiedemann immature stages. J. Agric. Food Chem. 2014, 62, 10084–10091. [Google Scholar] [CrossRef]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D. Variation in the components and antioxidant activity of Citrus medica L. var. Sarcodactylis essential oils at different stages of maturity. Ind. Crops Prod. 2013, 46, 311–316. [Google Scholar]
- Di Rauso Simeone, G.; Di Matteo, A.; Rao, M.A.; Di Vaio, C. Variations of peel essential oils during fruit ripening in four lemon (Citrus limon (L.) Burm. F.) cultivars. J. Sci. Food Agric. 2020, 100, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, S.; Baldi, E.; Crucitti, M.C.; Toselli, M.; Caboni, M.F. Effect of harvesting time on volatile compounds composition of bergamot (Citrus × Bergamia) essential oil. Flavour Fragr. J. 2019, 34, 426–435. [Google Scholar] [CrossRef]
- Bhuyan, N.; Barua, P.C.; Kalita, P.; Saikia, A. Physico-chemical variation in peel oils of Khasi mandarin (Citrus reticulata Blanco) during ripening. Indian J. Plant. Physiol. 2015, 20, 227–231. [Google Scholar] [CrossRef]
- Ghani, A.; Mohtashami, S.; Jamalian, S. Peel essential oil content and constituent variations and antioxidant activity of grapefruit (Citrus × paradisi var. red blush) during color change stages. J. Food Meas. Charact. 2021, 15, 4917–4928. [Google Scholar] [CrossRef]
- Rowshan, V.; Najafian, S. Changes of peel essential oil composition of Citrus aurantium L. during fruit maturation in Iran. J. Essent. Oil-Bearing Plants 2015, 18, 1006–1012. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Dhuique-Mayer, C.; Luro, F.; Casanova, J.; Morillon, R.; Ollitrault, P. Carotenoid diversity in cultivated citrus is highly influenced by genetic factors. J. Agric. Food Chem. 2006, 54, 4397–4406. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Morrone, L.A.; Rombolà, L.; Pelle, C.; Corasaniti, M.T.; Zappettini, S.; Paudice, P.; Bonanno, G.; Bagetta, G. The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: Implication of monoterpene hydrocarbons. Pharmacol. Res. 2007, 55, 255–262. [Google Scholar] [CrossRef]
- Kang, P.; Suh, S.H.; Min, S.S.; Seol, G.H. The essential oil of Citrus bergamia Risso induces vasorelaxation of the mouse aorta by activating K+ channels and inhibiting Ca2+ influx. J. Pharm. Pharmacol. 2013, 65, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Di Vaio, C.; Graziani, G.; Gaspari, A.; Scaglione, G.; Nocerino, S.; Ritieni, A. Essential oils content and antioxidant properties of peel ethanol extract in 18 lemon cultivars. Sci. Hortic. 2010, 126, 50–55. [Google Scholar] [CrossRef]
- Frassinetti, S.; Caltavuturo, L.; Cini, M.; Della Croce, C.M.; Maserti, B.E. Antibacterial and antioxidant activity of essential oils from Citrus spp. J. Essent. Oil Res. 2011, 23, 27–31. [Google Scholar] [CrossRef]
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of natural antioxidants and potential use of bergamot in treating rheumatoid arthritis. PharmaNutrition 2015, 3, 53–59. [Google Scholar] [CrossRef]
- Lamine, M.; Rahali, F.Z.; Hammami, M.; Mliki, A. Correlative metabolite profiling approach to understand antioxidant and antimicrobial activities from citrus essential oils. Int. J. Food Sci. Technol. 2019, 54, 2615–2623. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of some citrus essential oils on post-harvest shelf life and physicochemical quality of strawberries during cold storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Feng, L.X.; Zhang, B.Y.; Zhu, H.J.; Pan, L.; Cao, F. Bioactive metabolites from Talaromyces purpureogenus, an endophytic fungus from Panax notoginseng. Chem. Nat. Compd. 2020, 56, 974–976. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Locatelli, M.; Navarra, M.; Ventura, C.A.; Wolfram, J.; Carafa, M.; Morittu, V.M.; Britti, D.; Di Marzio, L.; et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf. B Biointerfaces 2013, 112, 548–553. [Google Scholar] [CrossRef]
- Dudai, N.; Weinstein, Y.; Krup, M.; Rabinski, T.; Ofir, R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005, 71, 484–488. [Google Scholar] [CrossRef]
- Xia, H.; Liang, W.; Song, Q.; Chen, X.; Chen, X.; Hong, J. The in vitro study of apoptosis in NB4 cell induced by citral. Cytotechnology 2013, 65, 49–57. [Google Scholar] [CrossRef]
- Subba, M.S.; Soumithri, T.C.; Rao, R.S. Antimicrobial action of citrus oils. J. Food Sci. 1967, 32, 225–227. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. In vitro inhibition of vancomycin-susceptible and vancomycin-resistant Enterococcus faecium and E. faecalis in the presence of citrus essential oils. Br. J. Biomed. Sci. 2009, 66, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Romano, L.; Battaglia, F.; Lopizzo, T.; De Carolis, E.; Fadda, G. In vitro activity of Citrus bergamia (bergamot) oil against clinical isolates of dermatophytes. J. Antimicrob. Chemother. 2007, 59, 305–308. [Google Scholar] [CrossRef]
- Sah, A.N.; Juyal, V.; Melkani, A.B. Antimicrobial activity of six different parts of the plant Citrus medica Linn. Pharmacogn. J. 2011, 3, 80–83. [Google Scholar] [CrossRef]
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial effects and mechanism of mandarin (Citrus reticulata L.) essential oil against Staphylococcus aureus. Molecules 2020, 25, 4956. [Google Scholar] [CrossRef]
- Moreira, M.R.; Ponce, A.G.; Del Valle, C.E.; Roura, S.I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT-Food Sci. Technol. 2005, 38, 565–570. [Google Scholar] [CrossRef]
- Conte, A.; Speranza, B.; Sinigaglia, M.; Del Nobile, M.A. Effect of lemon extract on foodborne microorganisms. J. Food Prot. 2007, 70, 1896–1900. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Álvarez, J. Antibacterial activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. J. Food Saf. 2008, 28, 567–576. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Bai, J.; Nong, L.; Ning, Z.; Hu, Z.; Xu, A.; Xu, C.P. Chemical composition and antibacterial activity of the essential oil of Citrus maxima (Burm.) Merr. Cv. Shatian Yu. J. Biol. Act. Prod. from Nat. 2018, 8, 228–233. [Google Scholar]
- Mehmood, T.; Afzal, A.; Anwar, F.; Iqbal, M.; Afzal, M.; Qadir, R. Variations in the composition, antibacterial and haemolytic activities of peel essential oils from unripe and ripened Citrus limon (L.) Osbeck fruit. J. Essent. Oil Bear. Plants 2019, 22, 159–168. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
- Mirzaei-Najafgholi, H.; Tarighi, S.; Golmohammadi, M.; Taheri, P. The effect of citrus essential oils and their constituents on growth of Xanthomonas citri subsp. citri. Molecules 2017, 22, 591. [Google Scholar] [CrossRef] [PubMed]
- Caccioni, D.R.L.; Guizzardi, M.; Biondi, D.M.; Renda, A.; Ruberto, G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int. J. Food Microbiol. 1998, 43, 73–79. [Google Scholar] [CrossRef]
- Kuate, J.; Foko, J.; Ndindeng, S.A.; Jazet-Dongmo, P.M.; Fouré, E.; Damesse, F.; Bella-Manga; Ducelier, D. Effect of essential oils from citrus varieties on in vitro growth and sporulation of Phaeoramularia angolensis causing citrus leaf and fruit spot disease. Eur. J. Plant. Pathol. 2006, 114, 151–161. [Google Scholar] [CrossRef]
- Chuah, T.S.; Tan, Y.Y.; Ismail, B.S. In vitro evaluation of the antifungal activity of some essential oils on post-harvest fungal pathogens of tropical fruits. Plant Prot. Q. 2010, 25, 162–164. [Google Scholar]
- Bosquez-Molina, E.; Ronquillo-de Jesús, E.; Bautista-Baños, S.; Verde-Calvo, J.R.; Morales-López, J. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Badawy, F.I.; Sallam, M.N.; Ibrahim, A.R.; Asran, M.R. Efficacy of some essential oils on controlling green mold of orange and their effects on postharvest quality parameters. Plant. Pathol. J. 2011, 10, 168–174. [Google Scholar] [CrossRef]
- Van Hung, P.; Chi, P.T.; Phi, N.T. Comparison of antifungal activities of Vietnamese citrus essential oils. Nat. Prod. Res. 2013, 27, 506–508. [Google Scholar] [CrossRef]
- Delkhoon, S.; Fahim, M.; Hosseinzadeh, J.; Panahi, O. Effect of lemon essential oil on the developmental stages of Trialeurodes vaporariorum West (Homoptera: Aleyrodidae). Arch. Phytopathol. Plant. Prot. 2013, 46, 569–574. [Google Scholar] [CrossRef]
- Sauer, A.V.; Santos, E.M.; Gonçalves-Zuliani, A.M.O.; Nocchi, P.T.R.; Nunes, W.M.C.; Bonato, C.M. Bacteriostatic and bactericidal activity in vitro of different essential oils as alternative treatments to control Xanthomonas citri subsp. citri. Acta Hortic. 2015, 1065, 931–936. [Google Scholar] [CrossRef]
- Bnina, E.B.; Hajlaoui, H.; Chaieb, I.; Daami-Remadi, M.; Said, M.B.; Jannet, H.B. Chemical composition, antimicrobial and insecticidal activities of the tunisian Citrus aurantium essential oils. Czech. J. Food Sci. 2019, 37, 81–92. [Google Scholar] [CrossRef]
- Pawar, V.C.; Thaker, V.S. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2006, 49, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, M.; Moharramipour, S.; Sefidkon, F.; Aghajanzadeh, S. Insecticidal and repellent activities of Citrus reticulata, Citrus limon and Citrus aurantium essential oils on Callosobruchus maculatus. Integr. Prot. Stored Prod. 2011, 69, 289–293. [Google Scholar]
- Lamine, M.; Gargouri, M.; Rahali, F.Z.; Mliki, A. Recovering and characterizing phenolic compounds from citrus by-product: A way towards agriculture of subsistence and sustainable bioeconomy. Waste Biomass Valor. 2021, 12, 4721–4731. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Kimbaris, A.C.; Papadopoulos, N.T.; Polissiou, M.G. Toxicity of citrus essential oils against Ceratitis capitata (Diptera: Tephritidae) larvae. Ann. Appl. Biol. 2009, 155, 381–389. [Google Scholar] [CrossRef]
- De Souza, E.L.; De Oliveira Lima, E.; De Luna Freire, K.R.; De Sousa, C.P. Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Brazilian Arch. Biol. Technol. 2005, 48, 245–250. [Google Scholar] [CrossRef]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 86–92. [Google Scholar] [CrossRef]
- Ammad, F.; Moumen, O.; Gasem, A.; Othmane, S.; Hisashi, K.N.; Zebib, B.; Merah, O. The potency of lemon (Citrus limon L.) essential oil to control some fungal diseases of grapevine wood. Comptes Rendus-Biol. 2018, 341, 97–101. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Subban, K.; Chelliah, J. Cloning and sequence analysis of 10-deacetylbaccatin III-10-O-acetyl transferase gene and WRKY1 transcription factor from taxol-producing endophytic fungus Lasiodiplodia theobromae. FEMS Microbiol. Lett. 2017, 364, fnx253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kabra, A.O.; Bairagi, G.B.; Mahamuni, A.S.; Wanare, R.S. In vitro antimicrobial activity and phytochemical analysis of the peels of Citrus medica L. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 34–37. [Google Scholar]
- Abd-Alla, M.A.; Haggag, W.M. Use of some plant essential oils as post-harvest botanical fungicides in the management of anthracnose disease of mango fruits (Mangifera indica L.) caused by Colletotrichum gloeosporioides (Penz). Int. J. Agric. For. 2013, 3, 1–6. [Google Scholar]
- Vilaplana, R.; Pazmiño, L.; Valencia-Chamorro, S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol. Technol. 2018, 138, 56–63. [Google Scholar] [CrossRef]
- Qadir, R.; Farooq Anwar, T.M.; Shahid, M.; Zahoor, S. Variations in chemical composition, antimicrobial and haemolytic activities of peel essential oils from three local Citrus cultivars. Pure Appl. Biol. 2018, 7, 282–291. [Google Scholar] [CrossRef]
- Tao, N.G.; Liu, Y.J.; Zhang, M.L. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck). Int. J. Food Sci. Technol. 2009, 44, 1281–1285. [Google Scholar] [CrossRef]
- Singh, G.; Upadhyay, R.K.; Narayanan, C.S.; Padmkumari, K.P.; Rao, G.P. Chemical and fungitoxic investigations on the essential oil of Citrus sinensis (L.) Pers/Untersuchungen über die chemie und fungitoxizität der ätherischen öle von Citrus sinensis (L.) Pers. zeitschrift für pflanzenkrankheiten und pflanzenschutz. J. Plant. Dis. Prot. 1993, 100, 69–74. [Google Scholar]
- Shukla, A.C.; Shani, S.K.; Dixit, A. Epicarp of Citrus sinensis: A potential source of natural pesticide. Ind. Phytopath. 2000, 53, 468–471. [Google Scholar]
- Sharma, N.; Tripathi, A. Fungitoxicity of the essential oil of Citrus sinensis on post-harvest pathogens. World J. Microbiol. Biotechnol. 2006, 22, 587–593. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rozwalka, L.C.; De Costa Lima, M.L.R.Z.; Mio, L.L.M.D.; Nakashima, T. Extracts, decoctions and essential oils of medicinal and aromatic plants in the inhibition of Colletotrichum gloeosporioides and Glomerella cingulata isolates from guava fruits. Ciência Rural 2008, 38, 301–307. [Google Scholar] [CrossRef]
- Velázquez-Nuñez, M.J.; Avila-Sosa, R.; Palou, E.; López-Malo, A. Antifungal activity of orange (Citrus sinensis var. Valencia) peel essential oil applied by direct addition or vapor contact. Food Control. 2013, 31, 1–4. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Chaisiri, C.; Luo, C.X.; Liu, X.Y.; Lin, Y.; Li, J.B.; Xiong, B. Phylogenetic analysis and development of molecular tool for detection of Diaporthe citri causing melanose disease of citrus. Plants 2020, 9, 329. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The thin line between pathogenicity and endophytism: The case of Lasiodiplodia theobromae. Agriculture 2020, 10, 488. [Google Scholar] [CrossRef]
- Phillips, C.A.; Laird, K.; Allen, S.C. The use of Citri-VTM ®-An antimicrobial citrus essential oil vapour for the control of Penicillium chrysogenum, Aspergillus niger and Alternaria alternata in vitro and on food. Food Res. Int. 2012, 47, 310–314. [Google Scholar] [CrossRef]
- Kumar, A.; Kudachikar, V.B. Antifungal properties of essential oils against anthracnose disease: A critical appraisal. J. Plant. Dis. Prot. 2018, 125, 133–144. [Google Scholar] [CrossRef]
- Karapinar, M. The effects of citrus oils and some spices on growth and aflatoxin production by Aspergillus parasiticus NRRL 2999. Int. J. Food Microbiol. 1985, 2, 239–245. [Google Scholar] [CrossRef]
- Rammanee, K.; Hongpattarakere, T. Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Bioprocess. Technol. 2011, 4, 1050–1059. [Google Scholar] [CrossRef]
- Bamba, R.; Sumbali, G. Co-occurrence of aflatoxin B1 and cyclopiazonic acid in sour lime (Citrus aurantifolia Swingle) during post-harvest pathogenesis by Aspergillus flavus. Mycopathologia 2005, 159, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Contreras, T.; Marín, S.; Villarruel-López, A.; Gschaedler, A.; Garrido-Sánchez, L.; Ascencio, F. Growth modeling of Aspergillus niger strains isolated from citrus fruit as a function of temperature on a synthetic medium from lime (Citrus latifolia T.) pericarp. J. Food Prot. 2017, 80, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Wang, F.; Xiao, C.L. Efficacy of natamycin against gray mold of stored Mandarin fruit caused by isolates of Botrytis cinerea with multiple fungicide resistance. Plant. Dis. 2020, 104, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Citrus and lemongrass essential oils inhibit Botrytis cinerea on ‘Golden Delicious’, ‘Pink Lady’ and ‘Granny Smith’ apples. J. Plant. Dis. Prot. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- Moraes Bazioli, J.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, D.M.; Kupper, K.C.; Augusto, F.; de Carvalho, J.E.; Fill, T.P. Biological control of citrus postharvest phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef]
- Rodov, V.; Ben-Yehoshua, S.; De Fang, Q.; Kim, J.J.; Ashkenazi, R. Preformed antifungal compounds of lemon fruit: Citral and its relation to disease resistance. J. Agric. Food Chem. 1995, 43, 1057–1061. [Google Scholar] [CrossRef]
- OuYang, Q.; Tao, N.; Jing, G. Transcriptional profiling analysis of Penicillium digitatum, the causal agent of citrus green mold, unravels an inhibited ergosterol biosynthesis pathway in response to citral. BMC Genomics 2016, 17, 599. [Google Scholar] [CrossRef]
- Suprapta, D.N.; Arai, K.; Iwai, H. Effects of volatile compounds on arthrospore germination and mycelial growth of Geotrichum candidum citrus race. Mycoscience 1997, 38, 31–35. [Google Scholar] [CrossRef]
- Klieber, A.; Scott, E.; Wuryatmo, E. Effect of method of application on antifungal efficacy of citral against postharvest spoilage fungi of citrus in culture. Australas. Plant. Pathol. 2002, 31, 329–332. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Klieber, A.; Scott, E.S. Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 2003, 51, 2637–2640. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control. 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Wu, Y.; OuYang, Q.; Tao, N. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus fruit. Lwt 2021, 141, 110924. [Google Scholar] [CrossRef]
- du Plooy, W.; Regnier, T.; Combrinck, S. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol. Technol. 2009, 53, 117–122. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Hink, W.F.; Fee, B.J. Toxicity of D-limonene, the major component of citrus peel oil, to all life stages of the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae). J. Med. Entomol. 1986, 23, 400–404. [Google Scholar] [CrossRef]
- da Cruz Silva, G.; de Oliveira Filho, J.G.; Ribeiro M de, M.M.; de Souza, C.W.O.; Ferreira, M.D. Antibacterial activity of nanoemulsions based on essential oils compounds against species of Xanthomonas that cause citrus canker. Biointerface Res. Appl. Chem. 2022, 12, 1835–1846. [Google Scholar] [CrossRef]
- Shimada, T.; Endo, T.; Fujii, H.; Rodríguez, A.; Peña, L.; Omura, M. Characterization of three linalool synthase genes from Citrus unshiu Marc. and analysis of linalool-mediated resistance against Xanthomonas citri subsp. citri and Penicilium italicum in citrus leaves and fruits. Plant Sci. 2014, 229, 154–166. [Google Scholar] [CrossRef]
- Shimada, T.; Endo, T.; Rodríguez, A.; Fujii, H.; Goto, S.; Matsuura, T.; Hojo, Y.; Ikeda, Y.; Mori, I.C.; Fujikawa, T.; et al. Ectopic accumulation of linalool confers resistance to Xanthomonas citri subsp. citri in transgenic sweet orange plants. Tree Physiol. 2017, 37, 654–664. [Google Scholar]
- Wang, H.; Tao, N.; Huang, S.; Liu, Y. Effect of Shatangju (Citrus reticulata Blanco) essential oil on spore germination and mycelium growth of Penicillium digitatum and P. italicum. J. Essent. Oil Bear. Plants 2012, 15, 715–723. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar] [CrossRef]
- Rodríguez, A.; Andrés, V.S.; Cervera, M.; Redondo, A.; Alquézar, B.; Shimada, T.; Gadea, J.; Rodrigo, M.J.; Zacarías, L.; Palou, L.; et al. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant. Physiol. 2011, 156, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, C.S.; Papadopoulos, N.T.; Kouloussis, N.A.; Tananaki, C.I.; Katsoyannos, B.I. Essential oils of citrus fruit stimulate oviposition in the Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). Physiol. Entomol. 2012, 37, 330–339. [Google Scholar] [CrossRef]
- Papanastasiou, S.A.; Bali, E.M.D.; Ioannou, C.S.; Papachristos, D.P.; Zarpas, K.D.; Papadopoulos, N.T. Toxic and hormetic-like effects of three components of citrus essential oils on adult Mediterranean fruit flies (Ceratitis capitata). PLoS ONE 2017, 12, e0177837. [Google Scholar] [CrossRef]
- Papanastasiou, S.A.; Ioannou, C.S.; Papadopoulos, N.T. Oviposition-deterrent effect of linalool–a compound of citrus essential oils–on female Mediterranean fruit flies, Ceratitis capitata (Diptera: Tephritidae). Pest. Manag. Sci. 2020, 76, 3066–3077. [Google Scholar] [CrossRef]
- Faraone, N.; De Cristofaro, A.; Maltese, M.; Vitagliano, S.; Caleca, V. First data on the repellent activity of essential oils of Citrus limon towards medfly (Ceratitis capitata). New Medit 2012, 11, 31–34. [Google Scholar]
- Kouloussis, N.A.; Katsoyannos, B.I.; Papadopoulos, N.T.; Ioannou, C.S.; Iliadis, I.V. Enhanced mating competitiveness of Ceratitis capitata males following exposure to citrus compounds. J. Appl. Entomol. 2013, 137, 30–38. [Google Scholar] [CrossRef]
- Niogret, J.; Epsky, N.D. Attraction of Ceratitis capitata (Diptera: Tephritidae) sterile males to essential oils: The importance of linalool. Environ. Entomol. 2018, 47, 1287–1292. [Google Scholar] [CrossRef]
- Hollingsworth, R.G. Limonene, a citrus extract, for control of mealybugs and scale insects. J. Econ. Entomol. 2005, 98, 772–779. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Wu, T.; Yuan, C.; Hu, Y. Antioxidant and antibacterial properties of coating with chitosan–citrus essential oil and effect on the quality of Pacific mackerel during chilled storage. Food Sci. Nutr. 2019, 7, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- El Guilli, M.; Hamza, A.; Clément, C.; Ibriz, M.; Barka, E.A. Effectiveness of postharvest treatment with chitosan to control citrus green mold. Agriculture 2016, 6, 12. [Google Scholar] [CrossRef]
- Bill, M.; Chidamba, L.; Gokul, J.K.; Korsten, L. Mango endophyte and epiphyte microbiome composition during fruit development and post-harvest stages. Horticulturae 2021, 7, 495. [Google Scholar] [CrossRef]
- Kumar, A.; Zhimo, Y.; Biasi, A.; Salim, S.; Feygenberg, O.; Wisniewski, M.; Droby, S. Endophytic microbiome in the carposphere and its importance in fruit physiology and pathology. In Postharvest Biology and Technology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 12, pp. 73–88. ISBN 978-3-030-56529-9. [Google Scholar]
- Nicoletti, R. Endophytic fungi of citrus plants. Agriculture 2019, 9, 247. [Google Scholar] [CrossRef]
- Wang, Z.; Sui, Y.; Li, J.; Tian, X.; Wang, Q. Biological control of postharvest fungal decays in citrus: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 861–870. [Google Scholar] [CrossRef]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agric 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Scalvenzi, L. New frontiers of essential oils research: Biotransformation of the phytocomplex and its pure compounds by endophytic fungi. Acta Hortic. 2012, 1030, 125–132. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Uma Shaanker, R. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef]
- Strobel, G.; Booth, E.; Schaible, G.; Mends, M.T.; Sears, J.; Geary, B. The Paleobiosphere: A novel device for the in vivo testing of hydrocarbon producing-utilizing microorganisms. Biotechnol. Lett. 2013, 35, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Pena, L.C.; Jung, L.F.; Savi, D.C.; Servienski, A.; Aluizio, R.; Goulin, E.H.; Galli-Terasawa, L.V.; de Noronha Sales Maia, B.H.L.; Annies, V.; Franco, C.R.C.; et al. A Muscodor strain isolated from Citrus sinensis and its production of volatile organic compounds inhibiting Phyllosticta citricarpa growth. J. Plant. Dis. Prot. 2017, 124, 349–360. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed]
- Suwannarach, N.; Kumla, J.; Bussaban, B.; Nuangmek, W.; Matsui, K.; Lumyong, S. Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop. Prot. 2013, 45, 63–70. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Able, A.J.; Ford, C.M.; Scott, E.S. Effect of volatile citral on the development of blue mould, green mould and sour rot on navel orange. Australas. Plant. Pathol. 2014, 43, 403–411. [Google Scholar] [CrossRef]
- Majeed, H.; Bian, Y.-Y.; Ali, B.; Jamil, A.; Majeed, U.; Khan, Q.F.; Iqbal, K.J.; Shoemaker, C.F.; Fang, Z. Essential oil encapsulations: Uses, procedures, and trends. RSC Adv. 2015, 5, 58449–58463. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli Rodriguez, M.; Demma Carà, P.; Lopez Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A step closer to circular bioeconomy for citrus peel waste: A review of yields and technologies for sustainable management of essential oils. J. Environ. Manag. 2021, 280, 111832. [Google Scholar] [CrossRef]
- Rodríguez, A.; Shimada, T.; Cervera, M.; Alquézar, B.; Gadea, J.; Gómez-Cadenas, A.; De Ollas, C.J.; Rodrigo, M.J.; Zacarías, L.; Peña, L. Terpene down-regulation triggers defense responses in transgenic orange leading to resistance against fungal pathogens. Plant Physiol. 2014, 164, 321–339. [Google Scholar] [CrossRef]
| Compound * | Citrus Species | Structure |
|---|---|---|
| Monoterpenes | ||
| Alcohols and derivatives | ||
| Borneol | C. aurantium [11], C. limon [11,21], C. reticulata [11], C. sinensis [11], C. tamurana [28] | 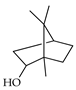 |
| Campherenol | C. bergamia [14] | 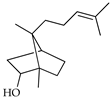 |
| Carvacrol | C. aurantium [11], C. limon [11], C. reticulata [11], C. sinensis [11], C. tamurana [28] |  |
| Carveol | C. limetta [19], C. medica [22] | 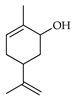 |
| trans-Carveol | C. limon [20,21], C. maxima [16], C. paradisi [17], C. tamurana [29] | 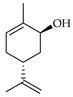 |
| cis-Carveol | C. limetta [20], C. maxima [16,17], C. paradisi [17], C. tamurana [28] | 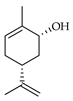 |
| Citronellol (= citronellyl alcohol) | C. aurantium [11], C. bergamia [14],C. limon [11], C. macroptera [16], C. medica [22,23], C. reticulata [11,24,26], C. sinensis [11], C. tamurana [28,29] |  |
| α-Citronellol | C. reticulata [26] | 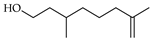 |
| d-Citronellol | C. hystrix [18] | 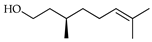 |
| Dehydrocarveol | C. maxima [17], C. paradisi [17], C. tamurana [28] | 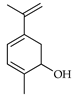 |
| Geraniol (=geranyl alcohol) | C. aurantifolia [8], C. latifolia [8], C. limetta [21], C. limon [8,11], C. macroptera [16], C. maxima [17], C. medica [23], C. paradisi [17], C. reticulata [11,25], C. tamurana [28,29] | 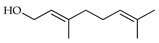 |
| Geraniol methyl ether | C. medica [22] | 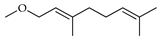 |
| Nerol (=Z-geraniol) | C. aurantium [10,11,12], C. aurantifolia [8], C. bergamia [14,15], C. latifolia [8],C. limetta [19], C. limon [8,11], C. maxima [17], C. medica [23], C. paradisi [17], C. reticulata [11,25,26], C. sinensis [11], C. tamurana [28,29] | 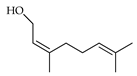 |
| Isopinocarveol (=pinocarveol) | C. limetta [19] | 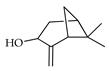 |
| Limonene-1,2-diol | C. tamurana [28,29], C. medica [22,23] | 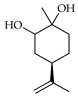 |
| Linalool | C. aurantifolia [8], C. aurantium [10,11,12], C. bergamia [13,14,15], C. hystrix [18], C. latifolia [8], C. limetta [19,20], C. limon [8,11,21], C. limonia [8], C. macroptera [16], C. maxima [16,17], C. medica [22,23], C. paradisi [17], C. reticulata [11,24,25], C. sinensis [11,27], C. tamurana [28,29], C. unshiu [30] | 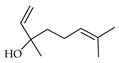 |
| l-Menthol | C. tamurana [28,29] | 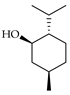 |
| trans-p-Mentha-2,8-dienol | C. maxima [17], C. paradisi [17] |  |
| trans-p-Menth-2,8-dien-1-ol | C. limetta [20], C. maxima [16,17], C. medica [23], C. paradisi [17], C. tamurana [28] | 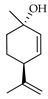 |
| cis-p-Menth-2,8-dien-1-ol | C. limetta [20] |  |
| p-Menth-2,8-dien-1-ol | C. limon [21] | 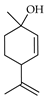 |
| p-Mentha-1,8-dien-10-ol | C. tamurana [28,29] | 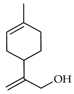 |
| p-Mentha-1-en-9-ol | C. maxima [17], C. paradisi [17], C. tamurana [28,29] | 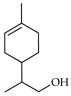 |
| Myrcenol | C. aurantium [10], C. tamurana [28] | 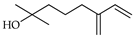 |
| trans-Myrtanol | C. limetta [19] | 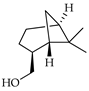 |
| 3,7-Nonadien-2-ol, 4,8-dimethyl | C. medica [22] | 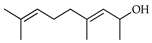 |
| Perillol | C. limon [21], C. maxima [17], C. paradisi [17] | 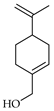 |
| trans-Piperitol | C. tamurana [28] | 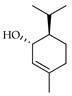 |
| cis-Piperitol | C. maxima [17], C. paradisi [17] | 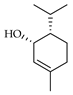 |
| Sabinol | C. limon [21] | 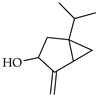 |
| trans-Sabinene hydrate | C. bergamia [15], C. limetta [19], C. paradisi [17], C. sinensis [27] | 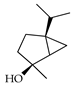 |
| cis-Sabinene hydrate | C. aurantium [11,12], C. bergamia [14], C. limetta [19], C. limon [11], C. reticulata [11], C. sinensis [11] | 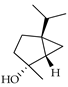 |
| Terpinen-4-ol | C. limetta [19], C. aurantifolia [8], C. limonia [8], C. latifolia [8], C. macroptera [16], C. maxima [16], C. hystrix [18], C. limon [8,11,21], C. reticulata [11,24], C. aurantium [11,12], C. sinensis [11,27], C. tamurana [28,29], C. bergamia [14,15], C. medica [22,23] | 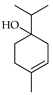 |
| α-Terpineol | C. aurantifolia [8,9], C. aurantium [10,11,12], C. bergamia [14,15], C. hystrix [18], C. latifolia [8], C. limetta [19], C. limon [8,11,21], C. limonia [8], C. macroptera [16], C. medica [22], C. paradisi [17], C. reticulata [11,24,25], C. sinensis [11,27], C. tamurana [28,29] | 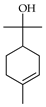 |
| Thymol | C. reticulata [26] | 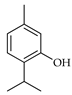 |
| cis-Verbenol | C. medica [23] |  |
| trans-Verbenol | C. limon [21] |  |
| Aldehydes | ||
| Citronellal | C. aurantium [12], C. bergamia [14], C. hystrix [18], C. limon [21], C. limonia [8], C. maxima [17], C. paradisi [17], C. reticulata [24,25], C. sinensis [27], C. tamurana [28,29] | 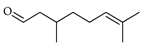 |
| Cumin aldehyde | C. maxima [17], C. paradisi [17], C. tamurana [28] | 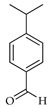 |
| Geranial (=E-citral) | C. aurantifolia [8], C. bergamia [14,15], C. latifolia [8], C. limetta [19], C. limon [8], C. macroptera [16], C. maxima [16,17], C. medica [22,23], C. paradisi [17], C. reticulata [24,25], C. sinensis [27] |  |
| Neral (=Z-citral) | C. aurantifolia [8], C. aurantium [12], C. bergamia [14,15], C. latifolia [8], C. limetta [19], C. limon [11,21], C. macroptera [16], C. maxima [17], C. medica [22], C. paradisi [17], C. reticulata [25], C. sinensis [27], C. tamurana [28,29] | 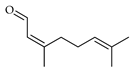 |
| Perillaldehyde | C. bergamia [14], C. limetta [19], C. maxima [17], C. paradisi [17], C. reticulata [26], C. reticulata [24], C. tamurana [28,29] | 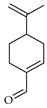 |
| Esters | ||
| Bornyl acetate | C. aurantium [11], C. limon [11], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 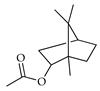 |
| Carveol propionate | C. limon [21] | 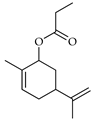 |
| Citronellol acetate | C. bergamia [14,15], C. hystrix [18], C. reticulata [25], C. tamurana [28] | 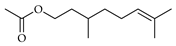 |
| Citronellol formate | C. macroptera [16], C. tamurana [28,29] |  |
| Geraniol acetate | C. aurantium [11,12], C. bergamia [14,15], C. latifolia [8], C. limetta [19], C. limon [8,11], C. medica [22], C. reticulata [11,25], C. sinensis [11], C. tamurana [28,29] |  |
| Geraniol formate | C. reticulata [25] | 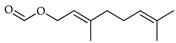 |
| Geraniol propionate | C. maxima [17], C. paradisi [17], C. tamurana [28,29] | 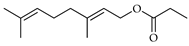 |
| Isobornyl acetate | C. bergamia [14] |  |
| Linalool acetate (=bergamiol) | C. aurantium [11,12], C. bergamia [13,14,15], C. limetta [19], C. limon [11], C. maxima [17], C. paradisi [17], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 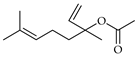 |
| Linalool butyrate | C. aurantium [10] | 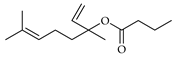 |
| p-Mentha-1-en-9-yl acetate | C. tamurana [28] | 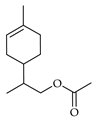 |
| Methyl geranate | C. bergamia [14] | 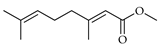 |
| Methylthymol | C. reticulata [24] | 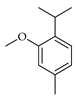 |
| Z-2-Octen-1-ol,3,7-dimethyl-, isobutyrate | C. medica [22] | 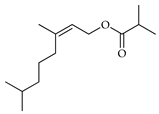 |
| Perillyl acetate | C. bergamia [15], C. paradisi [17] | 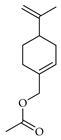 |
| Terpineol acetate (=terpinyl acetate) | C. aurantium [11,12], C. bergamia [14,15], C. limetta [19], C. limon [11], C. medica [23], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] |  |
| β-Terpinyl acetate | C. medica [23] | 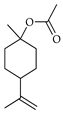 |
| Hydrocarbons | ||
| Camphene | C. aurantium [11,12], C. bergamia [14,15], C. hystrix [18], C. latifolia [8], C. limetta [19,20], C. limon [8,11], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 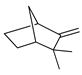 |
| 2-Carene | C. reticulata [24] | 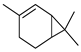 |
| 3-Carene | C. aurantium [10,11], C. bergamia [14], C. limon [11,21], C. medica [22], C. reticulata [11], C. sinensis [11,27], C. tamurana [28,29] | 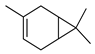 |
| o-Cymene | C. aurantium [12], C. limon [21], C. unshiu [30] | 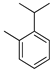 |
| m-Cymene | C. latifolia [8], C. limonia [8] | 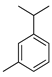 |
| p-Cymene | C. aurantifolia [8], C. aurantium [11,12], C. bergamia [14,15], C. latifolia [8], C. limetta [20], C. limon [8,11], C. limonia [8], C. reticulata [11], C. sinensis [11,27], C. tamurana [28,29] |  |
| α-Fenchene | C. aurantium [12], C. limon [21], C. tamurana [28,29] |  |
| Isolimonene | C. medica [23] | 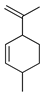 |
| Limonene | C. medica [23], C. latifolia [8], C. macroptera [16], C. paradisi [17], C. limon [8,11,21], C. aurantium [10,11,12], C. sinensis [8,11,27], C. bergamia [13,14,15], C. reticulata [11,25], C. tamurana [28,29], C. maxima [16,17], C. aurantifolia [8,9] | 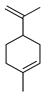 |
| d-Limonene | C. hystrix [18], C. limetta [19,20], C. medica [22,23], C. reticulata [24] | 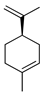 |
| l-Limonene | C. unshiu [30] |  |
| Myrcene (= β-myrcene) | C. aurantifolia [8], C. aurantium [10,11,12], C. bergamia [14,15], C. latifolia [8], C. limetta [19,20], C. limon [11], C. limonia [8], C. macroptera [16], C. maxima [16], C. medica [22,23], C. reticulata [11,24,25], C. sinensis [8,11,27], C. tamurana [28,29], C. unshiu [30] | 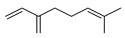 |
| Allo-Ocimene | C. medica [22] | 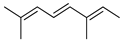 |
| E-β-Ocimene | C. aurantium [10,11], C. bergamia [14], C. limetta [11], C. reticulata [11,24,25], C. sinensis [11,27] |  |
| Z-β-Ocimene | C. bergamia [14,15], C. medica [22], C. reticulata [25], C. tamurana [28] | 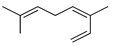 |
| Z-1,3,6-Octatriene,3,7-dimethyl- | C. medica [23] | 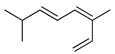 |
| α-Phellandrene | C. medica [22], C. latifolia [8], C. bergamia [14,15], C. tamurana [28,29] |  |
| β-Phellandrene | C. bergamia [15], C. reticulata [24], C. sinensis [27], C. tamurana [28] | 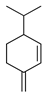 |
| α-Pinene | C. aurantifolia [8,9], C. aurantium [10,11,12], C. bergamia [14,15], C. hystrix [18], C. latifolia [8], C. limetta [19,20], C. limon [8,11], C. limonia [8], C. macroptera [16], C. maxima [16,17], C. medica [22,23], C. paradisi [17], C. reticulata [11,24,25], C. sinensis [11,27], C. tamurana [28,29] |  |
| β-Pinene | C. limetta [19], C. medica [22], C. limonia [8], C. latifolia [8], C. macroptera [16], C. hystrix [18], C. paradisi [17], C. aurantium [10,11,12], C. bergamia [13,14,15], C. limon [11,21], C. reticulata [11,24], C. sinensis [11,27], C. aurantifolia [8,9], C. tamurana [28,29], C. maxima [16,17] | 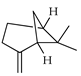 |
| Sabinene | C. aurantifolia [8,9], C. aurantium [11,12], C. bergamia [14,15], C. limetta [19], C. limon [8,11], C. limonia [8], C. macroptera [16], C. maxima [16,17], C. paradisi [17], C. reticulata [11,25], C. sinensis [11,27] |  |
| d-Sabinene | C. tamurana [28,29] | 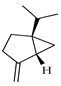 |
| α-Terpinene | C. aurantifolia [8,9], C. aurantium [11], C. bergamia [14,15], C. latifolia [8], C. limon [8,11], C. limonia [8], C. maxima [17], C. medica [22], C. paradisi [17], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 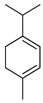 |
| γ-Terpinene | C. aurantifolia [8,9], C. aurantium [11,12], C. bergamia [13,14,15], C. hystrix [18], C. latifolia [8], C. limon [8,11,21], C. limonia [8], C. macroptera [16], C. maxima [16,17], C. medica [22], C. paradisi [17], C. reticulata [11,24], C. sinensis [11,27], C. tamurana [28,29], C. unshiu [30] | 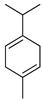 |
| Terpinolene | C. aurantifolia [8], C. aurantium [11], C. bergamia [14,15], C. latifolia [8], C. limon [11], C. limonia [8], C. paradisi [17], C. reticulata [11,24], C. sinensis [8,11,27], C. tamurana [28,29] | 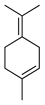 |
| α-Thujene | C. aurantifolia [8], C. aurantium [11,12], C. bergamia [14], C. hystrix [18], C. latifolia [8], C. limon [8,11], C. limonia [8], C. paradisi [17], C. reticulata [11,24], C. sinensis [11,27], C. tamurana [28,29] |  |
| β-Thujene | C. tamurana [28,29] | 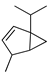 |
| Tricyclene | C. aurantium [11,12], C. bergamia [14], C. limon [11], C. reticulata [11], C. sinensis [11] | 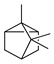 |
| Ketones | ||
| Camphor | C. aurantium [11], C. limetta [19], C. limon [11,21], C. reticulata [11,24], C. sinensis [11] | 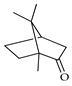 |
| δ-Camphor | C. tamurana [28,29] | 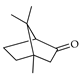 |
| Carvomenthone | C. medica [22] |  |
| l-Carvone | C. tamurana [28,29] | 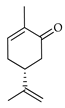 |
| Carvone | C. bergamia [15], C. maxima [16,17], C. paradisi [17], C. reticulata [25] | 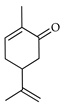 |
| cis-Dihydrocarvone | C. aurantium [11], C. limon [11], C. reticulata [11], C. sinensis [11] |  |
| Isopiperitone | C. tamurana [28,29] | 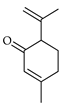 |
| Menthone | C. tamurana [28,29] | 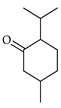 |
| Sabina ketone | C. maxima [17], C. paradisi [17] | 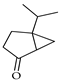 |
| 3-Terpinolenone (=piperitone) | C. limon [21] | 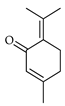 |
| Oxides | ||
| 4,5-Epoxycarene | C. medica [23] | 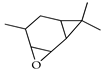 |
| Carvone oxide | C. tamurana [28] |  |
| 1,8-Cineole (= eucalyptol) | C. aurantifolia [9], C. aurantium [11,12], C. bergamia [15], C. limon [11], C. macroptera [16], C. reticulata [11,25], C. sinensis [11] | 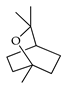 |
| cis-Limonene-1,2-epoxide | C. bergamia [14], C. maxima [16], C. reticulata [25], C. sinensis [27], C. tamurana [28] | 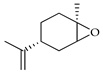 |
| trans-Limonene-1,2,-epoxide | C. bergamia [14,15], C. limon [21], C. reticulata [25], C. sinensis [27], C. tamurana [28] |  |
| Z-Linalool pyranoxide | C. tamurana [28,29] | 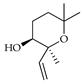 |
| cis-Linalool-oxide | C. aurantium [10,11], C. bergamia [15], C. hystrix [18], C. limetta [20], C. limon [11], C. macroptera [16], C. maxima [16], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 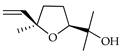 |
| trans-Linalool oxide | C. aurantium [12], C. bergamia [15], C. hystrix [18], C. macroptera [16], C. maxima [16], C. tamurana [28,29] | 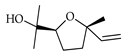 |
| Myrcene epoxide | C. paradisi [17] | 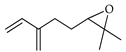 |
| Nerol oxide | C. tamurana [28,29] | 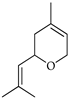 |
| 7-Oxabicycloheptane, 1-methyl-4-(1-methylethyl) | C. medica [23] | 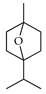 |
| Perillene | C. paradisi [17] | 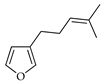 |
| α-Pinene oxide | C. limon [21] | 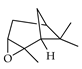 |
| Rose furan epoxide | C. reticulata [25] | 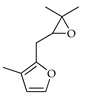 |
| Sesquiterpenes | ||
| Alcohols and derivatives | ||
| α-Bisabolol | C. bergamia [14,15], C. latifolia [8], C. limetta [19], C. limon [21], C. medica [22,23], C. tamurana [28,29] | 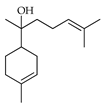 |
| β-Bisabolol | C. limon [21], C. limetta [19], C. medica [22,23] |  |
| α-Cadinol | C. macroptera [16], C. tamurana [28] | 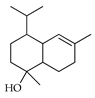 |
| Cedrenol | C. tamurana [28] | 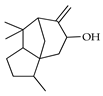 |
| Cedrol | C. bergamia [15], C. maxima [17], C. paradisi [17], C. tamurana [28,29] | 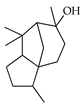 |
| Cubenol | C. macroptera [16] | 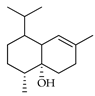 |
| Elemol | C. aurantifolia [9], C. hystrix [18], C. macroptera [16], C. maxima [17], C. paradisi [17], C. tamurana [28,29] |  |
| α-Eudesmol | C. aurantifolia [9] | 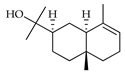 |
| β-Eudesmol | C. aurantifolia [9], C. macroptera [16], C. tamurana [28,29] | 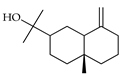 |
| γ-Eudesmol | C. aurantifolia [9], C. tamurana [28] | 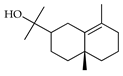 |
| 7-epi-α-Eudesmol | C. aurantifolia [9] | 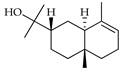 |
| 2E,6E-Farnesol | C. limetta [19], C. tamurana [28,29] | 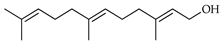 |
| 2Z,6E-Farnesol | C. aurantium [11], C. limon [11], C. paradisi [17], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 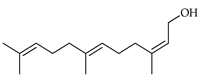 |
| Globulol | C. paradisi [17], C. tamurana [28,29] | 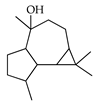 |
| Ledol | C. limon [21] |  |
| E-Nerolidol | C. reticulata [25], C. aurantium [12], C. bergamia [15], C. macroptera [16], C. tamurana [28,29] |  |
| Z-Nerolidol | C. paradisi [17], C. reticulata [26], C. tamurana [28,29] | 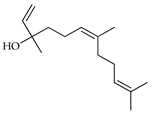 |
| α-Santalol | C. limetta [19] | 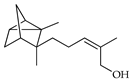 |
| E-β-santalol | C. limetta [19] | 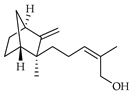 |
| E-sesquisabinene hydrate | C. bergamia [14] |  |
| Spathulenol | C. aurantium [11], C. limon [11], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 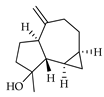 |
| Valerianol | C. aurantifolia [9] | 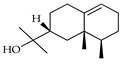 |
| Viridiflorol | C. tamurana [28] | 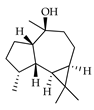 |
| Aldehydes | ||
| α-Sinensal | C. maxima [17], C. paradisi [17], C. reticulata [26] |  |
| β-Sinensal | C. maxima [17], C. paradisi [17], C. reticulata [26], C. tamurana [28] | 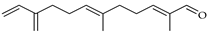 |
| Hydrocarbons | ||
| Aromadendrene | C. limetta [19] | 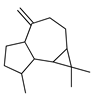 |
| α-Bergamotene | C. bergamia [15] | 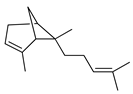 |
| cis-α-Bergamotene | C. latifolia [8], C. limon [21] |  |
| trans-α-Bergamotene | C. aurantifolia [8], C. bergamia [14], C. latifolia [8], C. limetta [19], C. limon [8], C. limonia [8], C. medica [22,23], C. reticulata [25] | 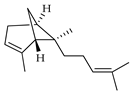 |
| Bicyclogermacrene | C. bergamia [14], C. macroptera [16] |  |
| α-Bisabolene | C. latifolia [8], C. limetta [21] | 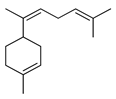 |
| β-Bisabolene | C. aurantifolia [8], C. bergamia [14,15], C. latifolia [8], C. limon [8], C. limonia [8], C. reticulata [25] | 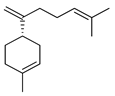 |
| E-γ-Bisabolene | C. bergamia [14] |  |
| Z-γ-Bisabolene | C. bergamia [14] |  |
| β-Cadinene | C. hystrix [18] | 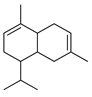 |
| δ-Cadinene | C. aurantifolia [8], C. aurantium [12], C. limonia [8], C. macroptera [16], C. medica [22,23], C. tamurana [28] |  |
| α-Cedrene | C. maxima [17], C. paradisi [17], C. tamurana [28,29] | 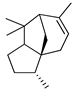 |
| α-Caryophyllene (=humulene) | C. aurantifolia [8], C. aurantium [11,12],C. bergamia [14], C. hystrix [18], C. latifolia [8], C. limon [11], C. macroptera [16], C. medica [23], C. reticulata [11,25], C. sinensis [11], C. tamurana [28,29] | 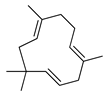 |
| β-Caryophyllene | C. aurantifolia [8], C. aurantium [12], C. bergamia [14,15], C. hystrix [18], C. latifolia [8], C. limetta [8], C. limon [8], C. macroptera [16], C. maxima [17], C. medica [22,23], C. paradisi [17], C. reticulata [25], C. tamurana [28,29] | 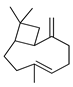 |
| α-Copaene | C. aurantium [12], C. hystrix [18], C. macroptera [16], C. maxima [17], C. paradisi [17], C. tamurana [28,29] | 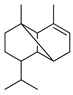 |
| α-Cubebene | C. maxima [17], C. paradisi [17], C. tamurana [28] |  |
| β-Cubebene | C. hystrix [18], C. macroptera [16], C. tamurana [28,29] |  |
| α-Curcumene | C. limon [21] | 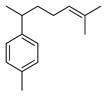 |
| β-Curcumene | C. limon [21] |  |
| β-Elemene | C. aurantifolia [8], C. aurantium [12], C. hystrix [18], C. latifolia [8], C. macroptera [16], C. sinensis [27], C. tamurana [28,29], C. unshiu [30] | 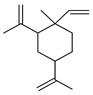 |
| δ-Elemene | C. aurantifolia [8], C. aurantium [12], C. bergamia [14], C. maxima [17], C. paradisi [17], C. reticulata [24], C. reticulata [25] | 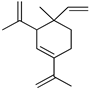 |
| γ-Elemene | C. reticulata [24], C. aurantium [12], C. tamurana [28,29] | 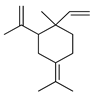 |
| E,E-α-Farnesene | C. limetta [19], C. macroptera [16], C. maxima [17], C. medica [23], C. paradisi [17], C. unshiu [30] |  |
| E-β-Farnesene | C. aurantifolia [8], C. bergamia [15], C. latifolia [8], C. limetta [19], C. tamurana [28,29] |  |
| Z-β-Farnesene | C. bergamia [14], C. medica [23], C. tamurana [28] |  |
| Germacrene B | C. reticulata [25,26] | 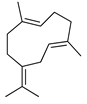 |
| Germacrene D | C. aurantifolia [8], C. aurantifolia [8], C. aurantium [11], C. bergamia [14,15], C. hystrix [18], C. latifolia [8], C. limon [8,11,21], C. macroptera [16], C. medica [22,23], C. reticulata [11,24], C. sinensis [11], C. tamurana [28,29] | 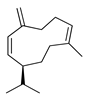 |
| α-Muurolene (=α-Cadinene) | C. macroptera [16], C. medica [22] | 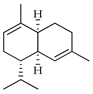 |
| Z-β-Santalene | C. bergamia [14], C. limetta [19], C. limon [21] |  |
| epi-β-Santalene | C. limetta [19] | 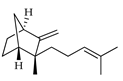 |
| Sesquiphellandrene | C. bergamia [14], C. limetta [21], C. tamurana [28,29] | 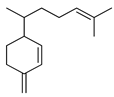 |
| Sesquithujene | C. bergamia [14] |  |
| Seychellene | C. limon [21] |  |
| Valecene | C. aurantium [11], C. limon [11], C. reticulata [11], C. sinensis [11], C. tamurana [28,29] | 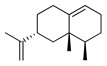 |
| α-Ylangene | C. tamurana [28] |  |
| β-Ylangene | C. tamurana [29] |  |
| Zizaene | C. limon [21] | 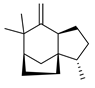 |
| Esters | ||
| Cedryl acetate | C. tamurana [28] | 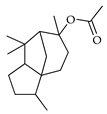 |
| 2E,6E-Farnesol acetate | C. aurantium [12], C. tamurana [28,29] |  |
| Methoprene | C. medica [22] | 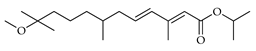 |
| Nerolidol acetate | C. maxima [17], C. paradisi [17] |  |
| Nerol acetate | C. aurantifolia [8], C. aurantium [12], C. bergamia [14,15], C. latifolia [8], C. limetta [19], C. medica [22,23], C. reticulata [25], C. tamurana [28,29] |  |
| Nerol formate | C. reticulata [25] |  |
| Ketones | ||
| β-Ionone | C. tamurana [28] | 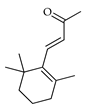 |
| Nootkatone | C. bergamia [14,15], C. paradisi [16,17] | 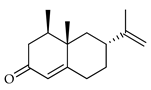 |
| Oxides | ||
| Caryophyllene oxide | C. aurantium [11,12], C. bergamia [15], C. limon [11], C. maxima [17], C. paradisi [17], C. reticulata [11,25], C. sinensis [11], C. tamurana [28,29] |  |
| Diterpenes | ||
| Geranyl α-terpinene | C. sinensis [27] | 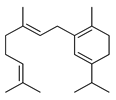 |
| E-Phytol | C. reticulata [25] | 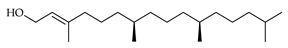 |
| Coumarins | ||
| Bergamottin | C. bergamia [13] | 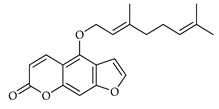 |
| Bergapten | C. bergamia [13] | 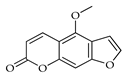 |
| Citropten | C. bergamia [13] | 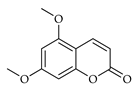 |
| 5-Geranyloxy-7-methoxycoumarin | C. bergamia [13] |  |
| Phenylpropanoids | ||
| Cinnamic aldehyde | C. paradisi [17] | 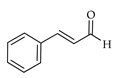 |
| Cinnamyl alcohol | C. tamurana [28,29] | 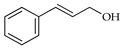 |
| α-Curcumin | C. aurantifolia [9] |  |
| Estragole | C. limon [21] |  |
| Ethyl cinnamate | C. limon [21] | 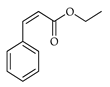 |
| Ethyl p-methoxycinnamate | C. limon [21] | 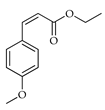 |
| Eugenol | C. tamurana [28] |  |
| Isoeugenol | C. tamurana [28,29] |  |
| Isosafrole | C. reticulata [26] | 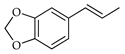 |
| Methyl eugenol | C. maxima [16] |  |
| Miscellaneous | ||
| E-Solanone | C. bergamia [15] |  |
| Sulcatone | C. bergamia [15], C. limon [21], C. reticulata [25], C. tamurana [28] | 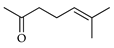 |
| Species | Bioactivity | References |
|---|---|---|
| C. aurantifolia | Acaricidal | [9] |
| Antibacterial | [67,68] | |
| Antifungal | [8,67,69,70,71,72,73,74] | |
| Insecticidal | [75] | |
| C. aurantium | Antibacterial | [12,47,49,68,76,77] |
| Antifungal | [49,77,78] | |
| Antioxidant | [12,47,49] | |
| Insecticidal | [77,79] | |
| C. bergamia | Antibacterial | [56,57] |
| Antifungal | [77,78,80] | |
| Antioxidant | [48] | |
| Insecticidal | [81] | |
| C. latifolia | Antifungal | [8,70] |
| C. limon | Antibacterial | [47,49,50,55,56,57,61,62,64,66,67] |
| Antifungal | [8,49,50,55,62,63,67,69,70,71,73,78,82,83,84] | |
| Antioxidant | [46,47,49] | |
| Insecticidal | [33,79,81] | |
| C. limonia | Antifungal | [8] |
| C. maxima | Antibacterial | [65] |
| Antifungal | [70,74,85] | |
| C. medica | Antibacterial | [86,87] |
| Antifungal | [59,88] | |
| C. paradisi | Antibacterial | [64,67] |
| Antifungal | [63,64,67,69,70,73,89] | |
| Insecticidal | [33] | |
| C. reticulata | Antibacterial | [47,49,50,60,64] |
| Antifungal | [24,25,49,50,63,69,70,74,90] | |
| Antioxidant | [47,49] | |
| Insecticidal | [79] | |
| C. sinensis | Antibacterial | [47,50,55,56,57,64,67,73,90,91] |
| Antifungal | [50,55,63,67,70,73,74,85,88,90,91,92,93,94,95,96,97] | |
| Antioxidant | [47] | |
| C. unshiu | Antibacterial | [30] |
| Antifungal | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, M.M.; Nicoletti, R.; Andolfi, A. Essential Oils in Citrus Fruit Ripening and Postharvest Quality. Horticulturae 2022, 8, 396. https://doi.org/10.3390/horticulturae8050396
Salvatore MM, Nicoletti R, Andolfi A. Essential Oils in Citrus Fruit Ripening and Postharvest Quality. Horticulturae. 2022; 8(5):396. https://doi.org/10.3390/horticulturae8050396
Chicago/Turabian StyleSalvatore, Maria Michela, Rosario Nicoletti, and Anna Andolfi. 2022. "Essential Oils in Citrus Fruit Ripening and Postharvest Quality" Horticulturae 8, no. 5: 396. https://doi.org/10.3390/horticulturae8050396
APA StyleSalvatore, M. M., Nicoletti, R., & Andolfi, A. (2022). Essential Oils in Citrus Fruit Ripening and Postharvest Quality. Horticulturae, 8(5), 396. https://doi.org/10.3390/horticulturae8050396








