Polyphenols in the Waste Water Produced during the Hydrodistillation of ‘Narcea Roses’ Cultivated in the Cibea River Valley (Northern Spain)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
2.2. Collection and Transport of Rose Flowers
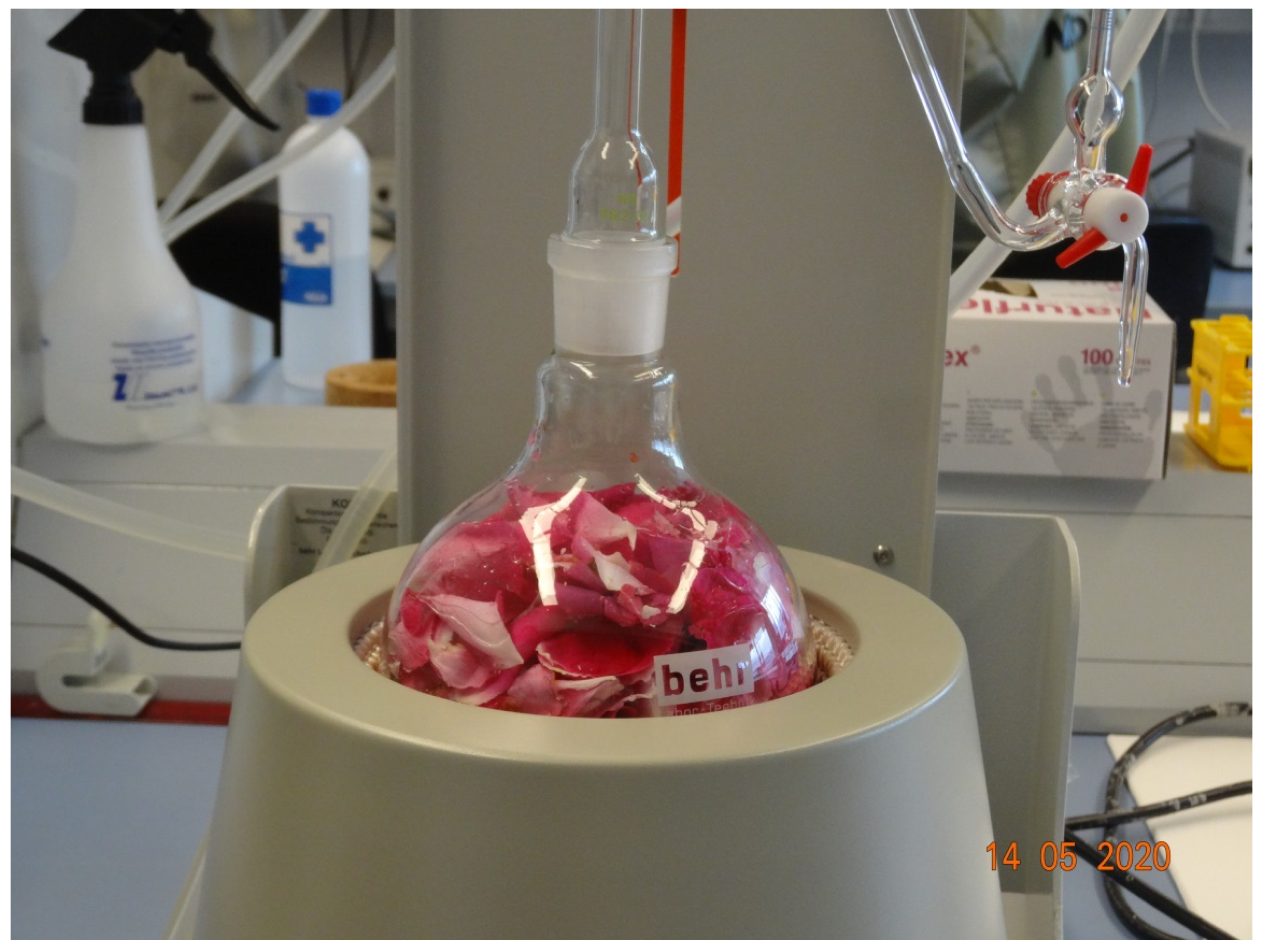
2.3. Hydrodistillation and the Waste Water Produced
2.4. Waste Water Analysis
Identification and Quantification
- Cyanidin-glucoside (for the derivatives of cyanidin);
- Kaempferol-glucoside (for the derivatives of kaempferol); and
- Quercetin-glucoside (for the derivatives of quercetin and other less abundant compounds).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, M.C.; Santiago, J.L.; Boso, S.; Gago, P.; Álvarez-Acero, I.; De Vega, M.E.; Martínez-Bartolomé, M.; Álvarez-Nogal, R.; Molíst, P.; Caser, M.; et al. Narcea-an unknown, ancient cultivated rose variety from northern Spain. Hortic. Res. 2020, 7, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedler, J.; Weston, A.; Rausenberger, J.; Butterweck, V. In vitro modulation of inflammatory target gene expression by a polyphenol-enriched fraction of rose oil distillation waste water. Fitoterapia 2016, 114, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkova, D.; Mihaylova, D.; Denev, P.; Krastanov, A. Antioxidant activity of some edible flowers water extracts from Bulgaria. Bull. UASVM Food Sci. Tech. 2020, 77, 54–61. [Google Scholar] [CrossRef]
- Dragoev, S.; Vlahova-Vangelova, D.; Balev, D.; Bozhilov, D.; Dagnon, S. Valorization of waste by-products of rose oil production as feedstuff phytonutrients. Bulg. J. Agric. Sci. 2021, 27, 209–219. [Google Scholar]
- Saint-Lary, K.; Roy, C.; Paris, J.P.; Martin, J.F.; Thomas, O.P.; Fernandez, X. Metabolomics as a tool for the authentication of rose extracts used in flavour and fragrance area. Metabolomics 2016, 12, 49. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wu, M. Characterization of key aroma compounds from different rose essential oils using gas chromatography-mass spectrometry, gas chromatography-olfactometry and partial least squares regression. Nat. Prod. Res. 2018, 32, 1567–1572. [Google Scholar] [CrossRef]
- Baydar, H.; Göktürk Baydar, N. The effects of harvest date, fermentation duration and Tween 20 treatment on essential oil content and composition of industrial oil rose (Rosa damascena Mill.). Ind. Crops Prod. 2005, 21, 251–255. [Google Scholar] [CrossRef]
- Yassa, N.; Masoomi, F.; Rouhani Rankouhi, S.; Hadjiakhoondi, A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. Daru J. Pharmac. Sci. 2009, 17, 175–180. [Google Scholar]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial Cultivation of Oil Bearing Rose and Rose Oil Production in Bulgaria during the 21st Century, Directions and Challenges. Biotechnology & Biotechnological Equipment. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar]
- Sharma, S.; Kumar, R. Influence of harvesting stage and distillation time of Damask rose (Rosa damascena Mill.) flowers of essential oil content and composition in the Western Himalayas. J. Essent. Oil Bear. Plants 2018, 21, 92–102. [Google Scholar] [CrossRef]
- Ginova, A.; Mihalev, K.; Kondakova, V. Antioxidant capacity of petals and leaves from different rose (Rosa damascena mill.) plantations in Bulgaria. Int. J. Pure Appl. Biosci. 2013, 1, 38–43. [Google Scholar]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreir, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Tech. 2019, 9, 244–258. [Google Scholar] [CrossRef]
- Ercişli, S.; Eşitken, A. Fruit characteristics of native rose hip (Rosa spp.) selections from the Erzurum province of Turkey. N. Z. J. Crop Hortic. Sci. 2004, 32, 51–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.Q.; Huang, X.D.; Wang, H.; Leung, A.K.N.; Chan, C.L.; Fong, D.W.F.; Yub, Z.L. Anti-inflammatory and analgesic effects of the ethanol extract of Rosa multiflora Thunb. hips. J. Ethnopharmacol. 2008, 118, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Nam, T.G.; Lee, I.; Shin, E.J.; Han, A.R.; Lee, P.; Lee, S.Y.; Lim, T.G. Skin anti-inflammatory activity of rose petal extract (Rosa gallica) through reduction of MAPK signaling pathway. Food Sci. Nutr. 2018, 6, 2560–2567. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, R.; Barros, L.; Duenas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; CFR-Ferreira, I. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [Green Version]
- Cendrowski, A.; Krasniewska, K.; Przybyl, J.L.; Zielinska, A.; Kalisz, S. Antibacterial and antioxidant activity of extracts from rose fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef] [Green Version]
- Nadpal, J.D.; Lesjak, M.M.; Mrkonjic, Z.O.; Majkic, T.M.; Cetojevic-Simin, D.D.; Mimica-Dukic, N.M.; Beara, I.N. Phytochemical composition and in vitro functional properties of three wild rose hips and their traditional preserves. Food Chem. 2018, 241, 290–300. [Google Scholar] [CrossRef]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Amenta, M.; Romeo, F.V. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous Sicilian flora. Food. Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound identification of selected Rose species and cultivars: An insight to petal and leaf phenolic profiles. J. Amer. Soc. Hort. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef] [Green Version]
- D´Angiolillo, F.; Mammano, M.M.; Fascella, G. Pigments, polyphenols and antioxidant activity of leaf extracts from four wild rose species grown in Sicily. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Vinokur, Y.; Rodov, V.; Reznick, N.; Goldman, G.; Horev, B.; Umiel, N. Rose petal tea as an antioxidant-rich beverage: Cultivar effects. J. Food Sci. 2006, 71, S42–S47. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Bari, S.S. Antioxidant activity and ultra-performance LC-electrospray ionization–quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol. 2009, 47, 361–367. [Google Scholar] [CrossRef]
- Kumar, S.; Gautam, S.; Sharma, A. Identification of antimutagenic properties of anthocyanins and other polyphenols from Rose (Rosa centifolia) petals and tea. J. Food Sci. 2013, 78, H948–H954. [Google Scholar] [CrossRef] [PubMed]
- Göktürk-Baydar, N.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Color and phenolic content changes during flower development in groundcover rose. J. Amer. Soc. Hort. Sci. 2010, 135, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Duthie, G.G.; Gardner, P.T.; Kyle, J.A. Plant polyphenols: Are they the new magic bullet? Proc. Nutr. Soc. 2003, 62, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Aubert, C.; Chalot, G. Chemical composition, bioactive com-pounds, and volatiles of six table grape varieties (Vitis vinifera L.). Food Chem. 2018, 240, 524–533. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Tech. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- De la Cerda Carrasco, A.; López Solís, R.; Nuñez Kalasic, H.; Peña Neira, A.; Obreque Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Food Waste Recovery Group (Eds.) Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Vienna, Austria, 2018; 456p. [Google Scholar]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta (BBA)-Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.I.; Wei, C.I.; Huang, Y.W.; Li, Y. Tannins and human healt. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, H.; Wang, Y.; Li, S.; Cao, Z.; Ji, S.; He, Y.; Zhang, H. Effect of dietary forage to concentrate ratios on dynamic profile changes and interactions of ruminal microbiota and metabolites in Holstein heifers. Front. Microbiol. 2017, 8, 2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.Y.; Humphries, D.J.; Cockman, D.A.; Givens, D.I.; Spencer, J.P.E. Accumulation of citrus flavanones in bobine milk following citrus pulp incorporation into the diet of dairy cows. EC Nutr. 2017, 7, 143–154. [Google Scholar]
- Kalantar, M. The importance of flavonoids in ruminant nutrition. Arch. Anim. Husb. Dairy Sci. 2018, 1, 1–4. [Google Scholar] [CrossRef]
- Ge, Q.; Ma, X. Composition and antioxidant activity of anthocyanins isolated from Yunnan edible rose (An Ning). Food Sci. Hum. Wellness 2013, 2, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Encinar, J.A.; Rodriguez-Diaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef] [PubMed]
- Stanila, A.; Diaconeasa, Z.; Roman, I.; Sima, N.; Maniutiu, D.; Roman, A.; Sima, R. Extraction and characterization of phenolic compounds from rose hip (Rosa canina L.) using liquid chromatography coupled with electrospray ionization—Mass spectrometry. Not. Bot. Horti. Agrobo. 2015, 3, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Chien, L.H.; Huang, B.M.; Chia, Y.C. Toona sinensis (aqueous leaf extracts) induces apoptosis through the generation of ROS and activation of intrinsic apoptotic pathways in human renal carcinoma cells. J. Funct. Foods 2014, 7, 362–372. [Google Scholar] [CrossRef]
- Fontana, A.R.M.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Kampa, M.; Theodoropoulou, K.M.; Mavromati, F.; Pelekanou, V.; Notas, G.; Lagoudaki, E.D.; Nifli, A.P.; Morel-Salmi, C.; Stathopoulos, E.N.; Vercauteren, J.; et al. Novel oligomeric proanthocyanidin derivatives interact with membrane androgen sites and induce regression of hormone-independent prostate cancer. J. Pharmacol. Exp. Ther. 2011, 337, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Athar, M. Grape seeds: Ripe for cancer chemoprevention. Cancer Prev. Res. 2013, 6, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Lachman, J.; Hejtmankova, A.; Hejtmankova, K.; Hornickova, S.; Pivec, V.; Skala, O.; Dedina, M.; Pribyl, J. Towards complex utilisation of winemaking residues: Characterisation of grape seeds by total phenols, tocols and essential elements content as a by-product of winemaking. Ind. Crops Prod. 2013, 49, 445–453. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G. Characterization of flavonoids in petals of Rosa damascena by HPLC and spectral analysis. J. Agric. Food Chem. 1991, 39, 463–467. [Google Scholar] [CrossRef]
- Santiago, J.L.; Gago, P.; Boso, S.; Álvarez-Acero, I.; Martínez-Bartolomé, M.; Martínez, M.C. Polyphenol content of the petals of the ’Rosa Narcea’ cultivated in the mountains of Asturias (northern Spain). IV International Symposium on woody ornamentals of the temperate zone. 3–4 March 2021 online symposium. Acta Hortic. 2021, 133, 233–238. [Google Scholar] [CrossRef]
- Mihailova, J.; Atanasova, R.; Tsvetkova, B.A. Direct gas chromatography of essential oil in the separate parts of the flower of the Kazanlik rose (Rosa damascena Mill. f. trigintipetala Dieck.). In Proceedings of the 7th International Congress of Essential Oils, Kyoto, Japan, 7–11 October 1977; pp. 219–221. [Google Scholar]
- Bayrak, A.; Akgul, A. Volatile oil composition of Turkish rose (Rosa damascena). J. Sci. Food Agric. 1994, 64, 441–448. [Google Scholar] [CrossRef]
- Poudel, P.; Tamura, H.; Kataoka, I.; Mochiola, R. Phenolic compounds and antioxidant activities of skins and seeds of five wild grapes and two hybrids native to Japan. J. Food Compos. Anal. 2008, 21, 622–625. [Google Scholar] [CrossRef]
- Martínez de Toda, F.; Ramos, M.C. Variability in grape composition and phenology of ’Tempranillo’ in zones located at different elevations and with differences in the climatic conditions. Vitis 2019, 58, 131–139. [Google Scholar]
- Gutiérrez-Gamboa, G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Koczka, N.; Stefanovits-Banyai, E.; Ombodi, A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]


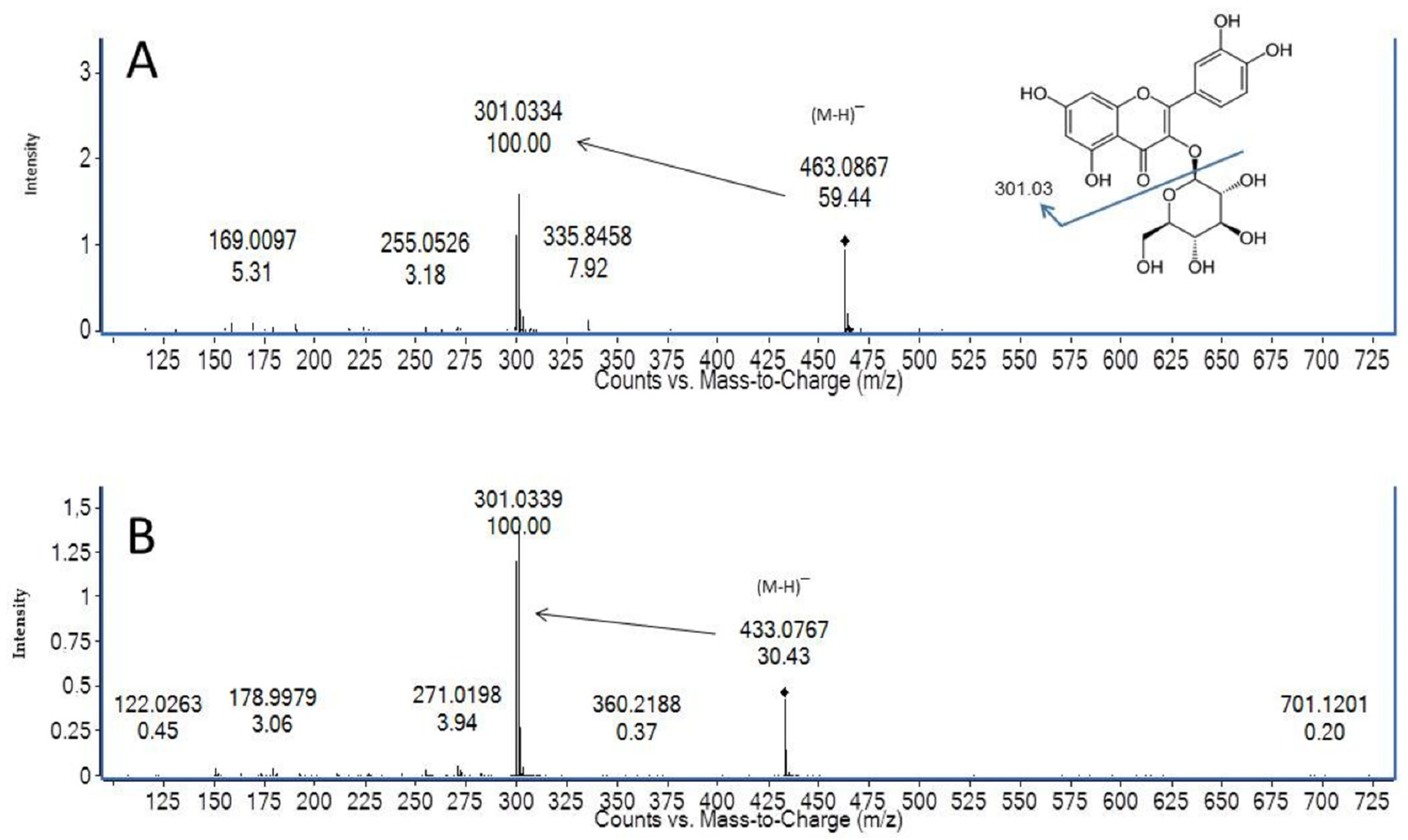
| ANTHOCYANINS | M-H | Formula | Score% | MS/MS | Fragment Identity | TR min | Means (µg/g) | S.D |
| CYANIDIN-DIGLUCOSIDE | 609.1461 | C27H31O16 | 97.9 | 285 | Cyanidin | 6.9 | 13.45 | 3.01 |
| FLAVONOLS 1 | M-H | Formula | Score% | MS/MS | Fragment identity | TR min | Means (µg/g) | S.D |
| KAEMPFEROL | 285.0405 | C15H10O6 | 97.6 | 34.2 | 13.76 | 2.53 | ||
| KAEMPFEROL-PENTOSIDE | 417.0827 | C20H18O10 | 97.9 | 285 | Kaempferol | 27.6 | 13.76 | 0.70 |
| KAEMPFEROL-PENTOSIDE | 417.0827 | C20H18O10 | 96.9 | 285 | Kaempferol | 28.2 | 37.99 | 1.88 |
| KAEMPFEROL-RHAMNOSIDE | 431.0984 | C21H20O10 | 96.4 | 285 | Kaempferol | 28.8 | 52.04 | 5,90 |
| KAEMPFEROL-HEXOSIDE (GALACTOSIDE) | 447.0933 | C21H20O11 | 98.9 | 285 | Kaempferol | 25.7 | 21.81 | 1.24 |
| KAEMPFEROL-RUTINOSIDE | 593.1301 | C30H26O13 | 99.2 | 285 | Kaempferol | 31.9 | 15.34 | 2.46 |
| TOTAL KAEMPFEROL DERIVATIVES | 154.70 | 14.71 | ||||||
| QUERCETIN | 301.0354 | C15H10O7 | 96.6 | 31.3 | 73,84 | 12.28 | ||
| QUERCETIN-PENTOSIDE | 433.0776 | C20H18O11 | 94.8 | 301 | Quercetin | 25.1 | 50.73 | 4.40 |
| QUERCETIN-PENTOSIDE | 433.0776 | C20H18O11 | 99.2 | 301 | Quercetin | 25.5 | 12.14 | 1.18 |
| QUERCETIN-PENTOSIDE | 433.0776 | C20H18O11 | 96.2 | 301 | Quercetin | 26.1 | 155.96 | 5.25 |
| QUERCETIN-RHAMNOSIDE | 447.0933 | C21H20O11 | 99.7 | 301 | Quercetin | 26.6 | 216.53 | 11.60 |
| QUERCETIN-HEXOSIDE (GALACTOSIDE) | 463.0882 | C21H20O12 | 99.6 | 301 | Quercetin | 23.7 | 239.84 | 7.24 |
| QUERCETIN-3-O-GLUCOSIDE | 463.0882 | C21H20O12 | 99.1 | 301 | Quercetin | 24.3 | 260.11 | 9.73 |
| QUERCETIN-HEXOSIDE-RHAMNOSIDE | 609.1250 | C30H26O14 | 97.9 | 301 | Quercetin | 30.2 | 40.91 | 5.14 |
| QUERCETIN-RUTINOSIDE | 609.1461 | C27H30O16 | 97.7 | 301 | Quercetin | 23.7 | 126.84 | 0.98 |
| TOTAL QUERCETIN DERIVATIVES | 1176.90 | 45.52 | ||||||
| FLAVANOLS AND PHENOLICS ACID | M-H | Formula | Score% | MS/MS | Fragment identity | TR min | Means (µg/g) | S.D |
| CATEQUIN | 289.0718 | C15H14O6 | 97.8 | 9.7 | 39.82 | 1.35 | ||
| PROCYANIDIN B1 | 577.1351 | C30H26O12 | 94.9 | 8.1 | 12.54 | 0.62 | ||
| ELLAGIC ACID | 300.9990 | C14H6O8 | 98.6 | 22.9 | 406.29 | 23.74 | ||
| DERIVATIVE ELLAGIC ACID | 425.0150 | C20H10O11 | 97.4 | 300 | Ellagic acid | 27.7 | 250.21 | 32.95 |
| GALIC ACID | 169.0142 | C7H6O5 | 81.1 | 2.9 | 726.96 | 23.47 | ||
| TOTAL FLAVANOLS AND PHENOLICS ACID | 1435.82 | 82.13 | ||||||
| TOTAL µg/g sample | 2780.87 | 145.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boso, S.; Gago, P.; Santiago, J.-L.; Álvarez-Acero, I.; Martinez Bartolomé, M.-A.; Martínez, M.-C. Polyphenols in the Waste Water Produced during the Hydrodistillation of ‘Narcea Roses’ Cultivated in the Cibea River Valley (Northern Spain). Horticulturae 2022, 8, 376. https://doi.org/10.3390/horticulturae8050376
Boso S, Gago P, Santiago J-L, Álvarez-Acero I, Martinez Bartolomé M-A, Martínez M-C. Polyphenols in the Waste Water Produced during the Hydrodistillation of ‘Narcea Roses’ Cultivated in the Cibea River Valley (Northern Spain). Horticulturae. 2022; 8(5):376. https://doi.org/10.3390/horticulturae8050376
Chicago/Turabian StyleBoso, Susana, Pilar Gago, José-Luis Santiago, Inmaculada Álvarez-Acero, Miguel-Angel Martinez Bartolomé, and María-Carmen Martínez. 2022. "Polyphenols in the Waste Water Produced during the Hydrodistillation of ‘Narcea Roses’ Cultivated in the Cibea River Valley (Northern Spain)" Horticulturae 8, no. 5: 376. https://doi.org/10.3390/horticulturae8050376
APA StyleBoso, S., Gago, P., Santiago, J.-L., Álvarez-Acero, I., Martinez Bartolomé, M.-A., & Martínez, M.-C. (2022). Polyphenols in the Waste Water Produced during the Hydrodistillation of ‘Narcea Roses’ Cultivated in the Cibea River Valley (Northern Spain). Horticulturae, 8(5), 376. https://doi.org/10.3390/horticulturae8050376








