Abstract
In recent years, the cultivation of tropical fruit crops has increased in the Mediterranean basin, especially in southern Italy. In surveys conducted from 2014 to 2019 woody canker and shoot blight were observed on mango plants (cvs. Kent, Keitt, Sensation, Osteen, and Kensington Pride) and litchi plants (cvs. Way Chee and Kwai Mai Pink) cultivated in Sicily. Botryosphaeriaceae and Diaporthaceae were consistently isolated from symptomatic samples. Morphological characterization and multi-locus phylogenies using three genomic loci (a portion of translation elongation factor 1-α gene, a portion of the β-tubulin gene, and an internal transcribed spacer) identified these fungi as Neofusicoccum parvum, Lasiodiplodia theobromae, Botryosphaeria dothidea, Diaporthe foeniculina, and Diaporthe baccae on mango and Diaporthe foeniculina and Diaporthe rudis on litchi. Pathogenicity tests on healthy mango (cv. Kensington Pride) and litchi (cv. Way Chee) plants demonstrated the pathogenicity of the isolates used in the study, and Koch’s postulates were fulfilled for all pathogens. To our knowledge, this is the first report of L. theobromae, B. dothidea, and Diaporthe species on mango in Italy and the first report worldwide of woody canker and shoot blight caused by D. foeniculina and D. rudis on litchi plants.
1. Introduction
In recent years, tropical crop cultivation has increased in the Mediterranean basin, and especially in Italy where the environmental conditions have allowed an intensification of their production. These crops represent a sector of great interest for their high nutritional value, antioxidant properties, and high income generated by the fruit market. In particular, the nutritional composition of some crops such as mango and litchi has been extensively studied for human nutrition, health, and other applications in the pharmaceutical and food industries [1,2,3]. Moreover, mango peels have recently been used to develop biodegradable or edible packing materials [4]. Due to their tropical origin, the cultivation of these crops is limited to southern Italy (Calabria) and insular Italy (Sardinia and Sicily). In Sicily, the cultivation of mango and litchi is mainly localized in the north-eastern coastal area of the island.
Mango (Mangifera indica L.) is one of the most popular fruit crops in the world and production is concentrated in Asia, Africa, America, and Oceania [5]. In Italy, the cultivation of mango for commercial purposes started during 1980–1990 in the Catania province (eastern Sicily). Thereafter, its cultivation expanded to the other provinces of the island reaching an area of approximately 200 ha [6]. In this species, the occurrence of fungal and bacterial infections pre- and post-harvest can compromise plant growth and fruit quality. Fortunately, only a few diseases have been reported to date in Italy. Among these, woody cankers and stem-end rot of fruit caused by Neofusicoccum spp. and decay and stem-end rot of fruit caused by some species of Colletotrichum are the major diseases limiting the production of mango [7,8]. These pathogens lead to substantial crop losses, quality changes, market value decreases, and in some cases post-harvest losses, especially during ripening and storage. Moreover, leaf spots caused by Pestalotiopsis uvicola and P. clavispora [9] and wilt caused by Verticillium dahliae were also reported [10]. Regarding bacterial diseases, apical necrosis caused by Pseudomonas syringae pv. syringae is a major disease in the Mediterranean area and it has been described as being present in different countries including Italy [11,12,13,14].
Southeast Asia (China, Vietnam, Thailand, and India) represents the main world producer of litchi (Litchi chinensis Sonn.), followed by Australia, Africa, the Mediterranean basin, and tropical America [15]. Several fungal diseases affecting litchi have been reported worldwide. Downy blight caused by Phytophthora litchii [16] and anthracnose and litchi pepper spot caused by Colletotrichum gloeosporioides, C. siamense, and C. simmondsii [17,18] are considered the major pre-harvest diseases. Moreover, leaf, panicle, and fruit blights caused by Alternaria alternata [19] and algal leaf spots caused by Cephaleuros virescens were reported to cause severe economic losses. In Italy, no fungal diseases were reported on litchi.
Mango and litchi have had a great economic impact on the European market, and few studies were conducted on these crops in Italy until now. For this reason, it is necessary to investigate the diseases affecting these crops, which could represent a limiting factor for their production, in order to develop effective management strategies. In recent years with the rapid expansion of their production, some diseases were observed in mango and litchi orchards. Surveys carried out since 2014 led us to detect severe symptoms of woody canker, shoot blight, and dieback on mango and litchi plants in the main growing area of north-eastern Sicily.
The aims of this study were to (i) evaluate the etiology of the disease by morphological and molecular characterization of causal agents associated with these symptoms and (ii) determine their pathogenicity.
2. Materials and Methods
2.1. Field Survey and Isolation
Between 2014 and 2019, surveys conducted in north-eastern Sicily (Catania and Messina provinces) revealed the presence of 3–4-year-old mango plants (cvs. Kent, Keitt, Sensation, Osteen, and Kensington Pride) and 7–8-year-old litchi plants (cvs. Way Chee and Kwai Mai Pink) showing woody canker and shoot blight. Symptomatic samples were collected and brought to the laboratory of the Department of Agriculture, Food and Environment, University of Catania, for further investigations. For culture isolation, symptomatic woody tissues were surface-disinfected for 1 min in 1.2% sodium hypochlorite, rinsed in sterile water, placed on potato dextrose agar (PDA 3.9%, Oxoid, Basingstoke Hampshire, UK) amended with 100 mg/L of streptomycin sulfate (Sigma-Aldrich, St. Louis, MO, USA), and then incubated at 25 ± 1 °C for three days. Subsequently, from the margin of each developing colony, a piece of mycelium was taken to obtain pure cultures. After about 5–7 days, a single conidium or hyphal tip from each pure culture was transferred to PDA and maintained at 25 ± 1 °C.
2.2. Morphological Characterization
For the morphological characterization of the pathogen, the isolates were transferred to PDA and malt extract agar (MEA; 2% malt extract, Merck, Darmstadt, Germany) supplemented with streptomycin sulfate (100 μg/mL) and incubated at 25 °C. In addition, technical agar (TA, 1.2% agar, Oxoid, Basingstoke Hampshire, UK) with sterilized pine needles placed onto the surface (PNA) and then maintained at room temperature under near-ultraviolet light (NUV) was used to encourage production of pycnidia. The size and shape of conidia and colour and shape of pycnidia/conidiomata were examined. Conidia were measured by mounting fungal structures in clear lactic acid on microscope slides and observing under an Olympus BX 61 light microscope. Fifty measurements of conidia were made for one representative isolate for each fungal species recovered.
2.3. Molecular Characterization
Mycelium was collected from 5-day-old fungal cultures grown on PDA and MEA medium. Total fungal DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA). The internal transcriber spacer region (ITS) of the rDNA was amplified with primers ITS4 and ITS5 [20], the primers EF1-728F and EF1-986R [21] were used to amplify part of the translation elongation factor 1alpha gene (tef1), and primer sets Bt-2a and Bt-2b [22] were used for the partial betatubulin gene (tub2). Amplification by polymerase chain reaction (PCR) was performed in a total volume of 25 µL using OneTaq 2X Master Mix with Standard Buffer (BioLabs, Ipswich, MA, USA), according to the manufacturer’s instructions on an Eppendorf AG 22331 Hamburg Mastercycler. One reaction was composed of 12.5 µL of OneTaq 2X Master Mix, 0.5 µL of each primer, and 10.5 µL of nuclease-free water. The thermal cycle consisted of an initial denaturation temperature of 94 °C for 120 s, followed by 35 cycles at the denaturation temperature of 94 °C for 30 s, the annealing temperature of 55 °C (ITS), 57–59 °C (tef1), or 58 °C (tub2) for 30 s, the elongation temperature of 72°C for 40 s (ITS), 1 min (tef1), or 50 s (tub2), and a final extension at 72 °C for 5 min. The amplification products were separated by 1% agarose gel electrophoresis, purified and sequenced by Macrogen Inc. (South Korea) using both PCR primers. Purified sequence reactions were analyzed by an Applied Biosystems 3730 × l DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The generated DNA sequences were analyzed, and consensus sequences were edited using the MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms [23] and submitted to GenBank.
2.4. Phylogenetic Analysis
New sequences obtained in this study were blasted against the NCBI’s GenBank nucleotide database to determine the closest sequences for a taxonomic arrangement of the studied isolates. Alignments of different gene regions, including sequences obtained from this study and sequences retrieved from GenBank, were initially performed with the MAFFT v. 7 online server (http://mafft.cbrc.jp/alignment/server/index.html, accessed on 15 February 2022) [24] and then manually adjusted in MEGA v. 7 [25]. A phylogenetic analysis was conducted as concatenated multilocus sequence analyses using the ITS, tef1, and tub2 combination for the Botryosphaeria, Diaporthe, Lasiodiplodia, and Neofusicoccum genera [26,27]. The ex-type strain of Neofusicoccum parvum (CMW9081) was used as an outgroup for the analysis of Botryosphaeria spp., Diaporthella corylina (CBS 121124) was used as an outgroup for Diaporthe spp., Neodeightonia phoenicum (CBS 122528) was used as an outgroup for Lasiodiplodia spp., and Botryosphaeria dothidea (CBS 110302) was used as an outgroup for Neofusicoccum spp. The phylogenies were based on Bayesian Inference (BI) for the multi-locus analyses. The best evolutionary model was determined for each partition using MrModeltest v. 2.3 [28] and incorporated into the analyses. MrBayes v. 3.2.5 [29] was used to generate dendrograms under optimal criteria per partition. The Markov Chain Monte Carlo (MCMC) analysis used four chains and started from a random tree topology. The heating parameter was set at 0.2 and trees were sampled every 1000 generations. Analyses stopped when the average standard deviation of split frequencies was below 0.01. Sequences generated in this study were deposited in GenBank (Table 1).

Table 1.
Fungal isolates collected in this study and their Genbank accession numbers.
2.5. Pathogenicity Tests
Pathogenicity tests were conducted in controlled conditions with one representative isolate of each fungal type isolated from mango and litchi plants, except for N. parvum. For each isolate, six potted plants of mango and litchi (2 years old, cvs. Kengsinton Pride and Way Chee, respectively) were wounded at 3 different points on the stem. Inoculations were conducted after removing bark portions with a 5 mm diam. cork borer, placing a 5 mm plug from a 10-day-old PDA culture of the test isolate into the wound, and covering with Parafilm® (Pechney Plastic Packaging Inc., Chicago, IL, USA) to prevent desiccation and contamination. An equal number of plants were inoculated in the same way using sterile PDA plugs for control. Inoculated plants were then incubated at 25 ± 1 °C and 95% relative humidity (RH) in an 18 h fluorescent light/16 h dark regime in a growth chamber. Symptom development was evaluated after 2 months. In order to fulfill Koch’s postulates, re-isolations were performed following the procedure described above. Then, each fungus obtained was identified through morphological and molecular characteristics.
3. Results
3.1. Field Survey and Morphological Characterization
Symptomatic mango plants (cvs. Kent, Keitt, Sensation, Osteen, and Kensington Pride) were observed in seven orchards (from Giarre to Fiumefreddo municipalities, Catania province), whereas litchi symptoms were observed in only one orchard in Caronia municipality (Messina province). On mango plants, disease incidence (DI) varied from 3% to 18% according to the plant cultivar and orchard investigated, with the lowest values for Kensington Pride and the highest values for Osteen. On litchi, the DI was approximately 30% for Way Chee and 1% for Kwai Mai Pink.
Field disease symptoms of mango and litchi plants are shown in Figure 1 and Figure 2. In detail, all investigated cultivars of mango showed various external disease symptoms including shoot and twig blight, branch dieback, woody canker, and cracking of the bark. Occasionally on mango, woody canker can completely girdle the rootstock Gomera-3 and kill the tree in one to several months. Internal observation of symptomatic tissues revealed a brown wood discoloration (Figure 1). Litchi plants (cvs. Way Chee and Kwai Mai Pink) showed woody canker and shoot and twig blight. Affected leaves showed a brownish color and wilting (Figure 2).

Figure 1.
Symptoms of woody canker and shoot blight on mango plants. (a–c) Plant death as a consequence of trunk canker on rootstock Gomera-3; (d) branch dieback; (e–g) different types of canker lesions; (h) internal discoloration under canker lesion.

Figure 2.
Symptoms of woody canker and shoot blight on litchi plants. (a) Twig blight and defoliation; (b) shoot blight; (c,d) leaf blight.
Different types of colonies were consistently obtained from symptomatic tissues. A total of 54 fungal isolates were established from single conidium or hyphal tip cultures on the PDA (Table 1). Thirty-five isolates were characterized by dark green to gray, fast-growing mycelium on the MEA. Moreover, the isolates produced pycnidia containing pigmented or hyaline conidia on the PNA within 4 weeks. These characteristics led us to identify the fungal isolates as Botryosphaeriaceae spp. based on the earlier family description [30]. Fifteen isolates were characterized by slow-growing colonies on the PDA, with white, sparse, aerial mycelium and greenish-yellow pigmentation developing on reverse from the center. These isolates produced pycnidia containing hyaline conidia on the PNA within 4 weeks. Based on these characteristics, the fungal isolates were identified as Diaporthe spp. [31]. The morphological characteristics of each species recognized are reported in Table 2.

Table 2.
Morphological characteristics of isolates collected in this study.
3.2. Molecular Characterization and Phylogenetic Analysis
The combined locus phylogeny of Botryosphaeria consisted of 21 sequences, Diaporthe consisted of 52 sequences, Lasiodiplodia 37 sequences, and Neofusicoccum 37 sequences including outgroups. A total of 1433 characters (ITS: 1–588, tef1: 591–976, and tub2: 980–1433) were included in the Botryosphaeria phylogenetic analyses. A total of 1412 characters (ITS: 1–397, tef1: 404–935, and tub2: 942–1412) were included in the Diaporthe phylogenetic analyses. The analyses for the Lasiodiplodia group consisted of 1094 nucleotides (ITS: 1–472, tef1: 479–758, and tub2: 765–1094) and the analyses of Neofusicoccum were based on a total of 1153 characters (ITS: 1–499, tef1: 506–890, and tub2: 897–1153). For both the Bayesian analyses, MrModeltest recommended the models SYM (symmetrical model) + I (proportion of invariable sites) + G (gamma distribution) for ITS, and GTR (generalized time-reversible model) + G for tef1 and tub2. Unique site patterns for each partition and all the parameters of the Bayesian analyses are reported in Table 3. In the Botryosphaeria species analysis, six isolates from symptomatic mango plants clustered with the ex-type and one reference strain of B. dothidea (Figure 3), whereas the final tree generated for Diaporthe showed that four isolates from litchi grouped with two reference strains of D. rudis, two from mango and three strains from litchi with the ex-type strain of D. baccae, and seven from mango and eight strains from litchi with the ex-type strain of D. foeniculina (Figure 4). Five isolates from mango clustered with the ex-type and one reference strain of L. theobromae (Figure 5), and 19 isolates clustered as N. parvum in the phylogenetic tree generated from the analysis of the Neofusicoccum genus (Figure 6).

Table 3.
Bayesian inference characteristics of the analyses conducted in this study.
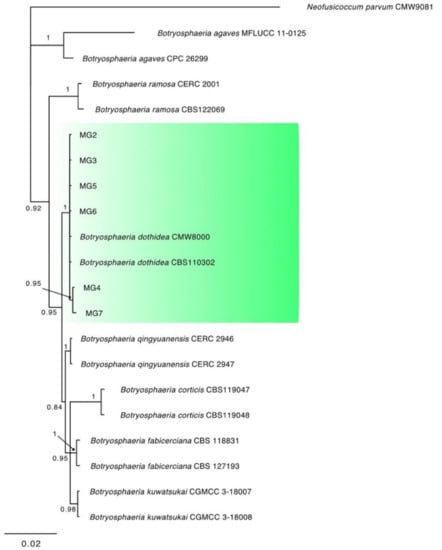
Figure 3.
Consensus phylogram of 1692 trees resulting from a Bayesian analysis of the combined ITS, tef1, and tub2 sequence of Botryosphaeria species. The tree was rooted to Neofusicoccum parvum (CMW9081). Bayesian posterior probability values are indicated at the nodes. The cluster containing the isolates obtained in this study is highlighted in green.
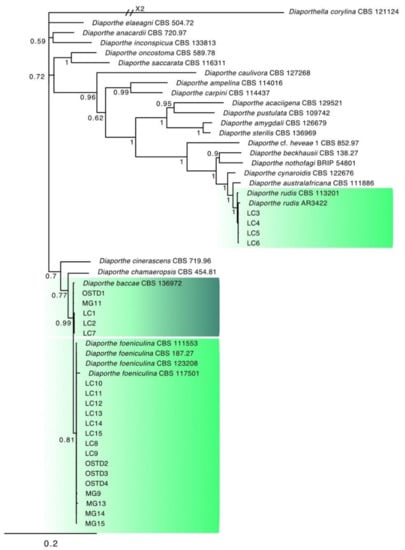
Figure 4.
Consensus phylogram of 3582 trees resulting from a Bayesian analysis of the combined ITS, tef1, and tub2 sequence of Diaporthe species. The tree was rooted to Diaporthella corylina (CBS121124). Bayesian posterior probability values are indicated at the nodes. The clusters containing the isolates obtained in this study are highlighted in green.
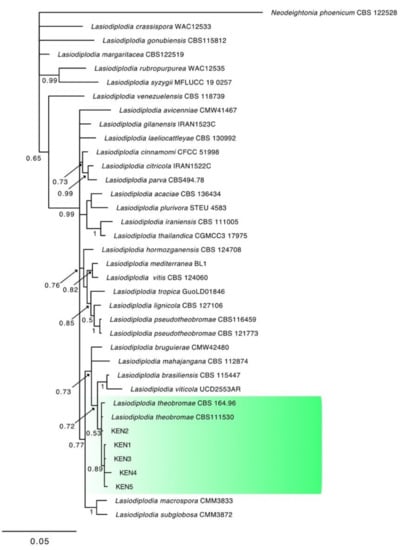
Figure 5.
Consensus phylogram of 1762 trees resulting from a Bayesian analysis of the combined ITS, tef1, and tub2 sequence of Lasiodiplodia species. The tree was rooted to Neodeightonia phoenicum (CBS122528). Bayesian posterior probability values are indicated at the nodes. The cluster containing the isolates obtained in this study is highlighted in green.
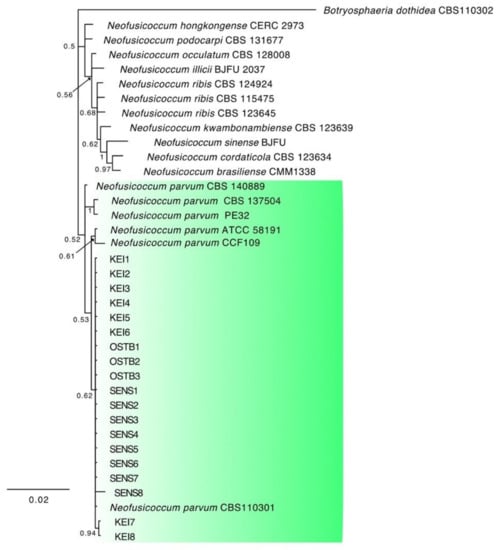
Figure 6.
Consensus phylogram of 6392 trees resulting from a Bayesian analysis of the combined ITS, tef1, and tub2 sequence of Neofusicoccum species. The tree was rooted to Botryosphaeria dothidea (CBS110302). Bayesian posterior probability values are indicated at the nodes. The cluster containing the isolates obtained in this study is highlighted in green.
3.3. Pathogenicity Tests
After 1 month, all the isolates of the different fungal species caused necrotic lesions on the wood of the inoculated plants. In detail, cankers and internal discoloration were observed in agreement with the inoculation points (Figure 7 and Figure 8). All the isolates induced similar lesions on the inoculated host species and no difference in length of the resulting lesions was observed among the inoculated isolates after 1 month. Otherwise, after 3 months infection resulted in apical dieback of the mango plants when inoculated with a D. baccae isolate. The other isolates killed the plants after more than 6 months. Control plants showed no symptoms. The fungi were re-isolated from some of the artificially inoculated plants in order to fulfill Koch’s postulates.

Figure 7.
Internal discoloration of woody cankers in mango plants cv. Kensington Pride 30 days after inoculation with (a) Botryosphaeria dothidea; (b) Lasiodiplodia theobromae; (c) Diaporthe foeniculina; (d) D. baccae.

Figure 8.
Woody cankers on litchi plants cv. Way Chee 30 days after inoculation with (a,b) Diaporthe rudis; (c) D. baccae.
4. Discussion
This study represents the first comprehensive survey of fungi associated with woody canker and shoot blight of mango and litchi plants in Sicily (southern Italy). Several cultivars of mango such as Kent, Keitt, Sensation, Osteen, and Kensington Pride from seven orchards and Way Chee and Kwai Mai Pink litchi from one orchard were inspected and sampled. Five different fungal species belonging to Botryosphaeriaceae and two species of Diaporthaceae were associated consistently with canker and dieback symptoms. Fifty-six fungal isolates were identified by morphological characterization and multi-locus phylogenies using ITS, tef1, and tub2. The discovered species included N. parvum, B. dothidea, L. theobromae, D. baccae, and D. foeniculina on mango and D. rudis and D. foeniculina on litchi. The pathogenicity of all Botryosphaeriaceae and Diaporthaceae species found for the first time in this study was confirmed through controlled inoculation experiments on mango and litchi plants. All isolates tested were able to cause cankers and necrotic lesions showing similar pathogenic behavior. Only D. baccae proved to be more aggressive killing the inoculated plants of mango after 3 months. To our knowledge, B. dothidea, L. theobromae, D. baccae, and D. foeniculina as well as D. rudis and D. foeniculina are reported here for the first time on mango and litchi plants in Italy.
Diaporthaceae and Botriophaeriaceae were already present in Italy and they were reported on other host species. Botryosphaeria dothidea has occurred on different species such as sycamore, red oak and English oak [32], grapevine [33,34], red eucalyptus in Sardinia [35], pistachio and Ficus microcarpa in Sicily [36,37]; D. baccae, D. rudis, and N. parvum have been reported on several Citrus spp. and on Vaccinum corymbosum [38,39,40]; D. foeniculina and N. parvum have also been reported on avocado and citrus [39,41,42]; L. theobromae was reported on grapevine and avocado [43,44]; N. parvum was also reported affecting Ficus carica [45], and D. rudis on grapes [46].
Some Botryophaeriaceae species obtained in this study, including B. dothidea and L. theobromae were reported as causal agents of fruit rot and the decline of mango in Florida, Brazil, and Australia [47,48,49,50,51,52]. Otherwise, dieback, decline, and stem-end rot of fruit caused by N. parvum, the main species found in this study, were already reported in Italy [7,41]. Botryosphaeriaceae include endophytes that infect the healthy tissues of woody plants and remain dormant until the occurrence of stress conditions [52,53,54,55]. Among these, environmental conditions such as temperature, rainfall, and wind could be involved in the development of infections increasing the stress on plants. Some studies have reported that climatic change affects the pathogen, the host, and the interaction between them [56,57]. Concerning Botryosphaeriaceae, B. dothidea was reported as a causal agent of serious infections on weak, stressed, or off-site trees [58]. Increased incidence and symptom severity of B. dothidea on apple trees have been related to water stress and winter injury in the eastern USA [59]. The symptom severity of diplodia shoot blight, caused by Diplodia sapinea, has consistently been associated with water stress [60,61,62].
Diaporthe species include endophytes associated with hosts present in temperate and tropical regions [63] as well as opportunistic plant pathogens. On mango, D. arecae, D. perseae, and D. pascoei have been reported in Malaysia, D. ueckerae and D. pseudomangiferae have been reported in Puerto Rico and other two species in Japan [64,65,66]. Several Diaporthe species are well-established in Europe and cause diseases of different crops [38,39,41,67]. Nevertheless, this study represents the first record of woody canker and shoot blight caused by D. baccae and D. foeniculina on mango. On litchi, Botryosphaeria sp., Diplodia sp., and Phomopis sp. were reported as causal agents of stem canker and dieback in Florida [68], but this study represents the first report worldwide of D. foeniculina and D. rudis on this host.
The occurrence of these infections in the field compromises plant growth as well as fruit quality. Indeed, Diaporthaceae and Botryosphaeriaceae have also been reported as causal agents of fruit stem-end rot, one of the most important diseases of fruit [41,50,64,66]. Therefore, these pathogens could also be a severe limiting factor for the production and export of fruit. Moreover, the pathogens’ recovery in this study confirms their high prevalence in Italy and the high infection risk for additional susceptible crops.
Since Sicily is not self-sufficient for the production of seedlings, young plants are imported from abroad, so the introduction of these pathogens in our areas could be due to the movement of infected plants from other countries. Moreover, the contamination of the material could occur during any of the production steps. For this reason, the use of certified propagative material and the elimination of dead wood or pruning residues are necessary to reduce potential inoculum sources, as well as following hygienic practices in order to avoid the inoculum spread.
Since these fungal infections could represent a major threat to the emerging tropical fruit crop cultivation in Sicily, it is necessary to conduct further research aimed at identifying the main stress factors influencing disease development and the potential damage to fruit, and to develop sustainable and effective strategies for disease management.
Author Contributions
D.A. performed the experiments and wrote the original manuscript; V.G. performed the experiments and the phylogenetic analysis; M.B.C., G.R.L. and F.E. performed the molecular analysis; G.P. (Giancarlo Perrone) performed the phylogenetic analysis; and G.P. (Giancarlo Polizzi) critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Programma Ricerca di Ateneo MEDIT-ECO UNICT 2020–2022 Linea 2-University of Catania (Italy).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Ara, R.; Motalab, M.; Uddin, M.N.; Fakhruddin, A.N.M.; Saha, B.K. Nutritional evaluation of different mango varieties available in Bangladesh. Int. Food Res. J. 2014, 21, 2169–2174. [Google Scholar]
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food. Sci. F. 2020, 19, 2139–2163. [Google Scholar] [CrossRef]
- Marçal, S.; Pintado, M. Mango peels as food ingredient/additive: Nutritional value, processing, safety and applications. Trends Food Sci. Technol. 2021, 114, 472–489. [Google Scholar] [CrossRef]
- FAO. Major Tropical Fruits—Statistical Compendium 2017; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; p. 38. [Google Scholar]
- Farina, V.; D’Asaro, A.; Mazzaglia, A.; Gianguzzi, G.; Palazzolo, E. Chemical-physical and nutritional characteristics of mature-green and mature-ripe ‘Kensington Pride’ mango fruit cultivated in Mediterranean area during cold storage. Fruits 2017, 72, 221–229. [Google Scholar] [CrossRef]
- Ismail, A.M.; Cirvilleri, G.; Lombard, L.; Crous, P.W.; Groenewald, J.Z.; Polizzi, G. Characterisation of Neofusicoccum species causing mango dieback in Italy. J. Plant Pathol. 2013, 95, 549–557. [Google Scholar]
- Ismail, A.M.; Cirvilleri, G.; Yaseen, T.; Epifani, F.; Perrone, G.; Polizzi, G. Characterisation of Colletotrichum species causing anthracnose disease of mango in Italy. J. Plant Pathol. 2015, 97, 167–171. [Google Scholar]
- Ismail, A.M.; Cirvilleri, G.; Polizzi, G. Characterisation and pathogenicity of Pestalotiopsis uvicola and Pestalotiopsis clavispora causing grey leaf spot of mango (Mangifera indica L.) in Italy. Eur. J. Plant Pathol. 2013, 135, 619–625. [Google Scholar] [CrossRef]
- Ahmed, Y.; Cirvilleri, G.; D’Onghia, A.M.; Yaseen, T. First report of Verticillium wilt of Mango (Mangifera indica) caused by Verticillium dahliae in Italy. Plant Dis. 2014, 98, 1156. [Google Scholar] [CrossRef]
- Trantas, E.A.; Mpalantinaki, E.; Pagoulatou, M.; Markakis, E.; Sarris, P.F.; Ververidis, F.; Goumas, D.E. First report of bacterial apical necrosis of mango caused by Pseudomonas syringae pv. syringae in Greece. Plant Dis. 2017, 101, 1541. [Google Scholar] [CrossRef]
- Torta, L.; Lo Piccolo, S.; Burruano, S.; Lo Cantore, P.; Iacobellis, N.S. Necrosi apicale del mango (Mangifera indica L.) causata da Pseudomonas syringae pv. syringae van Hall in Sicilia. Info. Fitopat. 2003, 11, 44–46. [Google Scholar]
- Aiello, A.; Ferrante, P.; Vitale, A.; Polizzi, G.; Scortichini, M.; Cirvilleri, G. Characterisation of Pseudomonas syringae pv. syringae isolated from mango in Sicily and occurrence of copper-resistant strains. J. Plant Pathol. 2015, 97, 273–282. [Google Scholar]
- Cazorla, F.M.; Tores, J.A.; Olalla, L.; Perez-Garcıa, A.; Farre, J.M.; de Vicente, A. Bacterial apical necrosis of mango in southern Spain: A disease caused by Pseudomonas syringae pv. syringae. Phytopathology 1998, 88, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Subhadrabandhu, S.; Mitra, S.K.; Ben-Arie, R.; Stern, R.A. Origin, History, Production and Processing. In Litchi and Longan. Botany, Production and Uses; Menzel, C.M., Waite, G.K., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 1–23. [Google Scholar]
- Ye, W.; Wang, Y.; Shen, D.; Li, D.; Pu, T.; Jiang, Z.; Zhang, Z.; Zheng, X.; Tyler, B.M.; Wang, Y. Sequencing of the litchi downy blight pathogen reveals it is a Phytophthora species with downy mildew-like characteristics. Mol. Plant-Microbe Interact. 2016, 29, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, J.F.; Song, X.B.; Xi, P.G.; Cheng, B.P.; Cui, Y.P.; Chen, X.; Peng, A.T.; Jiang, Z.D.; Zhang, L.H. Identification of Colletotrichum siamense causing litchi pepper spot disease in mainland China. Plant Pathol. 2019, 68, 1533–1542. [Google Scholar] [CrossRef]
- Anderson, J.M.; Aitken, E.A.B.; Dann, E.K.; Coates, L.M. Morphological and molecular diversity of Colletotrichum spp. causing pepper spot and anthracnose of lychee (Litchi chinensis) in Australia. Plant Pathol. 2013, 62, 279–288. [Google Scholar] [CrossRef]
- Kumar, V.; Anal, A.K.D.; Rai, S.; Nath, V. Leaf, panicle and fruit blight of litchi (Litchi chinensis) caused by Alternaria alternata in Bihar state, India. Can. J. Plant Pathol. 2018, 40, 84–89. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin–Felix, Y.; Hernández-Restrepo, M.; Wingfield, M.J.; Akulov, A.; Carnegie, A.J.; Cheewangkoon, R.; Gramaje, D.; Groenewald, J.Z.; Guarnaccia, V.; Halleen, F.; et al. Genera of phytopathogenic fungi: GOPHY2. Stud. Mycol. 2019, 92, 47–133. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v. 2. Program Distributed by the Author; Uppsala Evolutionary Biology Centre Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, A.J.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.M.; Phillips, A.J.L. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Divers. 2009, 34, 111–125. [Google Scholar]
- Turco, E.; Marianelli, L.; Vizzuso, C.; Ragazzi, A.; Gini, R.; Selleri, B.; Tucci, R. First report of Botryosphaeria dothidea on sycamore, red oak, and english oak in North western Italy. Plant Dis. 2006, 90, 1106. [Google Scholar] [CrossRef]
- Carlucci, A.; Lops, F.; Raimondo, M.L.; Gentile, V.; Mucci, M.; Frisullo, S. The Botryosphaeria species from vineyards of Apulia. Phytopathol. Mediterr. 2009, 48, 180. [Google Scholar]
- Carlucci, A.; Cibelli, F.; Lops, F.; Raimondo, M.L. Characterisation of Botryosphaeriaceae species as causal agents of trunk diseases on grapevines. Plant Dis. 2015, 99, 1678–1688. [Google Scholar] [CrossRef] [Green Version]
- Deidda, A.; Buffa, F.; Linaldeddu, B.T.; Pinna, C.; Scanu, B.; Deiana, V.; Satta, A.; Franceschini, A.; Floris, I. Emerging pests and diseases threaten Eucalyptus camaldulensis plantations in Sardinia, Italy. Forest 2016, 9, 883–891. [Google Scholar] [CrossRef]
- Gusella, G.; Lawrence, D.P.; Aiello, D.; Luo, Y.; Polizzi, G.; Michailides, T. Etiology of Botryosphaeria Panicle and Shoot Blight of Pistachio (Pistacia vera) caused by Botryosphaeriaceae in Italy. Plant Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, A.; Aiello, D.; Costanzo, M.B.; Gusella, G.; Polizzi, G. A New Disease for Europe of Ficus microcarpa Caused by Botryosphaeriaceae Species. Plants 2022, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Van Leeuwen, G.C.M.; Guarnaccia, V.; Polizzi, G.; Van Rijswick, P.C.; Rosendahl, K.C.H.M.; Gabler, J.; Crous, P.W. Diaporthe species associated with Vaccinium, with specific reference to Europe. Phytopathol. Mediterr. 2014, 53, 287–299. [Google Scholar]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by species of Diaporthe. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarnaccia, V.; Martino, I.; Tabone, G.; Brondino, L.; Gullino, M.L. Fungal pathogens associated with stem blight and dieback of blueberry in northern Italy. Phytopathol. Mediterr. 2020, 59, 229–245. [Google Scholar]
- Guarnaccia, V.; Vitale, A.; Cirvilleri, G.; Aiello, D.; Susca, A.; Epifani, F.; Perrone, G.; Polizzi, G. Characterisation and pathogenicity of fungal species associated with branch cankers and stem-end rot of avocado in Italy. Eur. J. Plant Pathol. 2016, 146, 963–976. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Crous, P.W.; Aiello, D.; Gullino, M.L.; Polizzi, G.; Guarnaccia, V. Genetic Diversity and Pathogenicity of Botryosphaeriaceae Species Associated with Symptomatic Citrus Plants in Europe. Plants 2021, 10, 492. [Google Scholar] [CrossRef]
- Burruano, S.; Mondello, V.; Conigliaro, G.; Alfonzo, A.; Spagnolo, A.; Mugnai, L. Grapevine decline in Italy caused by Lasiodiplodia theobromae. Phytopathol. Mediterr. 2008, 47, 132–136. [Google Scholar]
- Garibaldi, A.; Bertetti, D.; Amatulli, M.T.; Cardinale, J.; Gullino, M.L. First report of postharvest fruit rot in avocado (Persea americana) caused by Lasiodiplodia theobromae in Italy. Plant Dis. 2012, 96, 460. [Google Scholar] [CrossRef]
- Aiello, D.; Gusella, G.; Fiorenza, A.; Guarnaccia, V.; Polizzi, G. Identification of Neofusicoccum parvum causing Canker and Twig Blight on Ficus carica in Italy. Phytopathol. Mediterr. 2020, 59, 213–218. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. Diaporthe rudis associated with berry rot of postharvest grapes in Italy. Plant Dis. 2019, 103, 1030. [Google Scholar] [CrossRef]
- Ploetz, R.C. The major diseases of mango: Strategies and potential for sustainable management. Acta Hortic. 2004, 645, 137–150. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Benscher, D.; Vázquez, A.; Colls, A.; Nagel, J.; Schaffer, B. A re-examination of mango decline in Florida. Plant Dis. 1996, 80, 664–668. [Google Scholar] [CrossRef]
- Slippers, B.; Johnson, G.I.; Crous, P.W.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. Phylogenetic and Morphological Re-Evaluation of the Botryosphaeria Species Causing Diseases of Mangifera indica. Mycologia 2005, 97, 99–110. [Google Scholar] [CrossRef]
- Johnson, G.I. Status of mango postharvest disease management R&D: Options and solutions for the Australian mango industry. Hortic. Aust. 2008, 4, 1–130. [Google Scholar]
- de Oliveira Costa, V.S.; Michereff, S.J.; Martins, R.B.; Gava, C.A.T.; Mizubuti, E.S.G.; Camara, M.P.S. Species of Botryosphaeriaceae associated on mango in Brazil. Eur. J. Plant Pathol. 2010, 127, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Sakalidis, M.L.; Ray, J.D.; Lanoiselet, V.; Hardy, G.E.S.; Burgess, T.I. Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur. J. Plant Pathol. 2011, 130, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.; Wingield, M.J.; Crous, P.W.; Coutinho, T.A. Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. S. Afr. J. Bot. 1996, 62, 86–88. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.; Wingfield, M.J.; Petrini, O. Botryosphaeria dothidea endophytic in Eucalyptus grandis and Eucalyptus nitens in South Africa. For. Ecol. Manag. 1996, 89, 189–195. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Albers, A.J. Surveys for asymptomatic persistence of Sphaeropsis sapinea on or in stems of red pine seedlings from seven Great Lakes region nurseries. For. Pathol. 2005, 35, 233–244. [Google Scholar] [CrossRef]
- Lavalle, C.; Micale, F.; Houston, T.D.; Camia, A.; Hiederer, R.; Lazar, C.; Conte, C.; Amatulli, G.; Genovese, G. Climate change in Europe. 3. Impact on agriculture and forestry. A review. Agron. Sustain. Dev. 2009, 29, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Ma, Z.; Morgan, D.P.; Michailides, T.J. Effects of water stress on Botryosphaeria blight of pistachio caused by Botryosphaeria dothidea. Plant Dis. 2001, 85, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.A.; Hendrix, F.F. Pathogenicity and histopathology of Botryosphaeria dothidea on apple stems. Phytopathology 1981, 71, 375–379. [Google Scholar] [CrossRef]
- Blodgett, J.T.; Kruger, E.L.; Stanosz, G.R. Effects of moderate water stress on disease development by Sphaeropsis sapinea on red pine. Phytopathology 1997, 87, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Blodgett, J.T.; Kruger, E.L.; Stanosz, G.R. Sphaeropsis sapinea and water stress in a red pine plantation in central Wisconsin. Phytopathology 1997, 87, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, E.; Danti, R.; Strati, S. Pre- and post-inoculation water stress affects Sphaeropsis sapinea canker length in Pinus halepensis. For. Pathol. 2001, 31, 209–218. [Google Scholar] [CrossRef]
- Udayanga, D.; Liu, X.; Mckenzie, E.; Chukeatirote, E.; Bahkali, A.; Hyde, K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011, 50, 189–225. [Google Scholar] [CrossRef]
- Lim, L.; Mohd, M.H.; Zakaria, L. Identification and pathogenicity of Diaporthe species associated with stem-end rot of mango (Mangifera indica L.). Eur. J. Plant Pathol. 2019, 155, 687–696. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Rivera-Vargas, L.I.; French-Monar, R.D. First report of Diaporthe pseudomangiferae causing inflorescence rot, rachis canker and flower abortion on mango. Plant Dis. 2014, 98, 1004. [Google Scholar] [CrossRef] [PubMed]
- Ajitomi, A.; Minoshima, A.; Takushi, T.; Truong, H.H.; Ooshiro, A.; Yamashiro, M.; Arasaki, C.; Hirooka, Y. First report of mango (Mangifera indica) stem-end rot caused by two Diaporthe species and their susceptibility to procymidone. J. Gen. Plant Pathol. 2020, 86, 237–244. [Google Scholar] [CrossRef]
- Santos, J.M.; Vrandecic, K.; Cosic, J.; Duvnjak, T.; Phillips, A.J.L. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 2011, 27, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcmillan, J.R.R.T. Diseases of Litchi chinensis in south Florida. Proc. Fla. State Hortic. Soc. 1994, 107, 360–361. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).