Metagenomic Assessment Unravels Fungal Microbiota Associated to Grapevine Trunk Diseases
Abstract
:1. Introduction
2. Materials and Methods
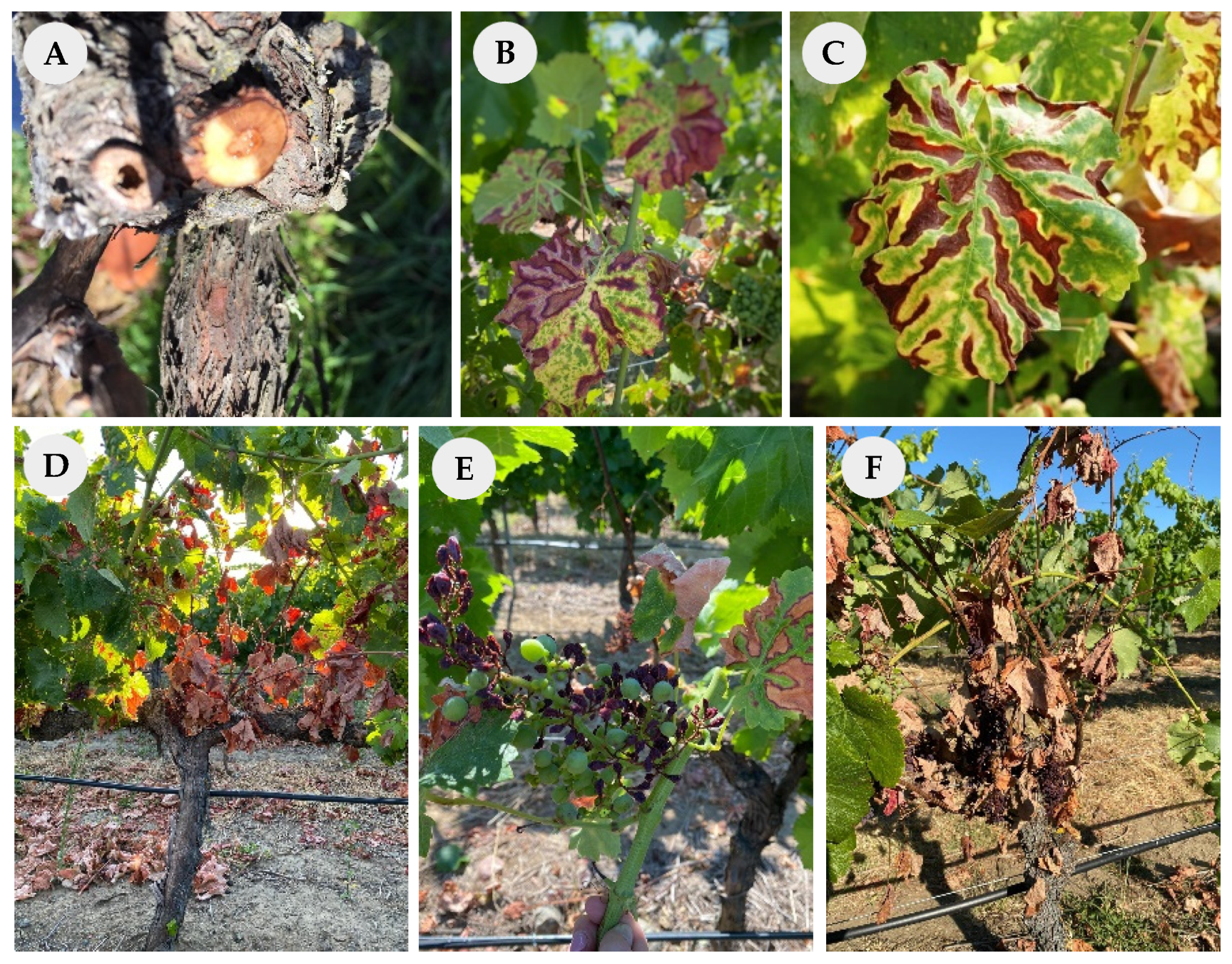
2.1. Study Site and Sample Collection
2.2. DNA Extraction and Sequencing
2.3. Bioinformatics Procedure
2.4. Statistical Data Analysis
3. Results
3.1. Grapevine Fungal Microbiota Overview
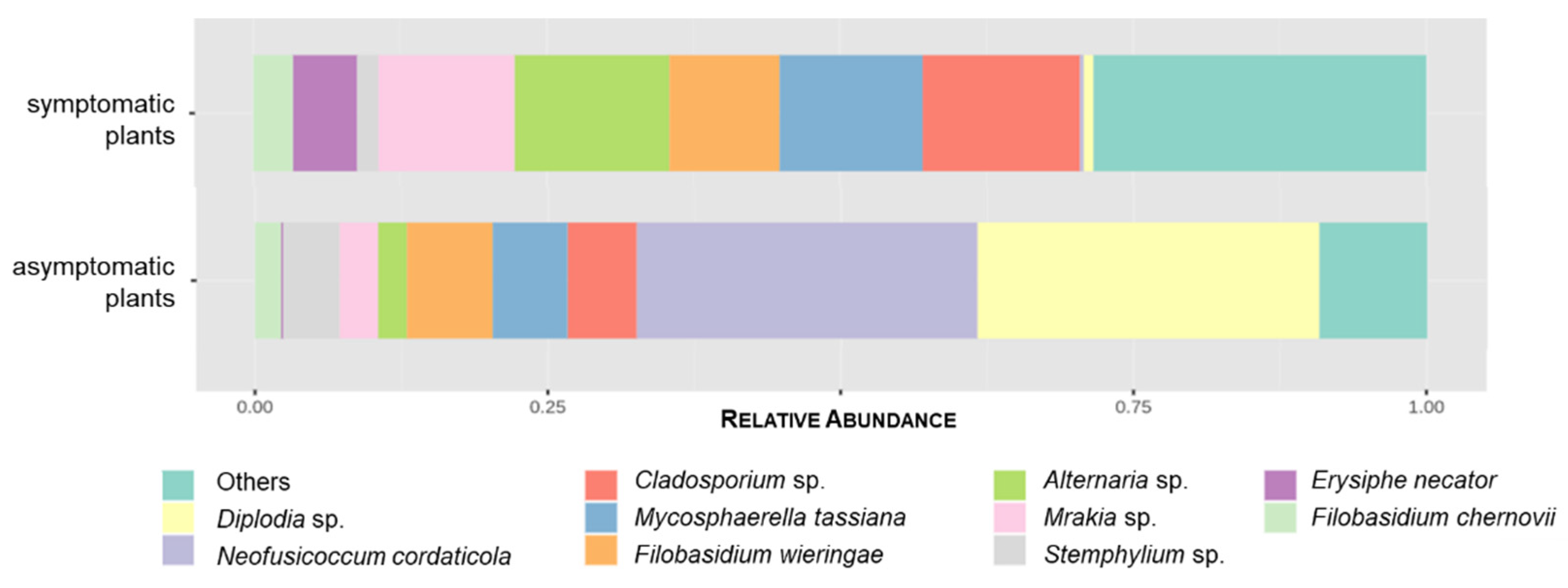
3.2. The Influence of Symptomatology on Grapevine Mycobiome
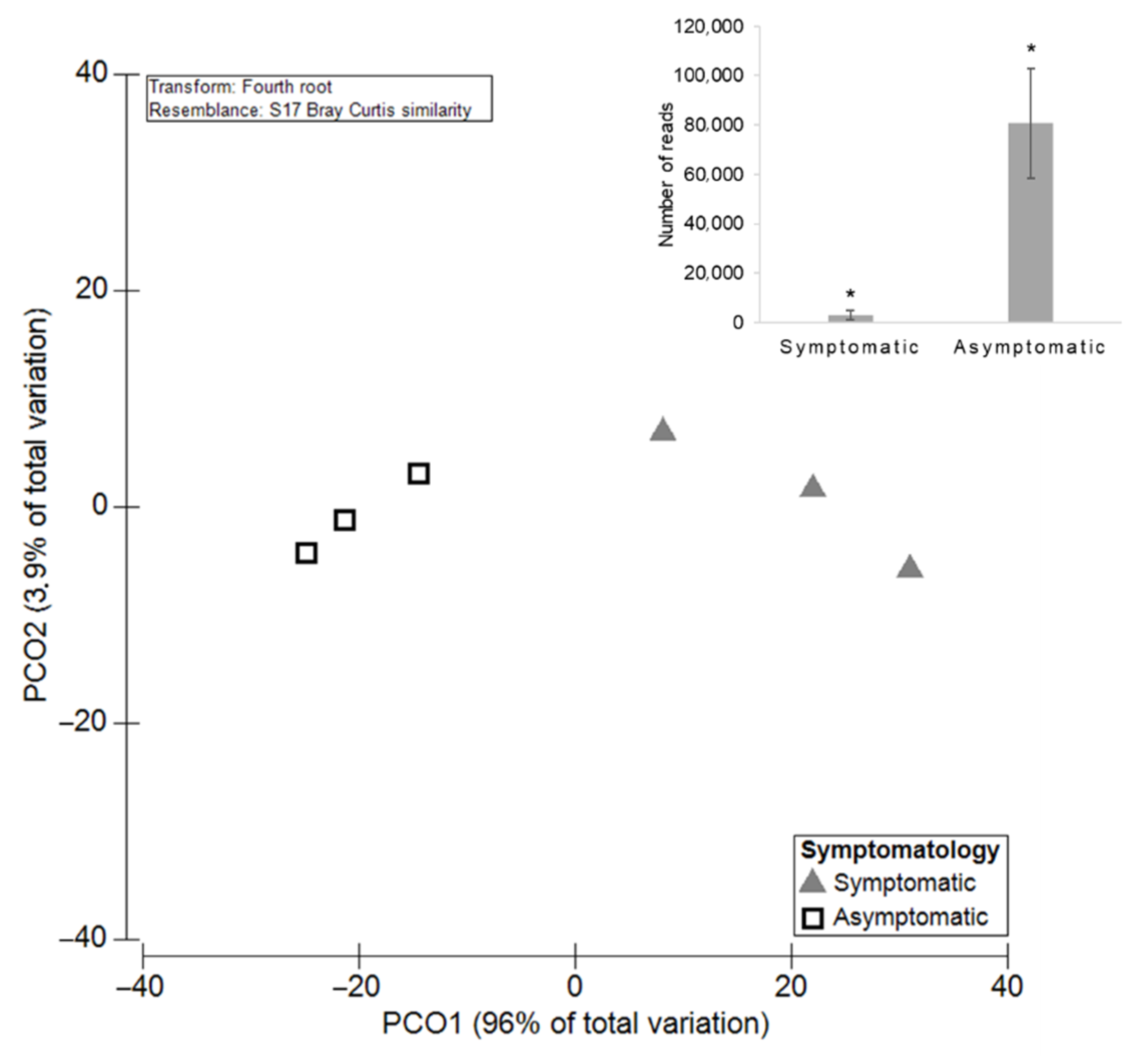
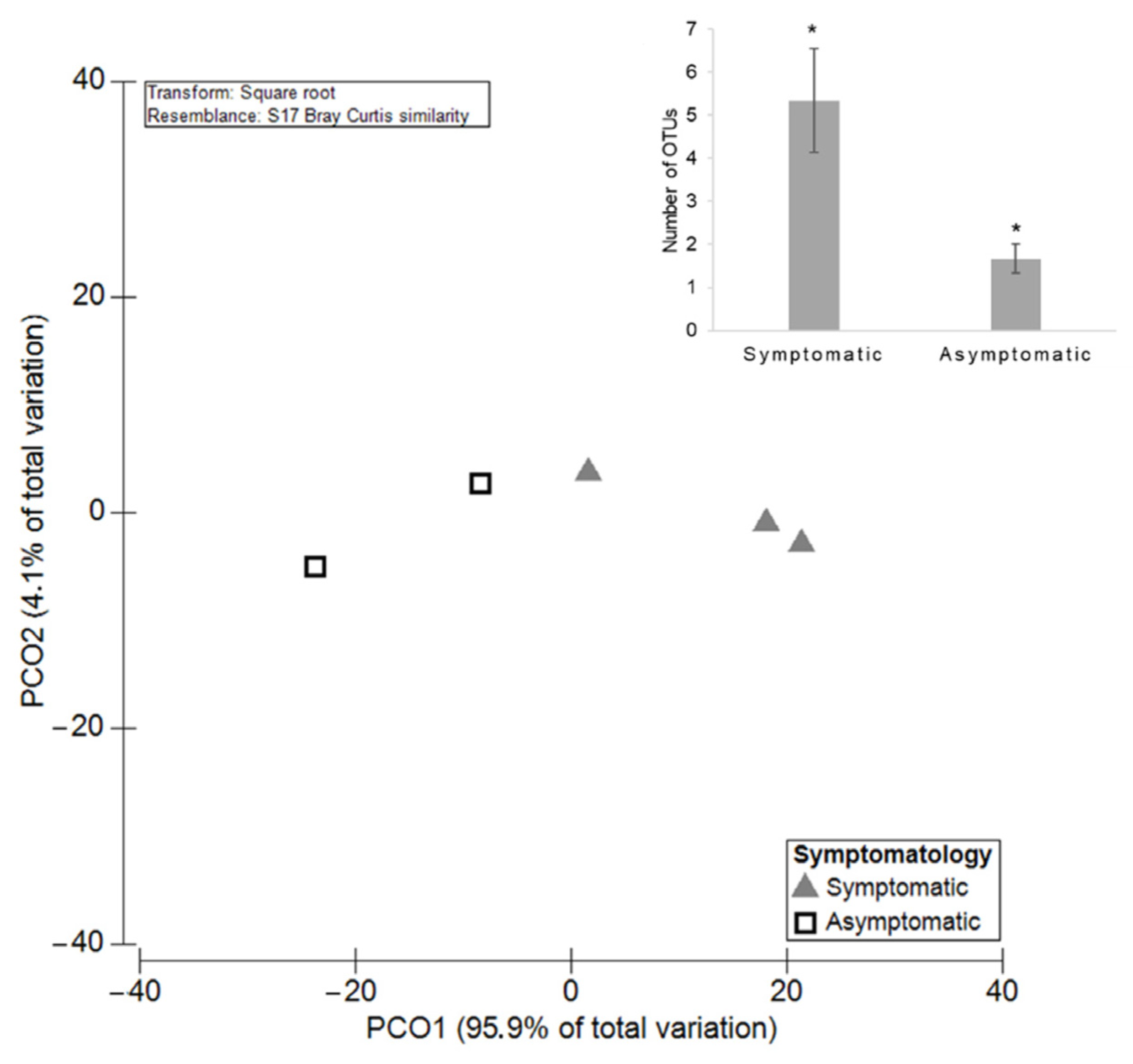
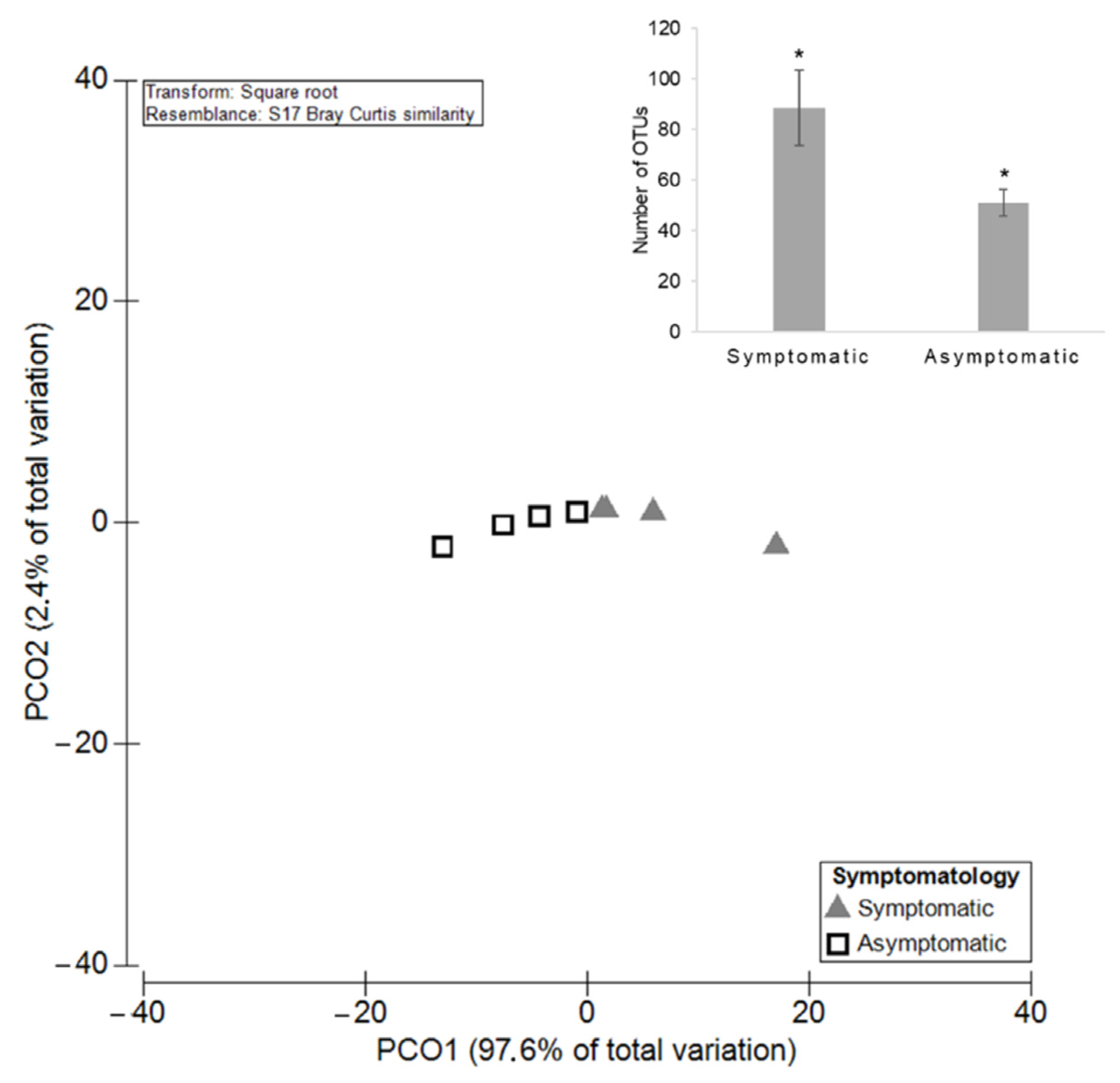
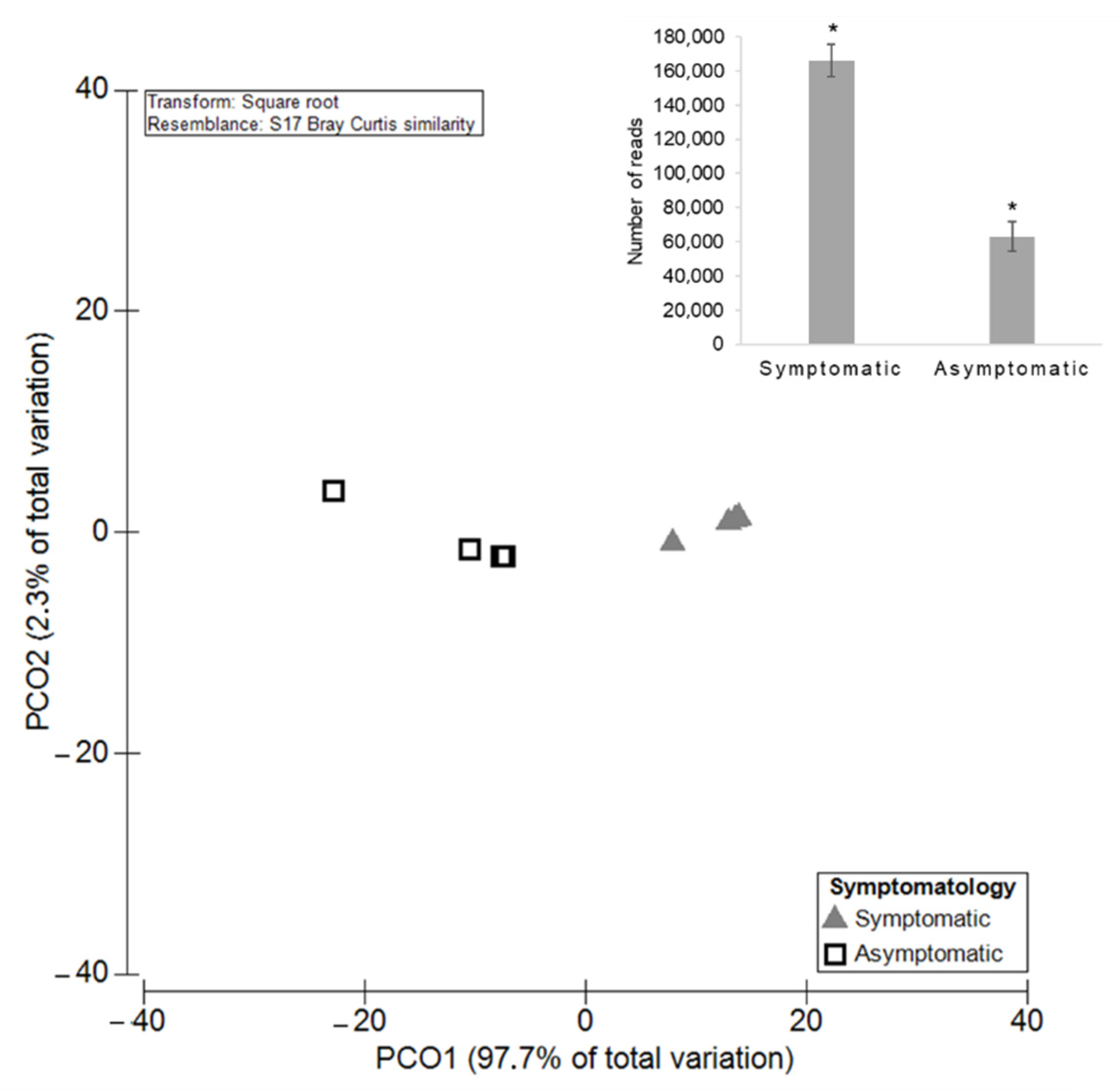
3.2.1. Diversity and Richness of the Fungal Microbiota
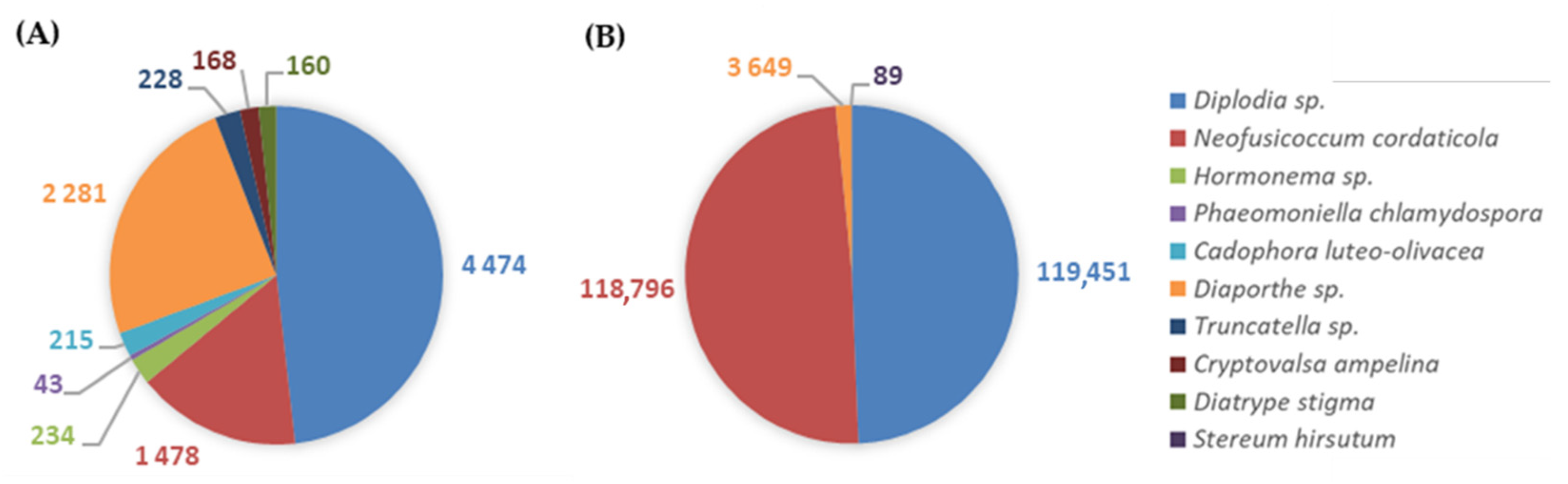
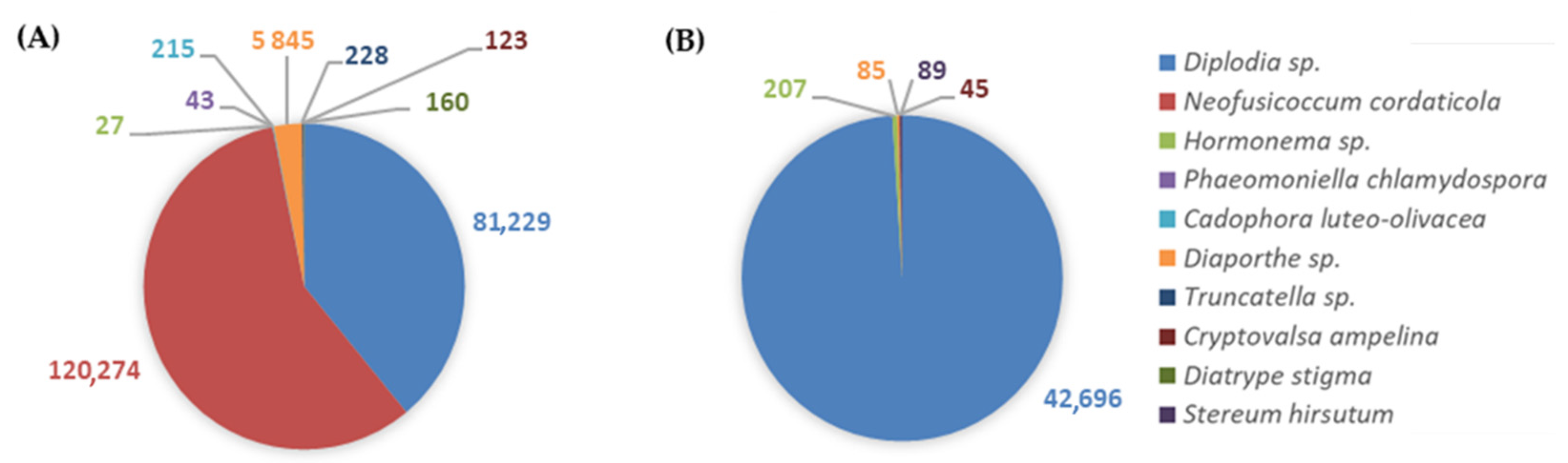
3.2.2. GTDs-Associated Fungi
3.2.3. Endophytic Community
3.3. The Influence of Cultivars on Grapevine Mycobiome
3.3.1. Diversity and Richness of the Fungal Microbiota
3.3.2. GTDs-Associated Fungi
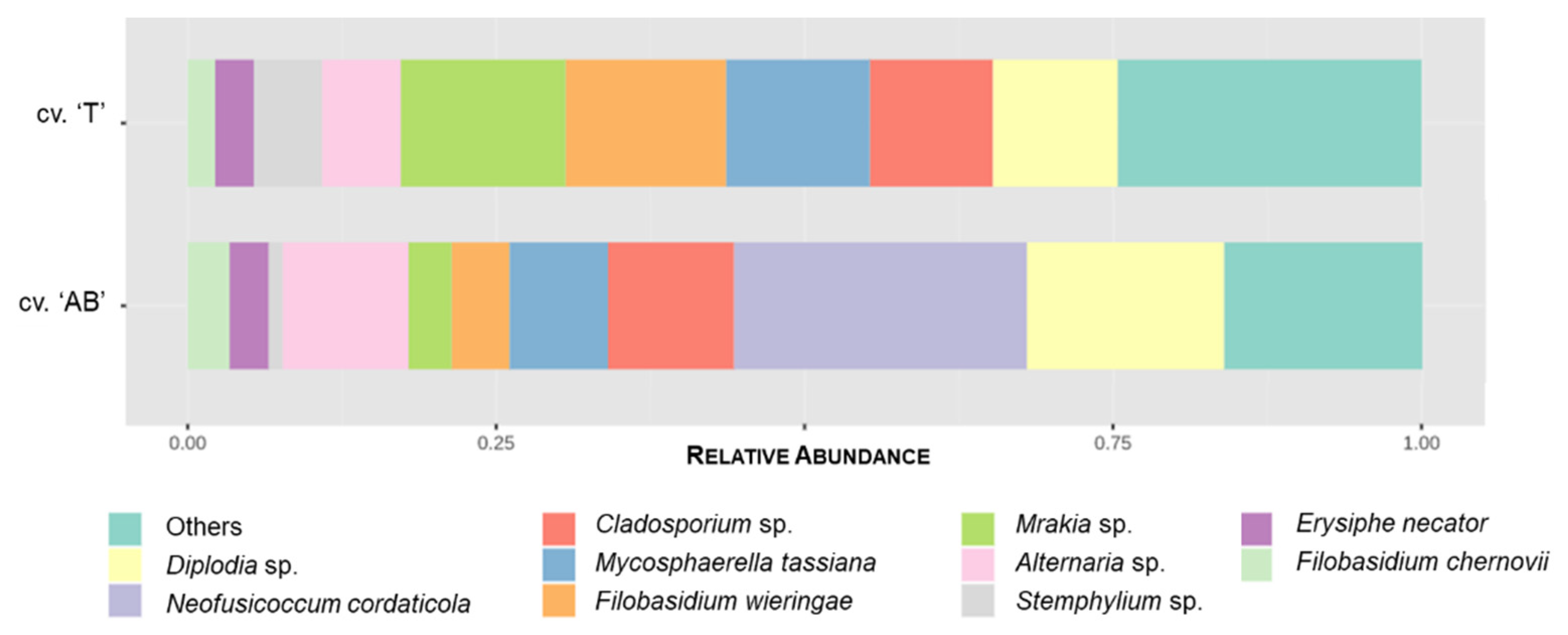
3.3.3. Endophytic Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, M.; Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Marie-France, C.-C. Grapevine Trunk Diseases. A Review; International Organisation of Vine and Wine: Paris, France, 2016; p. 24.
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2017, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef] [Green Version]
- Morales-Cruz, A.; Allenbeck, G.; Figueroa-Balderas, R.; Ashworth, V.E.; Lawrence, D.P.; Travadon, R.; Smith, R.J.; Baumgartner, K.; Rolshausen, P.E.; Cantu, D. Closed-reference metatranscriptomics enables in planta profiling of putative virulence activities in the grapevine trunk disease complex. Mol. Plant Pathol. 2018, 19, 490–503. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar] [CrossRef]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wery, J. Current knowledge on Grapevine Trunk Diseases with complex etiology: A systemic approach. Phytopathol. Mediterr. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Reis, P.; Pierron, R.; Larignon, P.; Lecomte, P.; Abou-Mansour, E.; Farine, S.; Bertsch, C.; Jacques, A.; Trotel-Aziz, P.; Rego, C.; et al. Vitis methods to understand and develop strategies for diagnosis and sustainable control of grapevine trunk diseases. Phytopathology 2019, 109, 916–931. [Google Scholar] [CrossRef]
- Surico, G. Towards a redefinition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar] [CrossRef]
- Lecomte, P.; Darrieutort, G.; Liminana, J.M.; Comont, G.; Muruamendiaraz, A.; Legorburu, F.J.; Choueiri, E.; Jreijiri, F.; El Amil, R.; Fermaud, M. New insights into Esca of grapevine: The development of foliar symptoms and their association with xylem discoloration. Plant Dis. 2012, 96, 924–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black Measles) and Brown Wood-Streaking: Two Old and Elusive Diseases of Grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Úrbez-Torres, J. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef]
- Bruez, E.; Baumgartner, K.; Bastien, S.; Travadon, R.; Guérin-Dubrana, L.; Rey, P. Various fungal communities colonise the functional wood tissues of old grapevines externally free from grapevine trunk disease symptoms. Aust. J. Grape Wine Res. 2016, 22, 288–295. [Google Scholar] [CrossRef]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Characterization of the Wood Mycobiome of Vitis vinifera in a Vineyard Affected by Esca. Spatial Distribution of Fungal Communities and Their Putative Relation With Leaf Symptoms. Front. Plant Sci. 2019, 10, 910. [Google Scholar] [CrossRef] [Green Version]
- Decoin, M. Grapevine products: News on withdrawals and restrictions. Phytoma 2001, 543, 28–33. [Google Scholar]
- Spinosi, J.; Févotte, J.; Vial, G. Éléments techniques sur l’exposition professionnelle aux pesticides arsenicaux. In Matrice Cultures-Expositions aux Pesticides Arsenicaux; Institut de Veille Sanitaire: Saint-Maurice, France, 2009; p. 19. [Google Scholar]
- Elena, G.; Luque, J. Seasonal susceptibility of grapevine pruning wounds and cane colonization in Catalonia, Spain following artificial infection with Diplodia seriata and Phaeomoniella chlamydospora. Plant Dis. 2016, 100, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.D.; Patanita, M.; Varanda, C.; Materatski, P.; Félix, M.R. Plant-Pathogen Interaction. Biology 2021, 10, 444. [Google Scholar] [CrossRef]
- Mondello, V.; Larignon, P.; Armengol, J.; Kortekamp, A.; Vaczy, K.; Prezman, F.; Serrano, E.; Rego, C.; Mugnai, L.; Fontaine, F. Management of grapevine trunk diseases: Knowledge transfer, current strategies and innovative strategies adopted in Europe. Phytopathol. Mediterr. 2018, 57, 369–383. [Google Scholar] [CrossRef]
- Mondello, V.; Lemaître-Guillier, C.; Trotel-Aziz, P.; Gougeon, R.; Acedo, A.; Schmitt-Kopplin, P.; Adrian, M.; Pinto, C.; Fernandez, O.; Fontaine, F. Assessment of a New Copper-Based Formulation to Control Esca Disease in Field and Study of Its Impact on the Vine Microbiome, Vine Physiology and Enological Parameters of the Juice. J. Fungi 2022, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- OIV. State of the World Vitivinicultural Sector in 2019; International Organisation of Vine and Wine: Paris, France, 2020; p. 14.
- Bruez, E.; Vallance, J.; Gerbore, J.; Lecomte, P.; Da Costa, J.-P.; Guerin-Dubrana, L.; Rey, P. Analyses of the Temporal Dynamics of Fungal Communities Colonizing the Healthy Wood Tissues of Esca Leaf-Symptomatic and Asymptomatic Vines. PLoS ONE 2014, 9, e95928. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [Green Version]
- Bettenfeld, P.; Cadena i Canals, J.; Jacquens, L.; Fernandez, O.; Fontaine, F.; van Schaik, E.; Courty, P.-E.; Trouvelot, S. The microbiota of the grapevine holobiont: A key component of plant health. J. Adv. Res. 2021; in press. [Google Scholar] [CrossRef]
- Pertot, I.; Prodorutti, D.; Colombini, A.; Pasini, L. Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. BioControl 2016, 61, 257–267. [Google Scholar] [CrossRef]
- Varanda, C.M.R.; Oliveira, M.; Materatski, P.; Landum, M.; Clara, M.I.E.; Félix, M.R. Fungal endophytic communities associated to the phyllosphere of grapevine cultivars under different types of management. Fungal Biol. 2016, 120, 1525–1536. [Google Scholar] [CrossRef]
- Del Frari, G.; Cabral, A.; Nascimento, T.; Boavida Ferreira, R.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 2019, 14, e0213273. [Google Scholar] [CrossRef] [Green Version]
- Billar de Almeida, A.; Concas, J.; Campos, M.D.; Materatski, P.; Varanda, C.M.R.; Patanita, M.; Murolo, S.; Romanazzi, G.; Félix, M.R. Endophytic fungi as potential biological control agents against grapevine trunk diseases in Alentejo region. Biology 2020, 9, 420. [Google Scholar] [CrossRef]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Fungicides and the Grapevine Wood Mycobiome: A Case Study on Tracheomycotic Ascomycete Phaeomoniella chlamydospora Reveals Potential for Two Novel Control Strategies. Front. Plant Sci. 2019, 10, 1405. [Google Scholar] [CrossRef]
- Morgan, H.H.; du Toit, M.; Setati, M.E. The Grapevine and Wine Microbiome: Insights from High-Throughput Amplicon Sequencing. Front. Microbiol. 2017, 8, 820. [Google Scholar] [CrossRef] [Green Version]
- Eichmeier, A.; Pečenka, J.; Peňázová, E.; Baránek, M.; Català-García, S.; León, M.; Armengol, J.; Gramaje, D. High-throughput amplicon sequencing-based analysis of active fungal communities inhabiting grapevine after hot-water treatments reveals unexpectedly high fungal diversity. Fungal Ecol. 2018, 36, 26–38. [Google Scholar] [CrossRef]
- Stefanini, I.; Cavalieri, D. Metagenomic Approaches to Investigate the Contribution of the Vineyard Environment to the Quality of Wine Fermentation: Potentials and Difficulties. Front. Microbiol. 2018, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Berlanas, C.; Berbegal, M.; Elena, G.; Laidani, M.; Cibriain, J.F.; Sagües, A.; Gramaje, D. The Fungal and Bacterial Rhizosphere Microbiome Associated with Grapevine Rootstock Genotypes in Mature and Young Vineyards. Front. Microbiol. 2019, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Diz, M.d.P.; Andrés-Sodupe, M.; Bujanda, R.; Díaz-Losada, E.; Eichmeier, A.; Gramaje, D. Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol. 2019, 41, 234–244. [Google Scholar] [CrossRef]
- Nerva, L.; Zanzotto, A.; Gardiman, M.; Gaiotti, F.; Chitarra, W. Soil microbiome analysis in an ESCA diseased vineyard. Soil Biol. Biochem. 2019, 135, 60–70. [Google Scholar] [CrossRef]
- Deyett, E.; Rolshausen, P.E. Endophytic microbial assemblage in grapevine. FEMS Microbiol. Ecol. 2020, 96, fiaa053. [Google Scholar] [CrossRef]
- Carbone, M.J.; Alaniz, S.; Mondino, P.; Gelabert, M.; Eichmeier, A.; Tekielska, D.; Bujanda, R.; Gramaje, D. Drought Influences Fungal Community Dynamics in the Grapevine Rhizosphere and Root Microbiome. J. Fungi 2021, 7, 686. [Google Scholar] [CrossRef]
- Dries, L.; Bussotti, S.; Pozzi, C.; Kunz, R.; Schnell, S.; Löhnertz, O.; Vortkamp, A. Rootstocks Shape Their Microbiome—Bacterial Communities in the Rhizosphere of Different Grapevine Rootstocks. Microorganisms 2021, 9, 822. [Google Scholar] [CrossRef]
- Bekris, F.; Vasileiadis, S.; Papadopoulou, E.; Samaras, A.; Testempasis, S.; Gkizi, D.; Tavlaki, G.; Tzima, A.; Paplomatas, E.; Markakis, E.; et al. Grapevine wood microbiome analysis identifies key fungal pathogens and potential interactions with the bacterial community implicated in grapevine trunk disease appearance. Environ. Microbiome 2021, 16, 23. [Google Scholar] [CrossRef]
- Gramaje, D.; Eichmeier, A.; Spetik, M.; Carbone, M.J.; Bujanda, R.; Vallance, J.; Rey, P. Exploring the temporal dynamics of the fungal microbiome in rootstocks, the lesser-known half of the grapevine crop. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Liu, D.; Howell, K. Community succession of the grapevine fungal microbiome in the annual growth cycle. Environ. Microbiol. 2021, 23, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Pacetti, A.; Moretti, S.; Pinto, C.; Compant, S.; Farine, S.; Bertsch, C.; Mugnai, L. Trunk Surgery as a Tool to Reduce Foliar Symptoms in Diseases of the Esca Complex and Its Influence on Vine Wood Microbiota. J. Fungi 2021, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Paolinelli, M.; Escoriaza, G.; Cesari, C.; Garcia-Lampasona, S.; Hernandez-Martinez, R. Characterization of Grapevine Wood Microbiome Through a Metatranscriptomic Approach. Microb. Ecol. 2021. [Google Scholar] [CrossRef]
- Lade, S.B.; Štraus, D.; Oliva, J. Variation in Fungal Community in Grapevine (Vitis vinifera) Nursery Stock Depends on Nursery, Variety and Rootstock. J. Fungi 2022, 8, 47. [Google Scholar] [CrossRef]
- Murolo, S.; Romanazzi, G. Effects of grapevine cultivar, rootstock and clone on esca disease. Australas. Plant Pathol. 2014, 43, 215–221. [Google Scholar] [CrossRef]
- Cardot, C.; Mappa, G.; La Camera, S.; Gaillard, C.; Vriet, C.; Lecomte, P.; Ferrari, G.; Coutos-Thévenot, P. Comparison of the Molecular Responses of Tolerant, Susceptible and Highly Susceptible Grapevine Cultivars During Interaction with the Pathogenic Fungus Eutypa lata. Front. Plant Sci. 2019, 10, 991. [Google Scholar] [CrossRef] [Green Version]
- CVRA. Relatório Annual—Gestão e Contas; Comissão Vitivinícola Regional Alentejana: Alentejo, Portugal, 2020; p. 85. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; Félix, M.R. Diversity of Colletotrichum Species Associated with Olive Anthracnose and New Perspectives on Controlling the Disease in Portugal. Agronomy 2018, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.D.; Patanita, M.; Campos, C.; Materatski, P.; Varanda, C.M.R.; Brito, I.; Félix, M.R. Detection and Quantification of Fusarium spp. (F. oxysporum, F. verticillioides, F. graminearum) and Magnaporthiopsis maydis in Maize Using Real-Time PCR Targeting the ITS Region. Agronomy 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Varanda, C.M.R.; Materatski, P.; Landum, M.; Campos, M.D.; Félix, M.R. Fungal Communities Associated with Peacock and Cercospora Leaf Spots in Olive. Plants 2019, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Materatski, P.; Varanda, C.M.R.; Carvalho, T.; Dias, A.; Campos, M.D.; Gomes, L.; Nobre, T.; Rei, F.; Félix, M.R. Effect of Long-Term Fungicide Applications on Virulence and Diversity of Colletotrichum spp. Associated to Olive Anthracnose. Plants 2019, 8, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E Ltd., Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Green, R.H. Statistical Design and Analysis for a ‘Biological Effects’ Study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Purahong, W.; Zhang, W.; Wubet, T.; Li, X.; Liu, M.; Zhao, W.; Hyde, K.D.; Liu, J.; Yan, J. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 2018, 90, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Perelló, A.; Aulicino, M.; Stenglein, S.A.; Labuda, R.; Moreno, M.V. Pseudopithomyces chartarum associated with wheat seeds in Argentina, pathogenicity and evaluation of toxigenic ability. Eur. J. Plant Pathol. 2017, 148, 491–496. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; Liu, J.K.; Lu, Y.Z.; et al. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 2019, 10, 1–186. [Google Scholar] [CrossRef]
- Al-Lami, H.F.D.; You, M.P.; Barbetti, M.J. Incidence, pathogenicity and diversity of Alternaria spp. associated with alternaria leaf spot of canola (Brassica napus) in Australia. Plant Pathol. 2019, 68, 492–503. [Google Scholar] [CrossRef]
- Schisler, D.A.; Janisiewicz, W.J.; Boekhout, T.; Kurtzman, C.P. Chapter 4—Agriculturally Important Yeasts: Biological Control of Field and Postharvest Diseases Using Yeast Antagonists, and Yeasts as Pathogens of Plants. In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 2011; pp. 45–52. [Google Scholar]
- Wang, X.; Schlatter, D.C.; Glawe, D.A.; Edwards, C.G.; Weller, D.M.; Paulitz, T.C.; Abatzoglou, J.T.; Okubara, P.A. Native yeast and non-yeast fungal communities of Cabernet Sauvignon berries from two Washington State vineyards, and persistence in spontaneous fermentation. Int. J. Food Microbiol. 2021, 350, 109225. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- González, V.; Tello, M.L. The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers. 2011, 47, 29–42. [Google Scholar] [CrossRef]
- Niem, J.M.; Billones-Baaijens, R.; Stodart, B.; Savocchia, S. Diversity Profiling of Grapevine Microbial Endosphere and Antagonistic Potential of Endophytic Pseudomonas Against Grapevine Trunk Diseases. Front. Microbiol. 2020, 11, 477. [Google Scholar] [CrossRef]
- Calzarano, F.; Osti, F.; Baránek, M.; Di Marco, S. Rainfall and temperature influence expression of foliar symptoms of grapevine leaf stripe disease (esca complex) in vineyards. Phytopathol. Mediterr. 2018, 57, 488–505. [Google Scholar] [CrossRef]
- Elena, G.; Bruez, E.; Rey, P.; Luque, J. Microbiota of grapevine woody tissues with or without esca-foliar symptoms in northeast Spain. Phytopathol. Mediterr. 2018, 57, 425–438. [Google Scholar] [CrossRef]
- Pancher, M.; Ceol, M.; Corneo, P.E.; Longa, C.M.O.; Yousaf, S.; Pertot, I.; Campisano, A. Fungal endophytic communities in grapevines (Vitis vinifera L.) respond to crop management. Appl. Environ. Microbiol. 2012, 78, 4308–4317. [Google Scholar] [CrossRef] [Green Version]
- Landum, M.C.; Félix, M.R.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F.; Varanda, C.M.R. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; Sanità di Toppi, L.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Karácsony, Z.; Golen, R.; Váczy, K.Z.; Geml, J. The compositional turnover of grapevine-associated plant pathogenic fungal communities are greater among intraindividual microhabitats and terroirs than among healthy and Esca-diseased plants. Phytopathology 2021. [Google Scholar] [CrossRef] [PubMed]
- Stranska, M.; Dzuman, Z.; Prusova, N.; Behner, A.; Kolouchova, I.; Lovecka, P.; Rezanka, T.; Kolarik, M.; Hajslova, J. Fungal Endophytes of Vitis vinifera—Plant Growth Promoters or Potentially Toxinogenic Agents? Toxins 2022, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Fourie, P.H.; Halleen, F. Proactive Control of Petri Disease of Grapevine Through Treatment of Propagation Material. Plant Dis. 2004, 88, 1241–1245. [Google Scholar] [CrossRef] [Green Version]
- Romanazzi, G.; Murolo, S.; Pizzichini, L.; Nardi, S. Esca in young and mature vineyards, and molecular diagnosis of the associated fungi. Eur. J. Plant Pathol. 2009, 125, 277–290. [Google Scholar] [CrossRef]
- Gramaje, D.; Di Marco, S. Identifying practices likely to have impacts on grapevine trunk disease infections: A European nursery survey. Phytopathol. Mediterr. 2015, 54, 313–324. [Google Scholar] [CrossRef]
- Surico, G.; Mugnai, L.; Marchi, G. Older and more recent observations on esca: A critical overview. Phytopathol. Mediterr. 2006, 45, 68–86. [Google Scholar] [CrossRef]
- Martos, S.; Andolfi, A.; Luque, J.; Mugnai, L.; Surico, G.; Evidente, A. Production of phytotoxic metabolites by five species of Botryosphaeriaceae causing decline on grapevines, with special interest in the species Neofusicoccum luteum and N. parvum. Eur. J. Plant Pathol. 2008, 121, 451–461. [Google Scholar] [CrossRef]
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, G.L.; Ippolito, M.P.; Mannerucci, F.; Bragazzi, L.; Tommasi, F. Physiological responses of ‘Italia’ grapevines infected with Esca pathogens. Phytopathol. Mediterr. 2021, 60, 321–336. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.; Báidez, A.G.; Ortuño, A.; Del Río, J.A. Grapevine xylem response to fungi involved in trunk diseases. Ann. Appl. Biol. 2016, 169, 116–124. [Google Scholar] [CrossRef]
- Kraus, C.; Rauch, C.; Kalvelage, E.M.; Behrens, F.H.; d’Aguiar, D.; Dubois, C.; Fischer, M. Minimal versus Intensive: How the Pruning Intensity Affects Occurrence of Grapevine Leaf Stripe Disease, Wood Integrity, and the Mycobiome in Grapevine Trunks. J. Fungi 2022, 8, 247. [Google Scholar] [CrossRef]
- Moretti, S.; Pacetti, A.; Pierron, R.; Kassemeyer, H.-H.; Fischer, M.; Péros, J.-P.; Perez-Gonzalez, G.; Bieler, E.; Schilling, M.; Marco, S.D.; et al. Fomitiporia mediterranea M. Fisch., the historical Esca agent: A comprehensive review on the main grapevine wood rot agent in Europe. Phytopathol. Mediterr. 2021, 60, 351–379. [Google Scholar] [CrossRef]
- Graniti, A.; Surico, G.; Mugnai, L. Esca of grapevine: A disease complex or a complex of diseases? Phytopathol. Mediterr. 2000, 39, 16–20. [Google Scholar] [CrossRef]
- Pacifico, D.; Squartini, A.; Crucitti, D.; Barizza, E.; Lo Schiavo, F.; Muresu, R.; Carimi, F.; Zottini, M. The Role of the Endophytic Microbiome in the Grapevine Response to Environmental Triggers. Front. Plant Sci. 2019, 10, 1256. [Google Scholar] [CrossRef] [Green Version]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R.; et al. A meta-analysis of global fungal distribution reveals climate-driven patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [Green Version]
- Del Frari, G.; Oliveira, H.; Boavida Ferreira, R. White Rot Fungi (Hymenochaetales) and Esca of Grapevine: Insights from Recent Microbiome Studies. J. Fungi 2021, 7, 770. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: III. Enzymes produced by the pathogens and their role in fungus-to-plant or in fungus-to-fungus interactions. Physiol. Mol. Plant Pathol. 2006, 69, 182–194. [Google Scholar] [CrossRef]
- Freitas, R.; Rego, C.; Oliveira, H.; Ferreira, R.B. Interactions among grapevine disease-causing fungi. The role of reactive oxygen species. Phytopathol. Mediterr. 2009, 48, 117–127. [Google Scholar] [CrossRef]
- Fischer, M.; Peighami-Ashnaei, S. Grapevine, esca complex, and environment: The disease triangle. Phytopathol. Mediterr. 2019, 58, 17–37. [Google Scholar] [CrossRef]
- Fourie, P.H.; Halleen, F.; van der Vyver, J.; Schrueder, W. Effect of Trichoderma treatments on the occurrence of decline pathogens on the roots and rootstocks of nursery plants. Phytopathol. Mediterr. 2001, 40, 473–478. [Google Scholar] [CrossRef]
- Casieri, L.; Hofstetter, V.; Viret, O.; Gindro, K. Fungal communities living in the wood of different cultivars of young Vitis vinifera plants. Phytopathol. Mediterr. 2009, 48, 73–83. [Google Scholar] [CrossRef]
- Travadon, R.; Lecomte, P.; Diarra, B.; Lawrence, D.; Renault, D.; Ojeda, H.; Rey, P.; Baumgartner, K. Grapevine pruning systems and cultivars influence the diversity of wood-colonizing fungi. Fungal Ecol. 2016, 24, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.D.; Félix, M.R.; Patanita, M.; Materatski, P.; Varanda, C. High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding. Hortic. Res. 2021, 8, 171. [Google Scholar] [CrossRef]
- Campos, M.D.; Félix, M.R.; Patanita, M.; Materatski, P.; Albuquerque, A.; Ribeiro, J.A.; Varanda, C. Defense Strategies: The Role of Transcription Factors in Tomato-Pathogen Interaction. Biology 2022, 11, 235. [Google Scholar] [CrossRef]

| Kingdom | Phylum | Class | Order | Family | Genus | Species | |
|---|---|---|---|---|---|---|---|
| % Sequences | 100 | 99.40 | 99.19 | 99.05 | 91.86 | 82.07 | 42.04 |
| Classified OTUs | 258 | 256 | 253 | 249 | 236 | 215 | 157 |
| Identified taxa | 1 | 2 | 17 | 46 | 102 | 157 | 157 |
| Phylum | Family | Genus/Species | Ecology and/or Pathogenic Potential | Relative Abundance (%) | |||
|---|---|---|---|---|---|---|---|
| Symptomatology | Cultivar | ||||||
| Symptomatic Plants | Asymptomatic Plants | ‘Alicante Bouschet’ | ‘Trincadeira’ | ||||
| Ascomycota | Botryosphaeriaceae | Diplodia sp. * | pathogen [18,64] | 0.38 | 10.22 | 6.95 | 3.65 |
| Neofusicoccum cordaticola * | pathogen [64] | 0.13 | 10.16 | 10.29 | – | ||
| Cladosporiaceae | Cladosporium sp. | endophyte; saprophyte [18,64] | 5.98 | 2.07 | 4.44 | 3.61 | |
| Mycosphaerellaceae | Mycosphaerella tassiana | endophyte; pathogen [45,64] | 5.43 | 2.23 | 3.46 | 4.20 | |
| Neodevriesiaceae | Neodevriesia capensis | unknown | 2.00 × 10−3 | 0.10 | 2.00 × 10−3 | 0.10 | |
| Teratosphaeriaceae | Catenulostroma hermanusense | saprophyte [64] | 0.27 | – | 0.27 | – | |
| Devriesia pseudoamericana | saprophyte [64] | 0.20 | 0.01 | 0.20 | 0.01 | ||
| Recurvomyces mirabilis | unknown | 0.10 | – | – | 0.10 | ||
| Amorosiaceae | Angustimassarina acerina | saprophyte [18] | 0.09 | 0.01 | 0.07 | 0.04 | |
| Didymosphaeriaceae | Pseudopithomyces chartarum | endophyte; pathogen; saprophyte [65,66] | 0.10 | 0.12 | 0.06 | 0.17 | |
| Spegazzinia tessarthra | unknown | 0.09 | 0.02 | 1.00 × 10−3 | 0.11 | ||
| Lentitheciaceae | Keissleriella sp. | unknown | 0.12 | – | 0.03 | 0.09 | |
| Phaeosphaeriaceae | Neosetophoma samararum | unknown | 0.07 | 0.06 | 0.06 | 0.07 | |
| Pleosporaceae | Alternaria sp. | endophyte; pathogen [18,64] | 5.88 | 0.86 | 4.44 | 2.31 | |
| Alternaria metachromatica | endophyte; pathogen [67] | 0.59 | 0.21 | 0.29 | 0.51 | ||
| Paradendryphiella arenariae | unknown | 0.13 | – | 0.05 | 0.07 | ||
| Stemphylium sp. | endophyte [64] | 0.80 | 1.67 | 0.48 | 1.99 | ||
| Chalastospora gossypii | endophyte [64] | 0.40 | 0.03 | 0.36 | 0.08 | ||
| Aspergillaceae | Aspergillus sp. | endophyte; pathogen; saprophyte [64] | 0.10 | 0.01 | 0.02 | 0.09 | |
| Penicillium lapidosum | unknown | 0.09 | 0.07 | 0.04 | 0.11 | ||
| Phaeomoniellaceae | Neophaeomoniella niveniae | unknown | 0.11 | – | 0.11 | – | |
| Erysiphaceae | Erysiphe necator | pathogen [64] | 2.45 | 0.07 | 1.39 | 1.13 | |
| Sclerotiniaceae | Monilinia laxa | saprophyte [64] | 0.94 | 0.05 | 4.00 × 10−3 | 0.99 | |
| Diaporthaceae | Diaporthe sp. * | pathogen [18,64] | 0.20 | 0.31 | 0.50 | 0.01 | |
| Nectriaceae | Fusarium sp. | pathogen; saprophyte [64] | 2.08 | 0.14 | 0.10 | 2.12 | |
| Gibberella intricans | pathogen; saprophyte [64] | 1.62 | 0.03 | 1.62 | 0.02 | ||
| Amphisphaeriaceae | Discostroma sp. | pathogen [64] | 0.64 | 0.03 | 0.57 | 0.10 | |
| Basidiomycota | Typhulaceae | Typhula sp. | saprophyte [64] | 0.29 | – | 0.29 | – |
| Malasseziaceae | Malassezia restricta | unknown | 0.12 | 0.03 | 0.04 | 0.12 | |
| Sporidiobolaceae | Sporobolomyces roseus | endophyte [68,69] | 1.56 | 0.67 | 0.53 | 1.69 | |
| Cystofilobasidiaceae | Cystofilobasidium macerans | unknown | 0.18 | 0.07 | 0.17 | 0.09 | |
| Mrakiaceae | Mrakia sp. | unknown | 5.17 | 1.16 | 1.51 | 4.82 | |
| Udeniomyces pyricola | unknown | 0.17 | – | 3.00 × 10−3 | 0.17 | ||
| Filobasidiaceae | Filobasidium chernovii | unknown | 1.49 | 0.77 | 1.46 | 0.80 | |
| Filobasidium magnum | endophyte [69] | 0.23 | 0.13 | 0.15 | 0.21 | ||
| Filobasidium wieringae | unknown | 4.19 | 2.56 | 2.04 | 4.70 | ||
| Filobasidium sp. | endophyte [69] | 0.94 | 0.14 | 0.28 | 0.80 | ||
| Holtermanniales incertae sedis | Holtermanniella takashimae | unknown | 0.10 | – | 0.10 | – | |
| Bulleribasidiaceae | Vishniacozyma dimennae | unknown | 0.10 | 0.05 | 0.09 | 0.06 | |
| Vishniacozyma victoriae | endophyte [69] | 0.86 | 0.53 | 0.89 | 0.50 | ||
| Phaeotremellaceae | Gelidatrema spencermartinsiae | unknown | 0.15 | 0.08 | 0.14 | 0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanita, M.; Albuquerque, A.; Campos, M.D.; Materatski, P.; Varanda, C.M.R.; Ribeiro, J.A.; Félix, M.d.R. Metagenomic Assessment Unravels Fungal Microbiota Associated to Grapevine Trunk Diseases. Horticulturae 2022, 8, 288. https://doi.org/10.3390/horticulturae8040288
Patanita M, Albuquerque A, Campos MD, Materatski P, Varanda CMR, Ribeiro JA, Félix MdR. Metagenomic Assessment Unravels Fungal Microbiota Associated to Grapevine Trunk Diseases. Horticulturae. 2022; 8(4):288. https://doi.org/10.3390/horticulturae8040288
Chicago/Turabian StylePatanita, Mariana, André Albuquerque, Maria Doroteia Campos, Patrick Materatski, Carla M. R. Varanda, Joana A. Ribeiro, and Maria do Rosário Félix. 2022. "Metagenomic Assessment Unravels Fungal Microbiota Associated to Grapevine Trunk Diseases" Horticulturae 8, no. 4: 288. https://doi.org/10.3390/horticulturae8040288
APA StylePatanita, M., Albuquerque, A., Campos, M. D., Materatski, P., Varanda, C. M. R., Ribeiro, J. A., & Félix, M. d. R. (2022). Metagenomic Assessment Unravels Fungal Microbiota Associated to Grapevine Trunk Diseases. Horticulturae, 8(4), 288. https://doi.org/10.3390/horticulturae8040288









