Abstract
Grapevine trunk diseases (GTDs) are among the most important problems that affect the longevity and productivity of vineyards in all the major growing regions of the world. They are slow-progression diseases caused by several wood-inhabiting fungi with similar life cycles and epidemiology. The simultaneous presence of multiple trunk pathogens in a single plant together with the inconsistent GTDs symptoms expression, their isolation in asymptomatic plants, and the absence of effective treatments make these diseases extremely complex to identify and eradicate. Aiming to gain a better knowledge of GTDs and search sustainable alternatives to limit their development, the present work studied the fungal community structure associated with GTDs symptomatic and asymptomatic grapevines, following a metagenomic approach. Two important cultivars from the Alentejo region with different levels of susceptibility to GTDs were selected, namely, ‘Alicante Bouschet’ and ‘Trincadeira’. Deep sequencing of fungal-directed ITS1 amplicon led to the detection of 258 taxa, including 10 fungi previously described as responsible for GTDs. Symptomatic plants exhibited a lower abundance of GTDs-associated fungi, although with significantly higher diversity of those pathogens. Our results demonstrated that trunk diseases symptoms are intensified by a set of multiple GTDs-associated fungi on the same plant. The composition of fungal endophytic communities was significantly different according to the symptomatology and it was not affected by the cultivar. This study opens new perspectives in the study of GTDs-associated fungi and their relation to the symptomatology in grapevines.
1. Introduction
Vitis vinifera L. is affected by several diseases with grapevine trunk diseases (GTDs) being the most widespread [1], influencing the productivity and longevity of vineyards in all the main growing regions of the world and causing great economic losses [1,2,3]. They are caused by wood-inhabiting fungi, namely, by 133 species described to date, belonging to nine families and 34 genera, with similar etiology and epidemiology [4,5,6]. The GTDs complex includes specific diseases such as Black foot disease, Botryosphaeria dieback, Esca, Eutypa dieback, Petri disease, and Phomopsis dieback [1,3,4,5,6,7]; these are categorized as slow-progression diseases with symptoms sometimes taking several years to appear after infection, making early detection difficult [8]. Black foot and Petri diseases typically occur in young grapevines (aged five years or younger), while Botryosphaeria dieback, Esca, Eutypa dieback, and Phomopsis dieback are usually found in older grapevines [7,8,9]. General symptoms of GTDs include leaf chlorosis, wood discoloration and necrosis, delayed bud-break, stunted growth, reduced vigour, canker formation, dieback, and the eventual death of symptomatic grapevine plants (Figure 1) [7,8,9,10]. This disease complex can be divided into different syndromes: tiger stripe foliar symptoms and apoplexy, depending on symptoms, vine age, pathogens involved, and environmental factors [9,11]. The apoplexy form involves complete wilting of grapevine canopies, leaves, and stems within a few days, leading to sudden grapevine death, while tiger stripe symptoms are milder, with interveinal discolorations and necrosis on leaf blades, affecting all or part of the grapevine foliage [9,12]. Nevertheless, some plants may remain only with foliar symptoms for many years, reaching apoplexy after the collapse of the entire plant hydraulic system, which occurs most frequently during hot days each year [9,13].
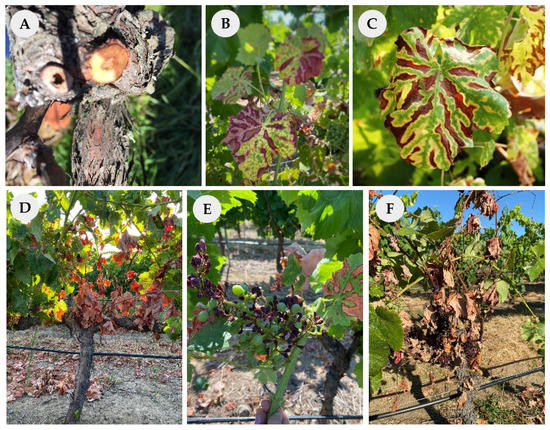
Figure 1.
Grapevine trunk diseases general symptoms: wood discoloration and necrosis (A), interveinal discolorations, chloroses and necrosis on leaf blades, affecting all or part of the grapevine foliage (B–D), stunted growth (E), dieback, and the eventual death of symptomatic grapevines (F). Pictures were taken in the frame of this study.
Grapevines can be simultaneously infected by different trunk pathogens through multiple infection opportunities that take place throughout a season and over the years [3]. Moreover, the inconsistent GTDs symptoms expression from year to year and the presence of these fungi in asymptomatic plants seem to indicate that some of GTDs-associated fungi act as latent pathogens [8,14]. In the asymptomatic phases of their life cycle, these fungi live as endophytes (saprobes, mutualists, or latent pathogens), and, at a specific point, they modify their behaviour and become pathogenic, leading to the expression of disease symptoms [15]. The transition from endophytic to pathogenic phases can be due to abiotic or biotic stress conditions [14,16]. Under stress, fungal growth is accelerated, and colonisation thresholds are reached sooner than under normal conditions [15]. Furthermore, when multiple GTDs-associated fungi are present within a grapevine, these thresholds could be reached earlier [8]. The simultaneous presence of multiple fungal species in a single plant also makes disease diagnosis difficult [3,4,17,18]. Currently, no effective treatments are known to control GTDs [3,4], since the use of effective chemical products against GTDs fungi was banned in Europe due to implemented European laws concerning pesticide restrictions [19,20,21,22]. Disease control is only possible through disease prevention and mitigation [14,23]; thereby, it is necessary to develop alternative, effective, and environmentally safe control strategies with minor impacts for human health. A recent study used a new low-copper-based formulation with good results in decreasing the incidence of GTDs in treated grapevines over the years, with no harmful effects on the microbiota and on the grapevine physiology [24].
Grapevine currently plays a key role in the economy of many countries; it is one of the most cultivated crops worldwide, with a total vineyard area of 7.3 mha [25] and numerous applications such as fresh table grape, dried fruit, and wine production [3]. Portugal is currently among the eleven major wine producers in the world, with 6.4 million hL produced, in a total vineyard area of 194 kha [25], and the Alentejo (south of Portugal) is the second largest wine-producer region in the country. Apart from the economic impact, this culture also presents significant environmental, social, and landscape importance in many countries. The study of fungal microbiota associated to GTDs, which constantly interact with each other and the grapevine, will facilitate the understanding of their complex interactions [26,27,28]. Knowledge of the composition of fungal communities in different cultivars associated with the presence or absence of symptoms can also aid the discovery of potential antagonists that can be exploited to design new effective biological strategies for disease management, resulting in more sustainable production systems [28]. The biological control using fungal endophytes has been the focus of several studies, which have already shown some beneficial effects against some important pathogens in Vitis vinifera [29,30,31,32,33], but further research is still required to limit the development of these diseases. Recent studies using metagenomic approaches have been used to investigate the grapevine microbiome and have allowed a deeper insight into endophytic microbial community changes [18,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. High-throughput sequencing technologies (HTS), combined with more efficient bioinformatics tools, have demonstrated significant improvements in the quality of results with reduced costs, revolutionising plant pathology [34].
Aiming to gain a better knowledge of GTDs, this work intended to investigate the fungal community structure (species richness and diversity) of symptomatic and asymptomatic grapevines to GTDs using HTS technology. Several factors may influence grapevine susceptibility to GTDs, mainly climate, vine age, soil fertilisation, rootstock, and cultivar [48,49,50]. In this sense, two important red grape cultivars from the Alentejo region were selected, ‘Alicante Bouschet’ and ‘Trincadeira’. These cultivars, despite comprising 17.2% and 15.8% of the total red grape vineyard area in this region, respectively [51], reveal different patterns of susceptibility to GTDs with distinct severity of foliar symptoms, with ‘Alicante Bouschet’ being more susceptible and ‘Trincadeira’ less susceptible [32]. Therefore, it was questioned whether the richness and diversity of the fungal communities associated with GTDs vary (i) according to the presence or absence of symptoms and (ii) among the cultivars to better understand the underlying mechanisms associated with the expression of trunk diseases symptoms. The need for new strategies for GTDs management and the lack of information on grapevine fungal microbiota, as well as on the susceptibility of cultivars to these diseases, led to this study.
2. Materials and Methods
2.1. Study Site and Sample Collection
This study focused on a cordon-pruned conventional 17-year-old vineyard propagated on 1103P rootstock and located in the Alentejo region (south of Portugal) (38°31′58.2″ N, 8°00′53.1″ W). The vineyard is managed under Integrated Pest Management (IPM) rules with a drip irrigation system. The sampled field has a history of trunk diseases, with many symptomatic grapevines, and is influenced by the Mediterranean climate. The mean annual temperature is 15.8 °C, the annual rainfall is 676 mm, and the soil is mostly of schist and calcareous origin.
In the early summer of 2019, 10 cm long spurs of symptomatic and asymptomatic plants for GTDs were collected from cv. ‘Alicante Bouschet’ and cv. ‘Trincadeira’. Four plants were randomly selected within each cultivar, two of them with GTDs symptoms and two asymptomatic. Therefore, total samples correspond to four plants from each symptomatology and four plants from each cultivar. The GTDs symptoms were as observed in Figure 1. Asymptomatic plants did not present visible GTDs symptoms on the wood and leaves. After sample collection, spurs were stored at 4 °C and further processed within the next 48 h. To suppress epiphytic microorganisms on the field-collected samples, spurs were surface disinfected, according to Varanda et al. [30], the rhytidome was removed, and cortical scrapings from cuttings were grounded into powder, separately for each sample, using sterile mortars and pestles, aiding the process with liquid nitrogen, and were stored at −80 °C until further analysis.
2.2. DNA Extraction and Sequencing
DNA extraction was performed using the CTAB (hexadecyltrimethylammonium bromide) method [52], with some modifications [30,53,54,55,56]. For each sample, total DNA was extracted from ca. 500 mg of the previously obtained plant material powder and DNA concentration was determined by using a Quawell Q9000 micro spectrophotometer (Quawell Technology, Beijing, China). DNA integrity was evaluated by 1.5% agarose gel electrophoresis and then samples were sent for next-generation sequencing (NGS) in Stab Vida, Lda. (Caparica, Portugal). The amplicon chosen to analyse the fungal community through metagenomic approach targets was the internal transcribed spacer 1 (ITS1) region. Library construction was performed using the Illumina metagenomic sequencing library preparation protocol and the generated DNA fragments were sequenced on the lllumina® MiSeq platform using 300 bp paired-end sequencing reads.
2.3. Bioinformatics Procedure
After sequencing, the generated raw data (3,386,406 reads) were downloaded and processed using QIIME2 v.2019.10 [57,58] and were denoised using the DADA2 plug-in [59]. Due to the target amplicon size, the plug-in was applied using single-read mode. Sequences were trimmed and quality filtered, full-length duplicate sequences were removed, and reads were sorted by abundance. In the case of ITS sequences, these were clustered followed by chimera filtering using the UNITE database as a reference. The reads were organised in features, which are essentially units of observation, named Operational Taxonomic Units (OTUs), and then classified by taxon using a fitted classifier. The scikit-learn classifier was used to train the classifier using the UNITE (release 8.0) database, with a clustering threshold of 97% similarity. For classification purposes, only OTUs containing at least 10 sequence reads were considered. The processed OTU table was composed of 1,168,974 reads from 258 OTUs (available in Table S1).
2.4. Statistical Data Analysis
Fungal diversity profiling data containing the total number of OTUs, total number of OTUs excluding GTDs-associated fungi, and total number of GTDs-associated fungi were generated to test separately for each factor: “symptomatology” (symptomatic and asymptomatic plants) and “cultivar” (‘Alicante Bouschet’ and ‘Trincadeira’).
Univariate and multivariate analyses were performed using the PRIMER v6 software [60] with the permutational analysis of variance (PERMANOVA) add-on package [61], to detect significant differences (p < 0.05) in the relative levels of the fungal microbiota, regarding the number of OTUs, for both factors. A one-way PERMANOVA was applied to test the hypothesis that significant differences existed within the “symptomatology” and “cultivar” groups. PERMANOVA analyses were carried out with the following one-factor design: “symptomatology”, symptomatic and asymptomatic (two levels, fixed), with four replicates from each level of symptomatology, and “cultivar”, ‘Alicante Bouschet’ and ‘Trincadeira’ (two levels, fixed), with four replicates from each cultivar. PERMANOVA analysis was conducted on a Bray–Curtis similarity matrix [62]. If the number of permutations was lower than 150, the Monte Carlo permutation p was used. Whenever significant interaction effects were detected, these were examined using a posteriori pairwise comparisons, using 9999 permutations under a reduced model. The similarities in fungal microbiota, regarding the number of OTUs, in “symptomatology” and “cultivar” were plotted by PCO using the Bray-Curtis similarity measure.
Alpha-diversity was determined using five diversity indexes (Margalef, Shannon, Pielou’s, Fisher, and Simpson), and the differences among factors were calculated using a PERMANOVA analysis based on the OTUs dataset.
Taxonomic profile graphics were analysed within the Marker Data Profiling module of MicrobiomeAnalyst [63], implementing the R version 3.6.1 (accessed on 29 April 2021). OTUs that occurred less than two times were removed from the full dataset. Features that appeared in only one sample were excluded. Unless mentioned, default settings were used for all procedures.
3. Results
3.1. Grapevine Fungal Microbiota Overview
Deep sequencing of fungal-directed ITS1 amplicon led to the detection of 1,168,974 high-quality reads distributed in 258 unique features (OTUs), 215 of which were assigned to genus level and 157 to species level, covering 82.07% and 42.04% of total reads, respectively. The overall composition is summarised in Table 1. The average number of reads per sample was 146,122, which allowed an adequate sequencing depth to unravel the complexity of the grapevine fungal microbiota. The Alpha rarefaction curve obtained with species richness is shown in Figure S1. Among the 258 annotated OTUs, 54 of them were represented in relative abundance (RA) greater than 0.10%, while the remaining 204 were considered rare taxa (RA < 0.10%). Considering only the OTUs assigned to genus or species level, 41 taxa with RA upper than 0.10% were identified (Table 2). From the 41 most abundant taxa, 33 were not detected in all samples, and among these, four only appeared in one sample (singletons). This fact highlights the high variability often encountered when comparing individual plants. The grapevine fungal microbiota were characterised by the presence of both ascomycetes and basidiomycetes (174 and 82 taxa, with 75.34% and 24.06% RA, respectively).

Table 1.
Overview of grapevine fungal microbiota composition.

Table 2.
Taxonomic classification of the most abundant taxa (with relative abundance ≥0.10%), identified to genus or species level, their ecology and/or pathogenic potential, and relative abundance in GTDs symptomatic and asymptomatic plants and in the studied grapevine cultivars (‘Alicante Bouschet’ and ‘Trincadeira’).
The most abundant families within the Ascomycota phylum were Botryosphaeriaceae (20.89%), Aureobasidiaceae (8.14%), and Cladosporiaceae (8.09%); within the Basidiomycota phylum they were Filobasidiaceae (10.46%), Mrakiaceae (6.49%), and Sporidiobolaceae (2.97%). Diplodia sp. and Neofusicoccum cordaticola, two members of the Botryosphaeriaceae, previously described as responsible for GTDs, were the most abundant taxa (with 10.60% and 10.29% RA, respectively). Other trunk disease pathogens, Hormonema sp., Phaeomoniella chlamydospora, Cadophora luteo-olivacea, Diaporthe sp., Truncatella sp., Cryptovalsa ampelina, Diatrype stigma, and one pathogenic basidiomycete, Stereum hirsutum, were also detected in lower abundance (≤0.51%), totalling ten GTDs-associated fungi, which together comprised nearly 21.49% of the total fungal density. OTUs that were not included in the previous group were classified as “endophytes”, microorganisms that live entirely within plant tissues without causing any apparent symptoms of disease to the host [30]. Since this study intended to assess the impact of GTDs-associated fungi, all the remaining fungi that were identified (other pathogens and other fungi without any previously described activity on grapevine plants) were considered as endophytes. Cladosporium sp., Alternaria sp., Clonostachys rosea, and Trichoderma atroviride were some of those endophytes that were found in the grapevine tissues.
3.2. The Influence of Symptomatology on Grapevine Mycobiome
3.2.1. Diversity and Richness of the Fungal Microbiota
The comparison of the fungal community in symptomatic and asymptomatic plants showed that a total of 83 taxa (95.90% RA) were detected in both groups, demonstrating the existence of a core grapevine mycobiome. Only 44 taxa were unique to asymptomatic plants (0.61% RA) and the remaining 131 were unique to symptomatic plants (3.49% RA). The average of the total OTUs number ± standard error (SE) was 92.75 ± 15.97 for symptomatic plants and 52.25 ± 5.25 for asymptomatic ones (Figure 2). PERMANOVA analysis revealed significant differences (p = 0.03), highlighting a higher diversity of fungal species in symptomatic grapevines. The compositional similarities of fungal microbiota between samples were estimated using Principal Coordinates Analysis (PCO), combined with a Bray-Curtis dissimilarity. The PCO ordination showed that the first two components (PCO1, 97.80% and PCO2, 2.20%) accounted for the total variability of the data (Figure 2). Regarding the fungal richness, for the symptomatic plants, the mean number of reads (±SE) was 168,631 ± 8125, and for asymptomatic it was 123,613 ± 21,515. In this case, the PERMANOVA analysis revealed no significant differences (p = 0.19).
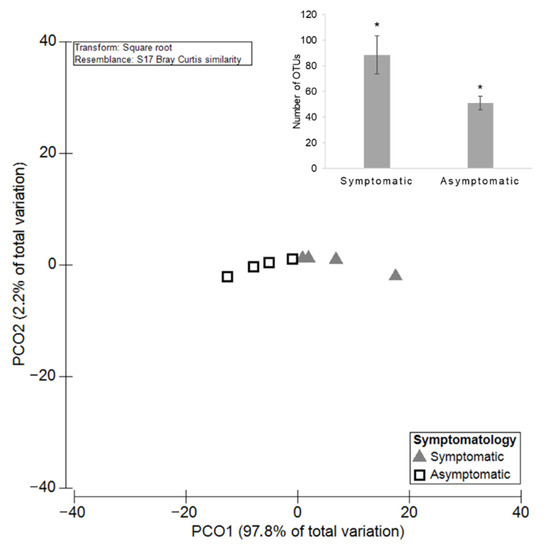
Figure 2.
Principal Coordinates Analysis (PCO) based on the diversity of the fungal communities among GTDs symptomatic and asymptomatic grapevines. The average of the total OTUs number for both groups is shown in bar plot and significant differences (p < 0.05) are indicated by asterisks.
Alpha-diversity analysis did not identify significant differences at the OTU level in terms of richness and evenness when comparing the fungal communities between both symptomatic and asymptomatic plants. Diversity indexes revealed a high similarity between samples. In fact, the PERMANOVA analysis showed no significant differences among both groups for each of the five diversity indexes (Margalef, p = 0.75; Shannon, p = 0.09; Pielou’s, p = 0.29; Fisher, p = 0.76; and Simpson, p = 0.14).
Cladosporium sp., Alternaria sp., and Mycosphaerella tassiana were the most common fungi found in symptomatic plants, with 5.98%, 5.88%, and 5.43% RA respectively. In asymptomatic plants, Diplodia sp. and N. cordaticola were the most abundant, with 10.22% and 10.16% RA, contrasting with 0.38% and 0.13% RA in symptomatic plants, respectively (Table 2). The relative abundance of the top 10 fungal species detected across all samples is represented in Figure 3. However, only the 215 OTUs assigned to the genus or species level were considered.
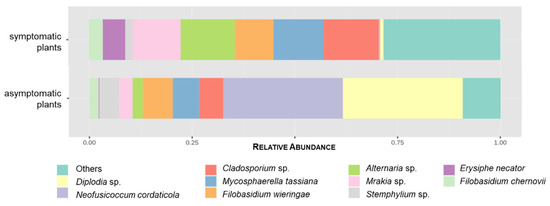
Figure 3.
Relative abundance of the top 10 fungal species according to the presence or absence of GTDs symptoms, considering the 215 OTUs assigned to genus or species level. Remaining OTUs are grouped in “Others”.
3.2.2. GTDs-Associated Fungi
Symptomatic plants presented 3079 ± 1749 mean reads of GTDs-associated fungi, while asymptomatic ones revealed a significantly higher average with 80,662 ± 22,079 reads (p = 0.01; Figure 4). Nevertheless, symptomatic plants exhibited greater diversity of GTDs phytopathogenic fungi when compared with asymptomatic plants. PERMANOVA showed significantly higher values of OTUs for the symptomatic grapevines (p = 0.03; Figure 5). In plants with symptoms, the mean number of OTUs (±SE) was 5.33 ± 1.20, and in plants without symptoms it was 1.67 ± 0.33. A total of nine fungi were detected in symptomatic grapevines, while in asymptomatic plants only four were identified (Figure 6). Diplodia sp., N. cordaticola and Diaporthe sp. were found in both symptomatic and asymptomatic plants. Hormonema sp., P. chlamydospora, C. luteo-olivacea, Truncatella sp., C. ampelina, and D. stigma were detected solely in symptomatic plants, while S. hirsutum was only found in asymptomatic plants, although with residual abundance.
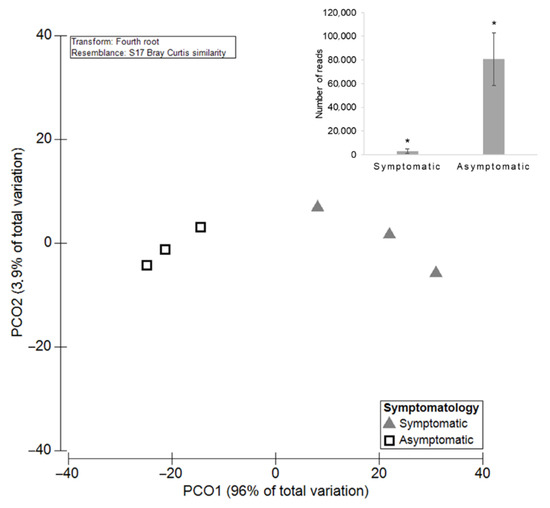
Figure 4.
Principal Coordinates Analysis (PCO) based on the diversity of the GTDs-associated fungi among symptomatic and asymptomatic grapevines. The average of the total number of reads for both groups is shown in bar plot and significant differences (p < 0.05) are indicated by asterisks.
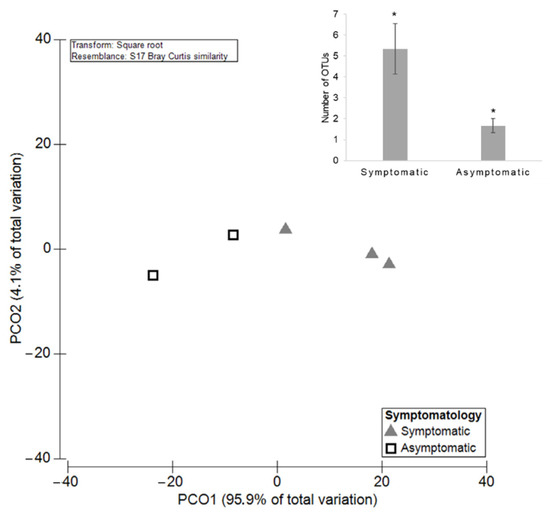
Figure 5.
Principal Coordinates Analysis (PCO) based on the diversity of the GTDs-associated fungi among symptomatic and asymptomatic grapevines. The average of the total OTUs number for both groups is shown in bar plot and significant differences (p < 0.05) are indicated by asterisks.
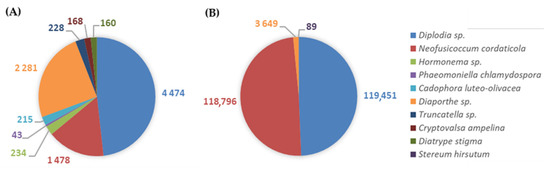
Figure 6.
Distribution of GTDs-associated fungi illustrating the respective number of reads identified in: (A) symptomatic and (B) asymptomatic grapevines to trunk diseases.
3.2.3. Endophytic Community
A total of 80 OTUs of the remaining fungal community were identified in both symptomatic and asymptomatic plants (74.50% RA). Cladosporium sp., M. tassiana, Alternaria sp., Mrakia sp., and Fusarium sp. are some examples. Only 43 taxa were unique to asymptomatic grapevines, such as Cystobasidium sp. and Cordyceps bassiana (0.60% RA), and the remaining 124 were solely detected in symptomatic grapevines (24.90% RA), as verified for Typhula sp. and Catenulostroma hermanusense. The mean number of OTUs (±SE) was 88.50 ± 14.85 for symptomatic plants and 51.00 ± 5.31 for asymptomatic ones (Figure 7). PERMANOVA analysis revealed significant differences (p = 0.03), highlighting a higher diversity of endophytes in symptomatic grapevines, as has already been seen for the overall fungal community. Regarding the richness of the endophytic community, a significantly higher (p = 0.0009) number of reads in symptomatic plants was observed, with the mean number of reads being (±SE) 166,310 ± 9506 compared with the asymptomatic ones with the mean number of 63,117 ± 8561 (Figure 8).
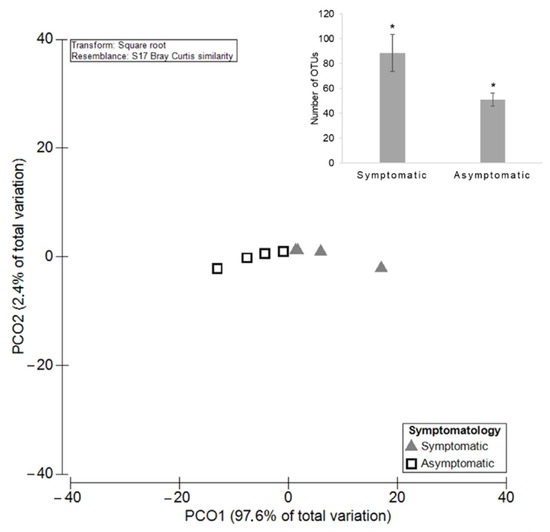
Figure 7.
Principal Coordinates Analysis (PCO) based on the diversity of the endophytic fungal communities among GTDs symptomatic and asymptomatic grapevines. The average of the total OTUs number for both groups is shown in bar plot and significant differences (p < 0.05) are indicated by asterisks.
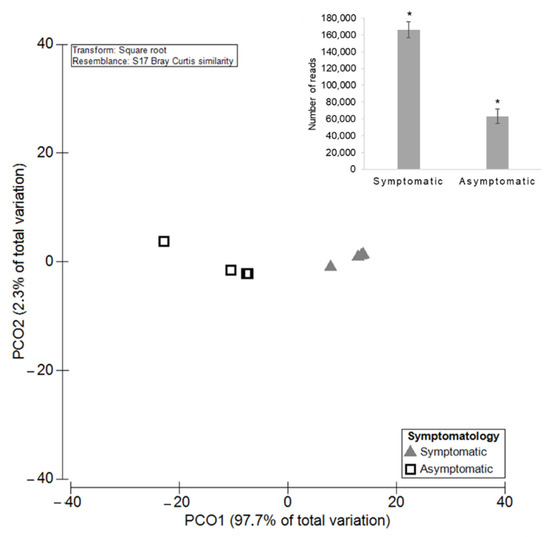
Figure 8.
Principal Coordinates Analysis (PCO) based on the diversity of the endophytic fungal communities among GTDs symptomatic and asymptomatic grapevines. The average of the total number of reads for both groups is shown in bar plot and significant differences (p < 0.05) are indicated by asterisks.
3.3. The Influence of Cultivars on Grapevine Mycobiome
3.3.1. Diversity and Richness of the Fungal Microbiota
The mycobiome of cv. ‘Trincadeira’ and cv. ‘Alicante Bouschet’ presented a total of 90 common taxa (86.58% RA), while 58 were exclusive of cv. ‘Trincadeira’ (0.96% RA) and 110 of cv. ‘Alicante Bouschet’ (12.46% RA), demonstrating the existence of a core grapevine mycobiome. For cv. ‘Trincadeira’, the least susceptible to GTDs, the mean number of OTUs (±SE) was 65.75 ± 4, and for cv. ‘Alicante Bouschet’, the most susceptible to GTDs, the mean number of OTUs (±SE) was 79.25 ± 23. PERMANOVA analysis revealed no statistical differences in terms of fungal diversity (p = 0.76). Regarding fungal richness, for cv. ‘Trincadeira’, the mean number of reads (±SE) was 133,341 ± 26,612, and for cv. ‘Alicante Bouschet’ it was 158,903 ± 7041. No significant differences were detected in terms of richness between both cultivars (p = 0.55). Alpha-diversity analysis did not identify significant differences at the OTU level in terms of richness and evenness when comparing the fungal communities between both cultivars. Diversity indexes revealed a high similarity between samples. PERMANOVA analysis showed no significant differences among both groups for each of the five diversity indexes (Margalef, p = 0.21; Shannon, p = 0.40; Pielou’s, p = 0.30; Fisher, p = 0.20; and Simpson, p = 0.30).
Mrakia sp., Filobasidium wieringae, M. tassiana, Diplodia sp., and Cladosporium sp. were the most common fungi, with RA over 3%, in cv. ‘Trincadeira’. In cv. ‘Alicante Bouschet’, N. cordaticola, Diplodia sp., Cladosporium sp., Alternaria sp., and M. tassiana were dominant (RA > 3%) (Table 2). The relative abundance of the top 10 fungal species detected across all samples, regarding cultivar, is shown in Figure 9.
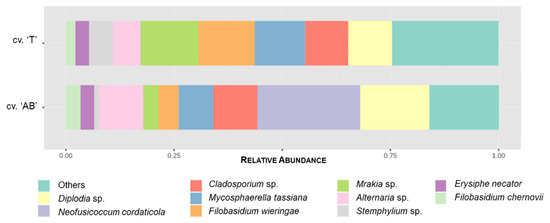
Figure 9.
Relative abundance of the top 10 fungal species according to the cultivar (’T’—Trincadeira; ‘AB’—Alicante Bouschet), considering the 215 OTUs assigned to the genus or species level. The remaining species are grouped in “Others”.
3.3.2. GTDs-Associated Fungi
A total of nine GTDs-associated fungi were identified in cv. ‘Alicante Bouschet’ and five in cv. ‘Trincadeira’. Diplodia sp., Hormonema sp., Diaporthe sp., and C. ampelina were found in both cultivars. N. cordaticola, P. chlamydospora, C. luteo-olivacea, Truncatella sp., and D. stigma were detected exclusively in cv. ‘Alicante Bouschet’, while S. hirsutum was only found in the ‘Trincadeira’ cultivar (Figure 10). The mean number of OTUs (±SE) was 1.25 ± 0.75 for cv. ‘Trincadeira’, and for cv. ‘Alicante Bouschet’ it was 4.00 ± 1.47. Although the mean number of OTUs in both cultivars was found to be numerically different, it was not enough to be statistically significant (p = 0.35). Considering the GTDs-associated fungal richness, cv. ‘Alicante Bouschet’ showed a higher mean number of reads compared with cv. ‘Trincadeira’. The most susceptible cultivar, ‘Alicante Bouschet’, presented 52,036 ± 28,677 mean reads, while the least susceptible, ‘Trincadeira’, presented a lower mean number of reads, 10,769 ± 10,516. Nevertheless, PERMANOVA analysis showed no statistically significant differences (p = 0.35).
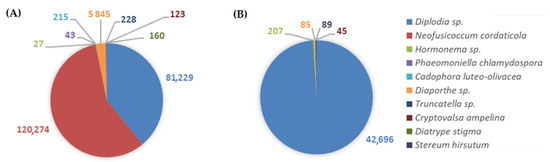
Figure 10.
Distribution of GTDs-associated fungi illustrating the respective number of reads identified in: (A) cv. ‘Alicante Bouschet’, the most susceptible to GTDs, and (B) cv. ‘Trincadeira’, the least susceptible to GTDs.
3.3.3. Endophytic Community
Endophytic fungal communities found in both cultivars were characterised by similar diversity and richness. A high percentage of taxa was detected simultaneously in both cultivars (75.44% RA), corresponding to 86 taxa, among which were Cladosporium sp., Alternaria sp., and Fusarium sp.; only 57 taxa were unique to cv. ‘Trincadeira’, such as Beauveria sp., and C. bassiana (0.95% RA). The remaining 104 were detected solely in cv. ‘Alicante Bouschet’ (23.61% RA), such as Typhula sp., C. hermanusense, and Cystobasidium sp.
The mean OTUs number (±SE) was 64.25 ± 3.45 for cv. ‘Trincadeira’ and 75.25 ± 21.23 for cv. ‘Alicante Bouschet’ with no statistically significant differences detected (p = 0.80). Regarding the richness of the endophytic community, a similar number of reads in both cultivars, 122,560 ± 30,563 for the least susceptible cultivar and 106,867 ± 31,038 for the most susceptible, was verified. PERMANOVA analysis showed no significant differences among taxa for their differential abundance when comparing both cultivars (p = 0.71).
4. Discussion
This study described the composition (richness and diversity) of the endophytic fungal communities associated with GTDs symptomatic and asymptomatic grapevines of two cultivars from the Alentejo region, ‘Trincadeira’ and ‘Alicante Bouschet’. The chosen cultivars are important highly used in wine production in this region, conferring valuable traits to wine and are present in the list of cultivars with the European nomenclature of Protected Designation of Origin (PDO). Moreover, these cultivars clearly demonstrate different levels of susceptibility to trunk diseases among them [32]. The present meta-analysis of the grapevine fungal microbiome considered two parameters (presence/absence of GTDs symptoms and cultivar) and elucidated some issues preventing a full understanding of the etiology of this disease complex, providing some guidelines about the underlying factors associated with the expression of trunk diseases symptoms.
The continuous development of high-throughput sequencing technologies, associated with an increasing accessibility, allowed, either through the lower cost of these methods or the simplification of the processes involved, the sequencing of distinct metagenomes and, among them, the grapevine fungal microbiome. Although the ITS region has some limitations in identifying highly specific genera, such as Aspergillus, Cladosporium, Fusarium, Penicillium, and Trichoderma, which have narrow or no barcode gaps in their ITS regions, it is still the primary fungal barcode marker for molecular identification [70,71]. In this study, a total of 258 taxa were detected, corroborating the diversity also verified in similar studies in grapevines using a NGS approach [18,37,41,64]. Furthermore, 60.85% of the total OTUs identified were assigned to species level. Overall fungal microbiota comprised the presence of both ascomycetes and basidiomycetes, with about 75% and 24% RA, respectively. The predominance of ascomycetes fungi over the basidiomycetes identified in this study is consistent with other endophytic studies concerning grapevine and other crops [18,28,30,33,37,43,55,72,73]. Within the Ascomycota phylum, the Botryosphaeriaceae was the most representative family (20.89% RA), especially due to Diplodia sp. and N. cordaticola, the most frequent GTDs-associated fungi among fungal microbiota. Moreover, Aureobasidiaceae and Cladosporiaceae, together with the Botryosphaeriaceae, comprised the main families of the grapevine endophytic mycobiota [72]. As expected, numerous GTDs-associated fungi were identified, as frequent (Diplodia sp., N. cordaticola, and Diaporthe sp.) or as rare taxa (Hormonema sp., P. chlamydospora, C. luteo-olivacea, Truncatella sp., C. ampelina, D. stigma, and S. hirsutum), totalling 10 trunk-disease pathogens, which together constituted nearly 21.49% of the total fungal density. These pathogens are involved in diseases commonly seen in mature and older grapevines, such as Botryosphaeria dieback, Esca, Eutypa dieback, and Phomopsis dieback, as is the case of the vineyard under study [8,9,32]. Some fungal genus/species often involved in GTDs complex, such as Eutypa lata, Neofusicoccum parvum, Phaeoacremonium spp., Fomitiporia spp., and Ilyonectria or closely related species [8,18], were not detected. However, it should be taken into consideration that the plant tissue analysed may influence the presence/absence of some fungal species [18].
The abundance of fungal endophytes was mostly comprised of frequent taxa (96.64% RA) rather than rare species, meaning that many species were repeatedly isolated and are, apparently, characteristic in grapevine, regardless of the presence/absence of symptoms or the cultivar. In general, the fungi isolated in this study have been previously reported in other studies as grapevine endophytes [18,30,73]. This result may be related to the fact that some endophytes become specialised on plant tissues and occupy a specific ecological role in the plant but, nevertheless, the colonisation of some rare species (RA < 0.10%) may occur [30].
Leaf symptoms are frequently associated with an advanced stage of infection, although this is not always clear due to the lack of homogenous symptoms across all leaves, their discontinuity, possible unidentified causal agents, and their roles and associations [9]. Although wood necrosis is present as a latent infection, GTDs leaf symptoms may be absent for many years, and then can fluctuate from year to year [74]. Since GTDs symptoms can be inconstant, it is interesting to understand whether there is a link between the fungal communities of the grapevine wood and the expression of leaf symptoms of GTDs. A total of 83 taxa within the fungal community (95.90% RA) were detected in both symptomatic and asymptomatic plants. These results suggest, as already been mentioned, that grapevine mycobiome is partially conserved. Although a higher diversity of fungal species was observed in symptomatic grapevines, no statistical differences were revealed regarding fungal richness between both symptomatic and asymptomatic plants. Other microbial ecology studies in symptomatic and asymptomatic grapevines reported opposite results, highlighting that the diversity of fungal communities was not affected by the manifestation of leaf symptoms [18,26,43,75]. Elena et al. (2018) characterised the grapevine fungal composition and diversity, in Spain, and did not find differences between fungal communities in symptomatic and asymptomatic plants [75]; Bruez et al. (2014), in France, also did not find differences between the endophytic microflora of both symptomatic and asymptomatic grapevines [26]. This fact may be related to the grapevine part sampled, health status of the wood, plant age, soil, cultivars, climate, and other environmental factors, which would have influenced the development of the fungal communities.
Cladosporium sp., Alternaria sp., and M. tassiana were the most common fungi in symptomatic plants. These species are usually the main fungal components of endophytic communities in grapevine, as well as in other plants [28,30,45,55,76,77], although no visible symptoms of plant disease are associated with these species in the studied grapevines. This fact proposes that the induction of disease by these species may depend not just on their presence, but also on the influence of the community or other plant factors [45], suggesting that these species are latent, behaving as plant endophytes. Whilst little is known about their associations with known pathogens, some fungal endophytes have revealed potential as biological control agents against GTDs-associated fungi and other grapevine pathogens [26,76,78]. Unexpectedly, asymptomatic plants revealed an abundance of GTDs-associated fungi significantly higher when compared with the symptomatic ones, with Diplodia sp. and N. cordaticola being the most abundant ones. Due to the reported isolation of these fungi from asymptomatic tissues [17], it is thought that some of GTDs phytopathogenic fungi may act also as latent pathogens [8,14], being able to survive, for part of their lives, as endophytes without causing any apparent symptoms to the host, but may become pathogenic under specific physiological or environmental conditions [79,80]. However, many of the hypotheses addressing the transition from endophytic to pathogenic phases remain untested. One of these may be the occurrence of biotic and/or abiotic stresses, which increase host susceptibility, facilitating the transition; other includes endophyte changes, including single point mutations, the transfer of virulence genes, and/or virus infections causing change to a pathogenic state [8]. Moreover, early infections or co-existence with other endophytic microorganisms, particularly those with biocontrol activity, which, either by the competition for nutrient and space or by the production of secondary metabolites, inhibit fungal growth, can also explain the long latency time of trunk diseases [75,77,81]. The presence of these fungi in asymptomatic grapevines can be quite problematic for growers, as infections can unnoticed spread in the field or in nurseries [82,83,84]. On the other hand, symptomatic grapevines exhibited a significantly greater diversity of GTDs phytopathogenic fungi (p = 0.03). A total of nine fungi were detected in symptomatic grapevines (Diplodia sp., N. cordaticola, Diaporthe sp., Hormonema sp., P. chlamydospora, C. luteo-olivacea, Truncatella sp., C. ampelina, and D. stigma), while in asymptomatic plants only four were identified (Diplodia sp., N. cordaticola, Diaporthe sp., and S. hirsutum). These results corroborate previous reports referring that GTDs symptoms are intensified by a set of multiple GTDs-associated fungi within a grapevine [8,11]. The main argument supporting this hypothesis is the action of phytotoxic compounds (toxins or secondary metabolites) produced by several fungi which would be released in the sap flow, disseminated, and reach the foliage [9,13,85]. The inciting factors could be a change in the micro-environment conditions, a fungal progression to infect previously healthy wood and functional parts, or an ineffective cellular response of the leaf tissues to the entering toxins [9,86,87,88]. In this context, with different fungi present in the same plant, there exists more competition between them and more phytotoxic compounds are released, which leads to more severe leaf symptoms. Furthermore, when grapevines become stressed, fungal growth is accelerated, and colonisation thresholds are reached sooner than under normal conditions [3,10,14,16]; therefore, the presence of multiple GTDs-associated fungi leads to an earlier reaching of these thresholds [8]. Nevertheless, several questions remain; it is not known if one or potentially all of these compounds cause symptoms and the threshold concentrations inducing toxicity have not yet been determined [9]. Changes in the plant morphology and physiology, as well as in biochemical functions, were also assessed as possible factors in the development of foliar symptoms. Ascorbate-Glutathione cycle was considered likely to be involved in grapevine susceptibility to fungi associated with the Esca complex [89]. Other hypotheses can contribute for the appearance of leaf symptoms, such as the disturbance of sap flow to the leaves on given xylem pathways altered by fungi, which is described as ‘hydraulic dysfunction’ [85,90,91], and annual infections by some fungi through pruning or green wounds [23,92]; however, more research is required to develop this hypothesis and to unravel the processes underlying colonization by these fungi [93].
It is important to consider that several factors can shape the grapevine microbiome and increase/decrease the susceptibility to GTDs, such as seasonality, climatic conditions, surrounding flora, cultural practices, plant genotype, plant age, and presence of pathogens, rootstock, and cultivar [48,50,85,92,93,94,95,96]. In addition, biotic and abiotic stresses might also be playing an important role in the expression of GTDs symptoms [97]. The relationships between the microbiota present on a plant can be more or less favourable to fungal pathogenicity [98]. Several studies suggest that interactions within the community of GTDs-associated fungi, as well as the grapevine physiology and the environment, can strongly affect the behaviour of each fungus [99,100]. Understanding the triple impact of host-pathogens-environment is critical to explain the evolution of trunk diseases [101]. Despite the similarities in the endophytic fungal community found in both symptomatic and asymptomatic grapevines, significant differences were observed between both, highlighting a higher diversity of endophytes in symptomatic grapevines, as has already been seen for the overall fungal community, as well as a significant higher abundance. Identification of the endophytic fungal community is essential not only to describe the fungi present in grapevines, but also to understand their relationships with GTDs-associated fungi and their potential as effective biocontrol agents so that control strategies may be developed. The role of endophytic communities in pathogen defence is attained through different mechanisms, namely the competition with pathogens for the same ecological niches in terms of nutrients and space; the production of secondary metabolites that inhibit fungal growth; the induction of systemic acquired resistance, through the accumulation of pathogenesis-related proteins; and the expression of plant defence genes [30,72]. The lower diversity and richness of endophytic fungi found on asymptomatic plants may lead to an increase of the development and expression of GTDs-associated fungi. However, symptoms are not visible, suggesting potential interactions between endophytes and GTDs-phytopathogenic fungi within a grapevine [26,29,76,78,102]. Cystobasidium sp., C. bassiana and other fungi found on asymptomatic grapevines may interact with the GTDs-associated fungi to mask or inhibit pathogen activity, which means that the endophytic community may constitute a source of biocontrol agents useful to regulate important grapevine diseases. The role of endophytes in GTDs symptoms expression still needs further research. Summing up, symptomatic and asymptomatic plants revealed differences in terms of endophyte fungal richness and diversity, GTDs fungal richness and diversity, and in terms of overall fungal diversity.
Overall results from comparison between cv. ‘Trincadeira’ (least susceptible to GTDs) and cv. ‘Alicante Bouschet’ (most susceptible) showed that the fungal communities of both cultivars did not differ significantly. Similar results were reported by Pancher et al. [76], using culture-dependent approaches with the ‘Chardonnay’ and ‘Merlot’ cultivars, while some other studies revealed differences in fungal communities when different grapevine cultivars were compared [30,43,48,72,103,104]. All these studies focused on a single cultivar in each vineyard and did not consider differences between cultivars on a same field. Differences on fungal composition of the different cultivars may be related to the different plant breeding and selection processes which cultivars have been exposed to, to differences in phenological stages, to different sugar content, pH, and nutrient composition, to the presence and abundance of secondary metabolites produced by the different cultivars, or to the biogeography [30,43]. Additionally, it should be noticed that the cultivars used in the present study belong to the same experimental field, which eventually may define the similarity on the grapevine endophytic community. In fact, it would be interesting to understand the reason that leads to visual differences among them regarding the symptomatology of trunk diseases. One of the hypotheses would be a greater diversity of GTDs-associated fungi in the most susceptible cultivar, which was not verified. Although the mean number of OTUs in both cultivars was found to be numerically different (nine in cv. ‘Alicante Bouschet’ and five in cv. ‘Trincadeira’), it was not statistically significant, as well as the GTDs fungal composition between cultivars. Additionally, the outcome of the statistical analyses of the mycobiome of cv. ‘Trincadeira’ and cv. ‘Alicante Bouschet’ revealed that the endophytic fungal communities were not affected by the cultivar. Without differences found in grapevine fungal composition among these cultivars, studies concerning grapevines’ differential gene expression are crucial to better understand the underlying mechanisms associated with the expression of trunk diseases symptoms. Key functional genes differentially expressed in response to different biotic stresses, notably during pathogen attack, have been identified in several plant species [50,105,106]. Understanding the vast fungal diversity in grapevines and the plant-pathogen interactions will indicate future research directions to advance understanding and management of GTDs and facilitate the development of sustainable and effective control strategies for grapevine protection.
5. Conclusions
The present study allowed an adequate sequencing depth to unravel the complexity of the grapevine fungal communities of the selected cultivars in a 17-year-old vineyard in the Alentejo region (south of Portugal) and updated the information on the richness and diversity of GTDs-associated fungi and their relationship with the symptomatology in plants. The results here presented, when we compared GTDs symptomatic vs. asymptomatic plants, revealed that the existence of the symptoms depends on the presence of multiple GTDs-associated fungi within a grapevine, reinforcing the importance of exploring fungal biodiversity in grapevine cultivars. The identified grapevine fungal communities comprised beneficial and phytopathogenic fungi that might have a significant impact on grapevine production. Endophytes found on asymptomatic grapevines may be playing an important role and should be further explored as antagonists of GTDs pathogens and possibly developed as effective biocontrol agents. This study contributes to a better understanding of plant-pathogen interactions and to the achievement of a better knowledge of GTDs and their expression, contributing to the mitigation and control of these diseases with such a high economic impact.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8040288/s1, Figure S1: Alpha rarefaction curve obtained with species richness. ABS1: cv. ‘Alicante Bouschet’ asymptomatic plant; ABS2: cv. ‘Alicante Bouschet’ asymptomatic plant; ABD1: cv. ‘Alicante Bouschet’ symptomatic plant; ABD2: cv. ‘Alicante Bouschet’ symptomatic plant; TS1: cv. ‘Trincadeira’ asymptomatic plant; TS2: cv. ‘Trincadeira’ asymptomatic plant; TD1: cv. ‘Trincadeira’ symptomatic plant; TD2: cv. ‘Trincadeira’ symptomatic plant. Table S1: Processed OTU table with the respective number of reads for each sample. ABS1: cv. ‘Alicante Bouschet’ asymptomatic plant; ABS2: cv. ‘Alicante Bouschet’ asymptomatic plant; ABD1: cv. ‘Alicante Bouschet’ symptomatic plant; ABD2: cv. ‘Alicante Bouschet’ symptomatic plant; TS1: cv. ‘Trincadeira’ asymptomatic plant; TS2: cv. ‘Trincadeira’ asymptomatic plant; TD1: cv. ‘Trincadeira’ symptomatic plant; TD2: cv. ‘Trincadeira’ symptomatic plant.
Author Contributions
Conceptualization, M.P., M.D.C. and M.d.R.F.; methodology, M.D.C., P.M., C.M.R.V. and M.d.R.F.; formal analysis, M.P., A.A. and P.M.; investigation, M.P., A.A., M.D.C., P.M., C.M.R.V. and M.d.R.F.; resources, M.P., A.A., M.D.C., P.M., C.M.R.V., J.A.R. and M.d.R.F.; writing—original draft preparation, M.P.; writing—review and editing, M.P., A.A., M.D.C., P.M., C.M.R.V., J.A.R. and M.d.R.F.; supervision, M.d.R.F.; funding acquisition, M.D.C., P.M., C.M.R.V. and M.d.R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Portuguese National Funds through FCT under the project UIDB/05183/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by Portuguese National Funds through FCT-MCTES under the PhD scholarship SFRH/BD/145321/2019, attributed to M.P., co-financed by the European Social Fund through the Regional Operational Program of the Alentejo. It was also supported by the project “Control of olive anthracnose through gene silencing and gene expression using a plant virus vector” (ALT20-03-0145-FEDER-028263 and PTDC/ASP-PLA/28263/2017) and by the project “Development of a new virus-based vector to control TSWV in tomato plants” (ALT20-03-0145-FEDER-028266 and PTDC/ASP-PLA/28266/2017), both projects co-financed by the European Union through the European Regional Development Fund, under the Alentejo 2020, Algarve 2020 and through the FCT, in its national component.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, M.; Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Marie-France, C.-C. Grapevine Trunk Diseases. A Review; International Organisation of Vine and Wine: Paris, France, 2016; p. 24.
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2017, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef] [Green Version]
- Morales-Cruz, A.; Allenbeck, G.; Figueroa-Balderas, R.; Ashworth, V.E.; Lawrence, D.P.; Travadon, R.; Smith, R.J.; Baumgartner, K.; Rolshausen, P.E.; Cantu, D. Closed-reference metatranscriptomics enables in planta profiling of putative virulence activities in the grapevine trunk disease complex. Mol. Plant Pathol. 2018, 19, 490–503. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar] [CrossRef]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wery, J. Current knowledge on Grapevine Trunk Diseases with complex etiology: A systemic approach. Phytopathol. Mediterr. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Reis, P.; Pierron, R.; Larignon, P.; Lecomte, P.; Abou-Mansour, E.; Farine, S.; Bertsch, C.; Jacques, A.; Trotel-Aziz, P.; Rego, C.; et al. Vitis methods to understand and develop strategies for diagnosis and sustainable control of grapevine trunk diseases. Phytopathology 2019, 109, 916–931. [Google Scholar] [CrossRef]
- Surico, G. Towards a redefinition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar] [CrossRef]
- Lecomte, P.; Darrieutort, G.; Liminana, J.M.; Comont, G.; Muruamendiaraz, A.; Legorburu, F.J.; Choueiri, E.; Jreijiri, F.; El Amil, R.; Fermaud, M. New insights into Esca of grapevine: The development of foliar symptoms and their association with xylem discoloration. Plant Dis. 2012, 96, 924–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black Measles) and Brown Wood-Streaking: Two Old and Elusive Diseases of Grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Úrbez-Torres, J. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef]
- Bruez, E.; Baumgartner, K.; Bastien, S.; Travadon, R.; Guérin-Dubrana, L.; Rey, P. Various fungal communities colonise the functional wood tissues of old grapevines externally free from grapevine trunk disease symptoms. Aust. J. Grape Wine Res. 2016, 22, 288–295. [Google Scholar] [CrossRef]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Characterization of the Wood Mycobiome of Vitis vinifera in a Vineyard Affected by Esca. Spatial Distribution of Fungal Communities and Their Putative Relation With Leaf Symptoms. Front. Plant Sci. 2019, 10, 910. [Google Scholar] [CrossRef] [Green Version]
- Decoin, M. Grapevine products: News on withdrawals and restrictions. Phytoma 2001, 543, 28–33. [Google Scholar]
- Spinosi, J.; Févotte, J.; Vial, G. Éléments techniques sur l’exposition professionnelle aux pesticides arsenicaux. In Matrice Cultures-Expositions aux Pesticides Arsenicaux; Institut de Veille Sanitaire: Saint-Maurice, France, 2009; p. 19. [Google Scholar]
- Elena, G.; Luque, J. Seasonal susceptibility of grapevine pruning wounds and cane colonization in Catalonia, Spain following artificial infection with Diplodia seriata and Phaeomoniella chlamydospora. Plant Dis. 2016, 100, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.D.; Patanita, M.; Varanda, C.; Materatski, P.; Félix, M.R. Plant-Pathogen Interaction. Biology 2021, 10, 444. [Google Scholar] [CrossRef]
- Mondello, V.; Larignon, P.; Armengol, J.; Kortekamp, A.; Vaczy, K.; Prezman, F.; Serrano, E.; Rego, C.; Mugnai, L.; Fontaine, F. Management of grapevine trunk diseases: Knowledge transfer, current strategies and innovative strategies adopted in Europe. Phytopathol. Mediterr. 2018, 57, 369–383. [Google Scholar] [CrossRef]
- Mondello, V.; Lemaître-Guillier, C.; Trotel-Aziz, P.; Gougeon, R.; Acedo, A.; Schmitt-Kopplin, P.; Adrian, M.; Pinto, C.; Fernandez, O.; Fontaine, F. Assessment of a New Copper-Based Formulation to Control Esca Disease in Field and Study of Its Impact on the Vine Microbiome, Vine Physiology and Enological Parameters of the Juice. J. Fungi 2022, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- OIV. State of the World Vitivinicultural Sector in 2019; International Organisation of Vine and Wine: Paris, France, 2020; p. 14.
- Bruez, E.; Vallance, J.; Gerbore, J.; Lecomte, P.; Da Costa, J.-P.; Guerin-Dubrana, L.; Rey, P. Analyses of the Temporal Dynamics of Fungal Communities Colonizing the Healthy Wood Tissues of Esca Leaf-Symptomatic and Asymptomatic Vines. PLoS ONE 2014, 9, e95928. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [Green Version]
- Bettenfeld, P.; Cadena i Canals, J.; Jacquens, L.; Fernandez, O.; Fontaine, F.; van Schaik, E.; Courty, P.-E.; Trouvelot, S. The microbiota of the grapevine holobiont: A key component of plant health. J. Adv. Res. 2021; in press. [Google Scholar] [CrossRef]
- Pertot, I.; Prodorutti, D.; Colombini, A.; Pasini, L. Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. BioControl 2016, 61, 257–267. [Google Scholar] [CrossRef]
- Varanda, C.M.R.; Oliveira, M.; Materatski, P.; Landum, M.; Clara, M.I.E.; Félix, M.R. Fungal endophytic communities associated to the phyllosphere of grapevine cultivars under different types of management. Fungal Biol. 2016, 120, 1525–1536. [Google Scholar] [CrossRef]
- Del Frari, G.; Cabral, A.; Nascimento, T.; Boavida Ferreira, R.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 2019, 14, e0213273. [Google Scholar] [CrossRef] [Green Version]
- Billar de Almeida, A.; Concas, J.; Campos, M.D.; Materatski, P.; Varanda, C.M.R.; Patanita, M.; Murolo, S.; Romanazzi, G.; Félix, M.R. Endophytic fungi as potential biological control agents against grapevine trunk diseases in Alentejo region. Biology 2020, 9, 420. [Google Scholar] [CrossRef]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Fungicides and the Grapevine Wood Mycobiome: A Case Study on Tracheomycotic Ascomycete Phaeomoniella chlamydospora Reveals Potential for Two Novel Control Strategies. Front. Plant Sci. 2019, 10, 1405. [Google Scholar] [CrossRef]
- Morgan, H.H.; du Toit, M.; Setati, M.E. The Grapevine and Wine Microbiome: Insights from High-Throughput Amplicon Sequencing. Front. Microbiol. 2017, 8, 820. [Google Scholar] [CrossRef] [Green Version]
- Eichmeier, A.; Pečenka, J.; Peňázová, E.; Baránek, M.; Català-García, S.; León, M.; Armengol, J.; Gramaje, D. High-throughput amplicon sequencing-based analysis of active fungal communities inhabiting grapevine after hot-water treatments reveals unexpectedly high fungal diversity. Fungal Ecol. 2018, 36, 26–38. [Google Scholar] [CrossRef]
- Stefanini, I.; Cavalieri, D. Metagenomic Approaches to Investigate the Contribution of the Vineyard Environment to the Quality of Wine Fermentation: Potentials and Difficulties. Front. Microbiol. 2018, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Berlanas, C.; Berbegal, M.; Elena, G.; Laidani, M.; Cibriain, J.F.; Sagües, A.; Gramaje, D. The Fungal and Bacterial Rhizosphere Microbiome Associated with Grapevine Rootstock Genotypes in Mature and Young Vineyards. Front. Microbiol. 2019, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Diz, M.d.P.; Andrés-Sodupe, M.; Bujanda, R.; Díaz-Losada, E.; Eichmeier, A.; Gramaje, D. Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol. 2019, 41, 234–244. [Google Scholar] [CrossRef]
- Nerva, L.; Zanzotto, A.; Gardiman, M.; Gaiotti, F.; Chitarra, W. Soil microbiome analysis in an ESCA diseased vineyard. Soil Biol. Biochem. 2019, 135, 60–70. [Google Scholar] [CrossRef]
- Deyett, E.; Rolshausen, P.E. Endophytic microbial assemblage in grapevine. FEMS Microbiol. Ecol. 2020, 96, fiaa053. [Google Scholar] [CrossRef]
- Carbone, M.J.; Alaniz, S.; Mondino, P.; Gelabert, M.; Eichmeier, A.; Tekielska, D.; Bujanda, R.; Gramaje, D. Drought Influences Fungal Community Dynamics in the Grapevine Rhizosphere and Root Microbiome. J. Fungi 2021, 7, 686. [Google Scholar] [CrossRef]
- Dries, L.; Bussotti, S.; Pozzi, C.; Kunz, R.; Schnell, S.; Löhnertz, O.; Vortkamp, A. Rootstocks Shape Their Microbiome—Bacterial Communities in the Rhizosphere of Different Grapevine Rootstocks. Microorganisms 2021, 9, 822. [Google Scholar] [CrossRef]
- Bekris, F.; Vasileiadis, S.; Papadopoulou, E.; Samaras, A.; Testempasis, S.; Gkizi, D.; Tavlaki, G.; Tzima, A.; Paplomatas, E.; Markakis, E.; et al. Grapevine wood microbiome analysis identifies key fungal pathogens and potential interactions with the bacterial community implicated in grapevine trunk disease appearance. Environ. Microbiome 2021, 16, 23. [Google Scholar] [CrossRef]
- Gramaje, D.; Eichmeier, A.; Spetik, M.; Carbone, M.J.; Bujanda, R.; Vallance, J.; Rey, P. Exploring the temporal dynamics of the fungal microbiome in rootstocks, the lesser-known half of the grapevine crop. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Liu, D.; Howell, K. Community succession of the grapevine fungal microbiome in the annual growth cycle. Environ. Microbiol. 2021, 23, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Pacetti, A.; Moretti, S.; Pinto, C.; Compant, S.; Farine, S.; Bertsch, C.; Mugnai, L. Trunk Surgery as a Tool to Reduce Foliar Symptoms in Diseases of the Esca Complex and Its Influence on Vine Wood Microbiota. J. Fungi 2021, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Paolinelli, M.; Escoriaza, G.; Cesari, C.; Garcia-Lampasona, S.; Hernandez-Martinez, R. Characterization of Grapevine Wood Microbiome Through a Metatranscriptomic Approach. Microb. Ecol. 2021. [Google Scholar] [CrossRef]
- Lade, S.B.; Štraus, D.; Oliva, J. Variation in Fungal Community in Grapevine (Vitis vinifera) Nursery Stock Depends on Nursery, Variety and Rootstock. J. Fungi 2022, 8, 47. [Google Scholar] [CrossRef]
- Murolo, S.; Romanazzi, G. Effects of grapevine cultivar, rootstock and clone on esca disease. Australas. Plant Pathol. 2014, 43, 215–221. [Google Scholar] [CrossRef]
- Cardot, C.; Mappa, G.; La Camera, S.; Gaillard, C.; Vriet, C.; Lecomte, P.; Ferrari, G.; Coutos-Thévenot, P. Comparison of the Molecular Responses of Tolerant, Susceptible and Highly Susceptible Grapevine Cultivars During Interaction with the Pathogenic Fungus Eutypa lata. Front. Plant Sci. 2019, 10, 991. [Google Scholar] [CrossRef] [Green Version]
- CVRA. Relatório Annual—Gestão e Contas; Comissão Vitivinícola Regional Alentejana: Alentejo, Portugal, 2020; p. 85. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; Félix, M.R. Diversity of Colletotrichum Species Associated with Olive Anthracnose and New Perspectives on Controlling the Disease in Portugal. Agronomy 2018, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.D.; Patanita, M.; Campos, C.; Materatski, P.; Varanda, C.M.R.; Brito, I.; Félix, M.R. Detection and Quantification of Fusarium spp. (F. oxysporum, F. verticillioides, F. graminearum) and Magnaporthiopsis maydis in Maize Using Real-Time PCR Targeting the ITS Region. Agronomy 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Varanda, C.M.R.; Materatski, P.; Landum, M.; Campos, M.D.; Félix, M.R. Fungal Communities Associated with Peacock and Cercospora Leaf Spots in Olive. Plants 2019, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Materatski, P.; Varanda, C.M.R.; Carvalho, T.; Dias, A.; Campos, M.D.; Gomes, L.; Nobre, T.; Rei, F.; Félix, M.R. Effect of Long-Term Fungicide Applications on Virulence and Diversity of Colletotrichum spp. Associated to Olive Anthracnose. Plants 2019, 8, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E Ltd., Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Green, R.H. Statistical Design and Analysis for a ‘Biological Effects’ Study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Purahong, W.; Zhang, W.; Wubet, T.; Li, X.; Liu, M.; Zhao, W.; Hyde, K.D.; Liu, J.; Yan, J. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 2018, 90, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Perelló, A.; Aulicino, M.; Stenglein, S.A.; Labuda, R.; Moreno, M.V. Pseudopithomyces chartarum associated with wheat seeds in Argentina, pathogenicity and evaluation of toxigenic ability. Eur. J. Plant Pathol. 2017, 148, 491–496. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; Liu, J.K.; Lu, Y.Z.; et al. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 2019, 10, 1–186. [Google Scholar] [CrossRef]
- Al-Lami, H.F.D.; You, M.P.; Barbetti, M.J. Incidence, pathogenicity and diversity of Alternaria spp. associated with alternaria leaf spot of canola (Brassica napus) in Australia. Plant Pathol. 2019, 68, 492–503. [Google Scholar] [CrossRef]
- Schisler, D.A.; Janisiewicz, W.J.; Boekhout, T.; Kurtzman, C.P. Chapter 4—Agriculturally Important Yeasts: Biological Control of Field and Postharvest Diseases Using Yeast Antagonists, and Yeasts as Pathogens of Plants. In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 2011; pp. 45–52. [Google Scholar]
- Wang, X.; Schlatter, D.C.; Glawe, D.A.; Edwards, C.G.; Weller, D.M.; Paulitz, T.C.; Abatzoglou, J.T.; Okubara, P.A. Native yeast and non-yeast fungal communities of Cabernet Sauvignon berries from two Washington State vineyards, and persistence in spontaneous fermentation. Int. J. Food Microbiol. 2021, 350, 109225. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- González, V.; Tello, M.L. The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers. 2011, 47, 29–42. [Google Scholar] [CrossRef]
- Niem, J.M.; Billones-Baaijens, R.; Stodart, B.; Savocchia, S. Diversity Profiling of Grapevine Microbial Endosphere and Antagonistic Potential of Endophytic Pseudomonas Against Grapevine Trunk Diseases. Front. Microbiol. 2020, 11, 477. [Google Scholar] [CrossRef]
- Calzarano, F.; Osti, F.; Baránek, M.; Di Marco, S. Rainfall and temperature influence expression of foliar symptoms of grapevine leaf stripe disease (esca complex) in vineyards. Phytopathol. Mediterr. 2018, 57, 488–505. [Google Scholar] [CrossRef]
- Elena, G.; Bruez, E.; Rey, P.; Luque, J. Microbiota of grapevine woody tissues with or without esca-foliar symptoms in northeast Spain. Phytopathol. Mediterr. 2018, 57, 425–438. [Google Scholar] [CrossRef]
- Pancher, M.; Ceol, M.; Corneo, P.E.; Longa, C.M.O.; Yousaf, S.; Pertot, I.; Campisano, A. Fungal endophytic communities in grapevines (Vitis vinifera L.) respond to crop management. Appl. Environ. Microbiol. 2012, 78, 4308–4317. [Google Scholar] [CrossRef] [Green Version]
- Landum, M.C.; Félix, M.R.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F.; Varanda, C.M.R. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; Sanità di Toppi, L.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Karácsony, Z.; Golen, R.; Váczy, K.Z.; Geml, J. The compositional turnover of grapevine-associated plant pathogenic fungal communities are greater among intraindividual microhabitats and terroirs than among healthy and Esca-diseased plants. Phytopathology 2021. [Google Scholar] [CrossRef] [PubMed]
- Stranska, M.; Dzuman, Z.; Prusova, N.; Behner, A.; Kolouchova, I.; Lovecka, P.; Rezanka, T.; Kolarik, M.; Hajslova, J. Fungal Endophytes of Vitis vinifera—Plant Growth Promoters or Potentially Toxinogenic Agents? Toxins 2022, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Fourie, P.H.; Halleen, F. Proactive Control of Petri Disease of Grapevine Through Treatment of Propagation Material. Plant Dis. 2004, 88, 1241–1245. [Google Scholar] [CrossRef] [Green Version]
- Romanazzi, G.; Murolo, S.; Pizzichini, L.; Nardi, S. Esca in young and mature vineyards, and molecular diagnosis of the associated fungi. Eur. J. Plant Pathol. 2009, 125, 277–290. [Google Scholar] [CrossRef]
- Gramaje, D.; Di Marco, S. Identifying practices likely to have impacts on grapevine trunk disease infections: A European nursery survey. Phytopathol. Mediterr. 2015, 54, 313–324. [Google Scholar] [CrossRef]
- Surico, G.; Mugnai, L.; Marchi, G. Older and more recent observations on esca: A critical overview. Phytopathol. Mediterr. 2006, 45, 68–86. [Google Scholar] [CrossRef]
- Martos, S.; Andolfi, A.; Luque, J.; Mugnai, L.; Surico, G.; Evidente, A. Production of phytotoxic metabolites by five species of Botryosphaeriaceae causing decline on grapevines, with special interest in the species Neofusicoccum luteum and N. parvum. Eur. J. Plant Pathol. 2008, 121, 451–461. [Google Scholar] [CrossRef]
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, G.L.; Ippolito, M.P.; Mannerucci, F.; Bragazzi, L.; Tommasi, F. Physiological responses of ‘Italia’ grapevines infected with Esca pathogens. Phytopathol. Mediterr. 2021, 60, 321–336. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.; Báidez, A.G.; Ortuño, A.; Del Río, J.A. Grapevine xylem response to fungi involved in trunk diseases. Ann. Appl. Biol. 2016, 169, 116–124. [Google Scholar] [CrossRef]
- Kraus, C.; Rauch, C.; Kalvelage, E.M.; Behrens, F.H.; d’Aguiar, D.; Dubois, C.; Fischer, M. Minimal versus Intensive: How the Pruning Intensity Affects Occurrence of Grapevine Leaf Stripe Disease, Wood Integrity, and the Mycobiome in Grapevine Trunks. J. Fungi 2022, 8, 247. [Google Scholar] [CrossRef]
- Moretti, S.; Pacetti, A.; Pierron, R.; Kassemeyer, H.-H.; Fischer, M.; Péros, J.-P.; Perez-Gonzalez, G.; Bieler, E.; Schilling, M.; Marco, S.D.; et al. Fomitiporia mediterranea M. Fisch., the historical Esca agent: A comprehensive review on the main grapevine wood rot agent in Europe. Phytopathol. Mediterr. 2021, 60, 351–379. [Google Scholar] [CrossRef]
- Graniti, A.; Surico, G.; Mugnai, L. Esca of grapevine: A disease complex or a complex of diseases? Phytopathol. Mediterr. 2000, 39, 16–20. [Google Scholar] [CrossRef]
- Pacifico, D.; Squartini, A.; Crucitti, D.; Barizza, E.; Lo Schiavo, F.; Muresu, R.; Carimi, F.; Zottini, M. The Role of the Endophytic Microbiome in the Grapevine Response to Environmental Triggers. Front. Plant Sci. 2019, 10, 1256. [Google Scholar] [CrossRef] [Green Version]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R.; et al. A meta-analysis of global fungal distribution reveals climate-driven patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [Green Version]
- Del Frari, G.; Oliveira, H.; Boavida Ferreira, R. White Rot Fungi (Hymenochaetales) and Esca of Grapevine: Insights from Recent Microbiome Studies. J. Fungi 2021, 7, 770. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: III. Enzymes produced by the pathogens and their role in fungus-to-plant or in fungus-to-fungus interactions. Physiol. Mol. Plant Pathol. 2006, 69, 182–194. [Google Scholar] [CrossRef]
- Freitas, R.; Rego, C.; Oliveira, H.; Ferreira, R.B. Interactions among grapevine disease-causing fungi. The role of reactive oxygen species. Phytopathol. Mediterr. 2009, 48, 117–127. [Google Scholar] [CrossRef]
- Fischer, M.; Peighami-Ashnaei, S. Grapevine, esca complex, and environment: The disease triangle. Phytopathol. Mediterr. 2019, 58, 17–37. [Google Scholar] [CrossRef]
- Fourie, P.H.; Halleen, F.; van der Vyver, J.; Schrueder, W. Effect of Trichoderma treatments on the occurrence of decline pathogens on the roots and rootstocks of nursery plants. Phytopathol. Mediterr. 2001, 40, 473–478. [Google Scholar] [CrossRef]
- Casieri, L.; Hofstetter, V.; Viret, O.; Gindro, K. Fungal communities living in the wood of different cultivars of young Vitis vinifera plants. Phytopathol. Mediterr. 2009, 48, 73–83. [Google Scholar] [CrossRef]
- Travadon, R.; Lecomte, P.; Diarra, B.; Lawrence, D.; Renault, D.; Ojeda, H.; Rey, P.; Baumgartner, K. Grapevine pruning systems and cultivars influence the diversity of wood-colonizing fungi. Fungal Ecol. 2016, 24, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.D.; Félix, M.R.; Patanita, M.; Materatski, P.; Varanda, C. High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding. Hortic. Res. 2021, 8, 171. [Google Scholar] [CrossRef]
- Campos, M.D.; Félix, M.R.; Patanita, M.; Materatski, P.; Albuquerque, A.; Ribeiro, J.A.; Varanda, C. Defense Strategies: The Role of Transcription Factors in Tomato-Pathogen Interaction. Biology 2022, 11, 235. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).