Decontamination of Tomato Brown Rugose Fruit Virus-Contaminated Shoe Soles under Practical Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of ToBRFV and Plant Material
2.2. Inoculum Preparation and Mechanical Inoculation
2.3. Germ Carriers
2.4. Selection of Disinfectants and Preparation of Disinfectant Solutions
2.5. Experimental Design
2.5.1. Experiment 1: Suspension Decontamination (Laboratory Conditions)
2.5.2. Experiment 2: Simulation—Boot Decontamination (Laboratory Conditions)
2.5.3. Experiment 3: Boot Decontamination (Practical Conditions)
- (a)
- Shoe sole disinfection
- (b)
- Disinfection inside the mat
2.6. Detection of ToBRFV
2.7. Data Analysis
3. Results
3.1. Suspension Decontamination under Laboratory Conditions (Experiment 1)
3.2. Simulated Inactivation of ToBRFV on Shoe Soles under Laboratory Conditions (Experiment 2)
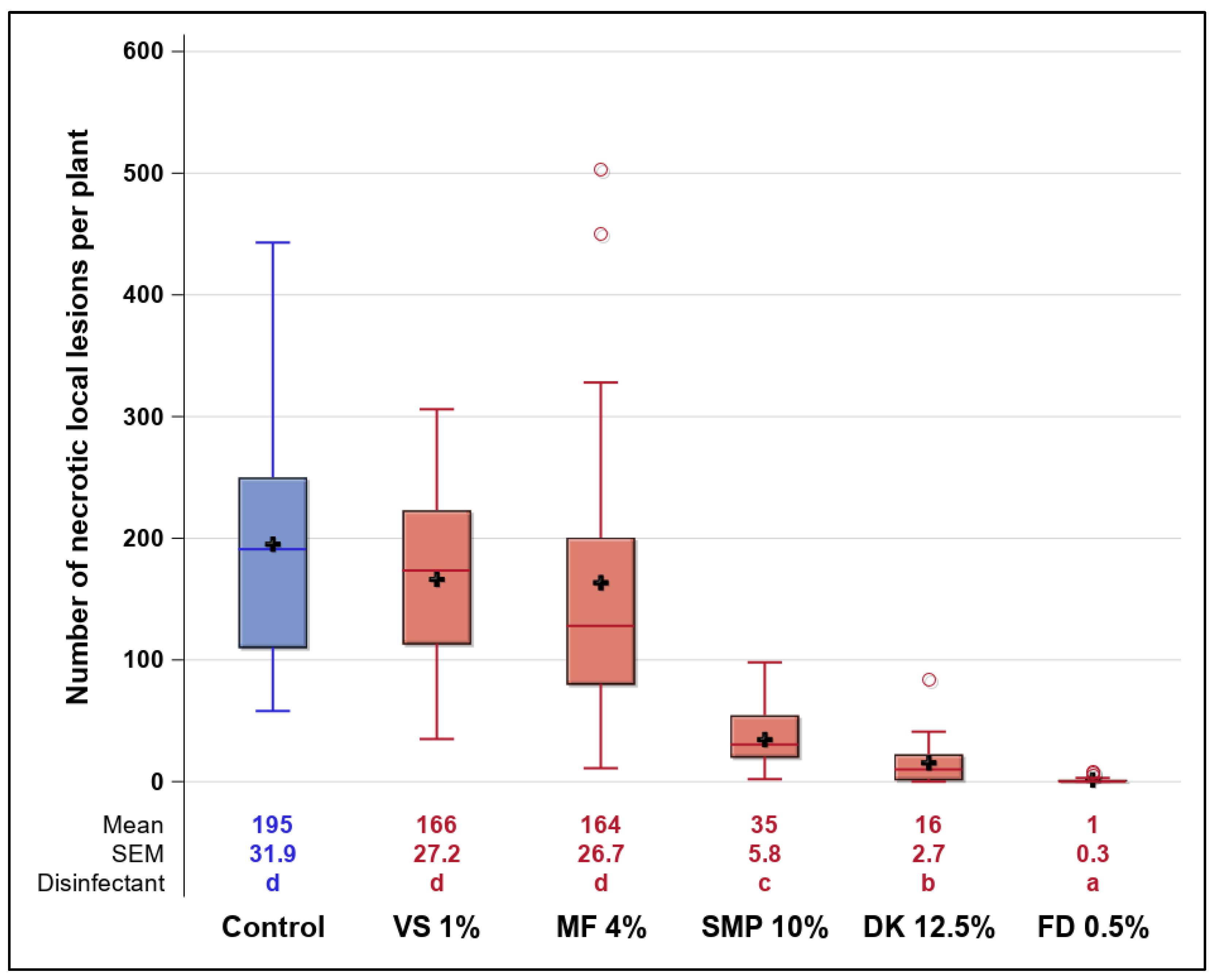
3.3. Shoe-Sole Decontamination under Practical Conditions (Experiment 3.1)
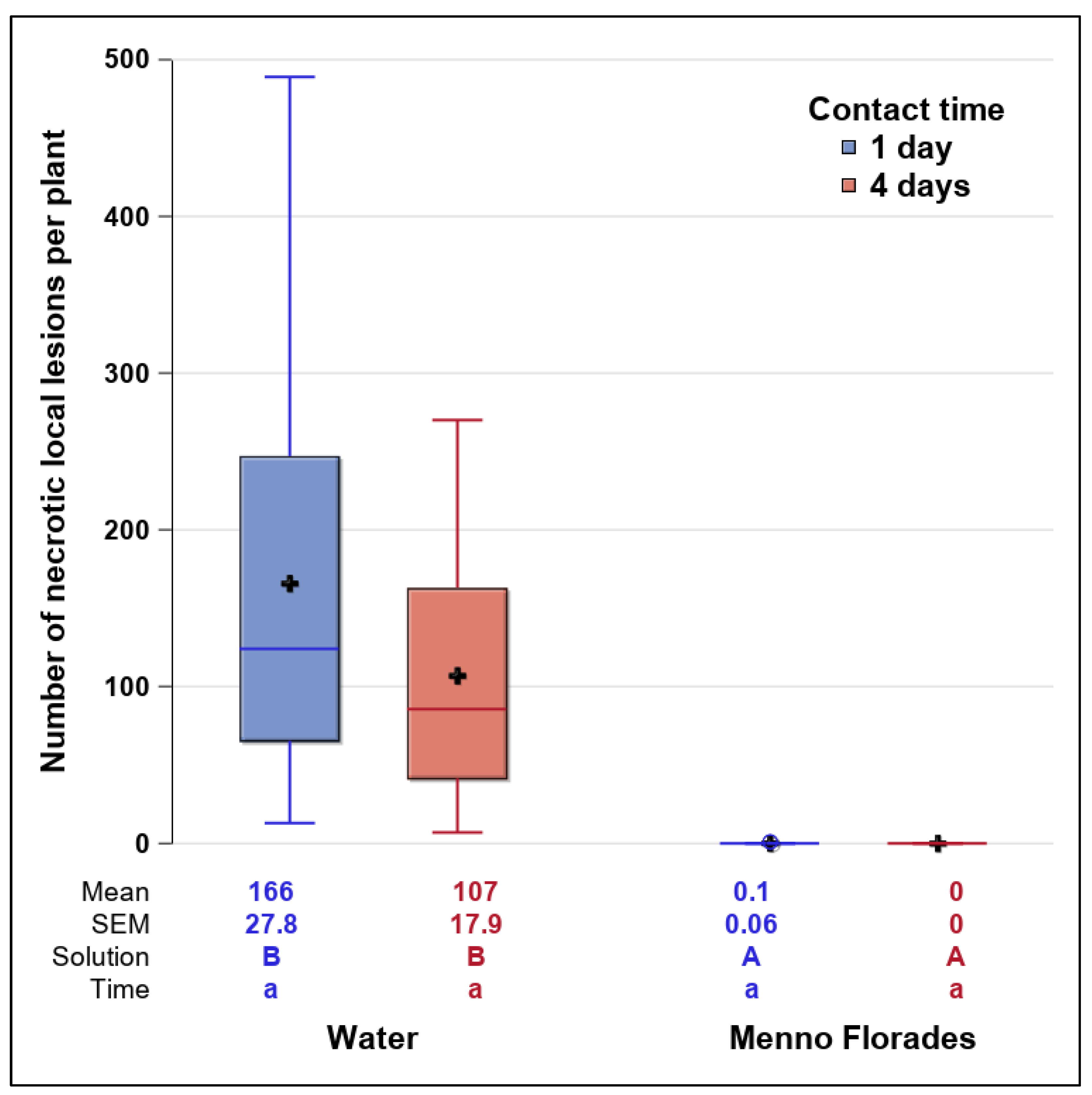
3.4. Inactivation of ToBRFV Inside the Disinfection Mat under Practical Conditions (Experiment 3.2)
- Disinfection inside the mat
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avni, B.; Gelbart, D.; Sufrin-Ringwald, T.; Zinger, A.; Chen, L.; Machbash, Z.; Bekelman, I.; Segoli, M.; Dombrovsky, A.; Kamenetsky, R. Tomato genetic resistance to tobamoviruses is compromised. In Proceedings of the VI International Symposium on Tomato Diseases: Managing Tomato Diseases in the Face of Globalization and Climate Change, Taichung, Taiwan, 6–9 May 2019; pp. 89–98. [Google Scholar]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A. A new Israeli Tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019, 39, 1. [Google Scholar] [CrossRef]
- Ling, K.-S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of tomato brown rugose fruit virus infecting greenhouse tomato in the United States. Plant Dis. 2019, 103, 1439. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Ma, H.-Y.; Han, S.-L.; Geng, C.; Tian, Y.-P.; Li, X.-D. First report of tomato brown rugose fruit virus infecting tomato in China. Plant Dis. 2019, 103, 2973. [Google Scholar] [CrossRef]
- Camacho-Beltrán, E.; Pérez-Villarreal, A.; Leyva-López, N.; Rodríguez-Negrete, E.; Ceniceros-Ojeda, E.; Méndez-Lozano, J. Occurrence of Tomato brown rugose fruit virus Infecting Tomato Crops in Mexico. Plant Dis. 2019, 103, 1440. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Castillo, P.; Sanahuja, E.; Rodríguez-Salido, M.; Font, M.I. First report of Tomato brown rugose fruit virus in tomato in Spain. Plant Dis. 2021, 105, 515. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Caruso, A.; Davino, S. First report of tomato brown rugose fruit virus on tomato crops in Italy. Plant Dis. 2019, 103, 1443. [Google Scholar] [CrossRef]
- Fidan, H.; Sarikaya, P.; Calis, O. First report of Tomato brown rugose fruit virus on tomato in Turkey. New Dis. Rep 2019, 39, 2044-0588.2019. [Google Scholar] [CrossRef]
- González-Concha, L.F.; Ramírez-Gil, J.G.; García-Estrada, R.S.; Rebollar-Alviter, Á.; Tovar-Pedraza, J.M. Spatiotemporal analyses of tomato brown rugose fruit virus in commercial tomato greenhouses. Agronomy 2021, 11, 1268. [Google Scholar] [CrossRef]
- Ehlers, J.; Nourinejhad Zarghani, S.; Kroschewski, B.; Büttner, C.; Bandte, M. Cleaning of Tomato brown rugose fruit virus (ToBRFV) from Contaminated Clothing of Greenhouse Employees. Horticulturae 2022, 8, 751. [Google Scholar] [CrossRef]
- Dunowska, M.; Morley, P.S.; Patterson, G.; Hyatt, D.R.; Van Metre, D.C. Evaluation of the efficacy of a peroxygen disinfectant-filled footmat for reduction of bacterial load on footwear in a large animal hospital setting. J. Am. Vet. Med. Assoc. 2006, 228, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, S.A.; Spijkerman, I.J.; Birnie, M.F.; Goslings, J.C.; Schepers, T. Preoperative disinfection of foot and ankle: Microbiological evaluation of two disinfection methods. Arch. Orthop. Trauma Surg. 2018, 138, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, S.B.; Alsos, I.G.; Ware, C. Prevention of microbial species introductions to the Arctic: The efficacy of footwear disinfection measures on cruise ships. NeoBiota 2018, 37, 37–49. [Google Scholar] [CrossRef]
- Bartlett, J.C.; Radcliffe, R.J.; Convey, P.; Hughes, K.A.; Hayward, S.A. The effectiveness of Virkon® S disinfectant against an invasive insect and implications for Antarctic biosecurity practices. Antarct. Sci. 2021, 33, 1–9. [Google Scholar] [CrossRef]
- Amass, S.F.; Ragland, D.; Spicer, P. Evaluation of the efficacy of a peroxygen compound, Virkon (R) S, as a boot bath disinfectant. J. Swine Health Prod. 2001, 9, 121–123. [Google Scholar]
- Amass, S.F.; Arighi, M.; Kinyon, J.M.; Hoffman, L.J.; Schneider, J.L.; Draper, D.K. Effectiveness of using a mat filled with a peroxygen disinfectant to minimize shoe sole contamination in a veterinary hospital. J. Am. Vet. Med. Assoc. 2006, 228, 1391–1396. [Google Scholar] [CrossRef]
- Nasr, S.A.; Ismael, E.; Laban, S.E.; Ismail, E.M.; Hamoud, M.M.; Zaki, M.M.; Zahran, O.M. Effectiveness of Some Disinfectants Commonly Used in footbaths inside Poultry Farms. IOSR J. Agric. Vet. Sci. (IOSR-JAVS) Vol. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Rashid, T.; VonVille, H.; Hasan, I.; Garey, K. Shoe soles as a potential vector for pathogen transmission: A systematic review. J. Appl. Microbiol. 2016, 121, 1223–1231. [Google Scholar] [CrossRef]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Galanti, R.; Lutgen, H. Greenhouse and Nursery Sanitation Tools, Equipment, Workers, and Visitors. 2021. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiawu-3mf77AhU-X_EDHWfuB1cQFnoECA8QAQ&url=https%3A%2F%2Fwww.ctahr.hawaii.edu%2Foc%2Ffreepubs%2Fpdf%2FOF-54.pdf&usg=AOvVaw1UatwnMcJUvW4UxOcWCJnG (accessed on 13 October 2022).
- O’Neill, T.; Lole, M.; Drakes, D.; Irving, R. Use of Chemical Disinfectants in Protected Ornamental Plant Production. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjl6rrHi_v6AhVcQvEDHYDSBqUQFnoECA4QAQ&url=https%3A%2F%2Fprojectblue.blob.core.windows.net%2Fmedia%2FDefault%2FHorticulture%2FDiseases%2F03_14%2520Use%2520of%2520chemical%2520disinfectants%2520in%2520protected%2520ornamental%2520plant%2520production.pdf&usg=AOvVaw0Skha4Q3hPH_H9c32X-j3N (accessed on 25 October 2022).
- Broadbent, L.; Fletcher, J. The epidemiology of tomato mosaic: IV. persistence of virus on clothing and glasshouse structures. Ann. Appl. Biol. 1963, 52, 233–241. [Google Scholar] [CrossRef]
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato brown rugose fruit virus: Seed transmission rate and efficacy of different seed disinfection treatments. Plants 2020, 9, 1615. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.M.; Sulaiman, A.; Samarah, N.; Turina, M.; Vallino, M. Localization and mechanical transmission of tomato brown rugose fruit virus in tomato seeds. Plant Dis. 2022, 106, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Liu, S.S.; Gu, Q.S. Transmission efficiency of Cucumber green mottle mosaic virus via seeds, soil, pruning and irrigation water. J. Phytopathol. 2016, 164, 300–309. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Mor, N.; Gantz, S.; Lachman, O.; Smith, E. Disinfection Efficacy of Tobamovirus-Contaminated Soil in Greenhouse-Grown Crops. Horticulturae 2022, 8, 563. [Google Scholar] [CrossRef]
- Lovelock, D.; Mintoff, S.; Kurz, N.; Neilsen, M.; Patel, S.; Constable, F.; Tran-Nguyen, L. Investigating the Longevity and Infectivity of Cucumber green mottle mosaic virus in Soils of the Northern Territory, Australia. Plants 2022, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Ellouze, W.; Mishra, V.; Howard, R.J.; Ling, K.-S.; Zhang, W. Preliminary study on the control of cucumber green mottle mosaic virus in commercial greenhouses using agricultural disinfectants and resistant cucumber varieties. Agronomy 2020, 10, 1879. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Barone, S.; Lo Bosco, G.; Rangel, E.A.; Davino, S. Spread of tomato brown rugose fruit virus in sicily and evaluation of the spatiotemporal dispersion in experimental conditions. Agronomy 2020, 10, 834. [Google Scholar] [CrossRef]
- Eppo. Tomato Brown Rugose Fruit Virus (ToBRFV) Categorization. Available online: https://gd.eppo.int/taxon/TOBRFV/categorization (accessed on 3 August 2022).
- Anonymous. Commission Implementing Regulation (EU) 2021/1809 of 13 October 2021 amending Implementing Regulation (EU) 2020/1191 on measures to prevent the introduction into and the spread within the Union of Tomato brown rugose fruit virus (ToBRFV). Off. J. Eur. Union 2021, 41–45. [Google Scholar]
- Li, R.; Baysal-Gurel, F.; Abdo, Z.; Miller, S.A.; Ling, K.-S. Evaluation of disinfectants to prevent mechanical transmission of viruses and a viroid in greenhouse tomato production. Virol. J. 2015, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Chanda, B.; Shamimuzzaman, M.; Gilliard, A.; Ling, K.-S. Effectiveness of disinfectants against the spread of tobamoviruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Darzi, E.; Lachman, O.; Smith, E.; Koren, A.; Klein, E.; Pass, N.; Frenkel, O.; Dombrovsky, A. Paths of cucumber green mottle mosaic virus disease spread and disinfectant-based management. Ann. Appl. Biol. 2020, 177, 374–384. [Google Scholar] [CrossRef]
- Wales, A.D.; Gosling, R.J.; Bare, H.L.; Davies, R.H. Disinfectant testing for veterinary and agricultural applications: A review. Zoonoses Public Health 2021, 68, 361–375. [Google Scholar] [CrossRef]
- Kindermann, J.; Karbiener, M.; Leydold, S.M.; Knotzer, S.; Modrof, J.; Kreil, T.R. Virus disinfection for biotechnology applications: Different effectiveness on surface versus in suspension. Biologicals 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Coutts, B.; Kehoe, M.; Jones, R. Zucchini yellow mosaic virus: Contact transmission, stability on surfaces, and inactivation with disinfectants. Plant Dis. 2013, 97, 765–771. [Google Scholar] [CrossRef]
- Holmes, F.O. Local lesions in tobacco mosaic. Bot. Gaz. 1929, 87, 39–55. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, C.I.; Zamora-Macorra, E.J.; Ochoa-Martínez, D.L.; González-Garza, R. Disinfectants effectiveness in Tomato brown rugose fruit virus (ToBRFV) transmission in tobacco plants. Mex. J. Phytopathol. 2022, 40, 240–253. [Google Scholar] [CrossRef]
- DSMZ. Double Antibody Sandwich ELISA (DAS-ELISA). Available online: https://www.dsmz.de/fileadmin/_migrated/content_uploads/DAS-ELISA_01.pdf (accessed on 15 March 2022).
- Stroup, W.W.; Milliken, G.A.; Claassen, E.A.; Wolfinger, R.D. SAS for Mixed Models: Introduction and Basic Applications; SAS Institute: Cary, NC, USA, 2018. [Google Scholar]
- Ahmed, S.M.; Chandhana, S. Contaminant Sole Disinfectant—A Methodical Approach to Reduce the Spread of Covid. In Modern Approaches in Machine Learning & Cognitive Science: A Walkthrough; Springer: Berlin/Heidelberg, Germany, 2022; pp. 335–342. [Google Scholar]
- Jurado, C.; Martinez-Aviles, M.; De La Torre, A.; Štukelj, M.; de Carvalho Ferreira, H.C.; Cerioli, M.; Sánchez-Vizcaíno, J.M.; Bellini, S. Relevant measures to prevent the spread of African swine fever in the European Union domestic pig sector. Front. Vet. Sci. 2018, 5, 77. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; CDC: Atlanta, GA, USA, 2008. [Google Scholar]
- Lewandowski, D.J.; Hayes, A.J.; Adkins, S. Surprising results from a search for effective disinfectants for Tobacco mosaic virus–contaminated tools. Plant Dis. 2010, 94, 542–550. [Google Scholar] [CrossRef]
- Chipley, J.R. Sodium benzoate and benzoic acid. In Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 41–88. [Google Scholar]
- Hernndez, A.; Martro, E.; Matas, L.; Martın, M.; Ausina, V. Assessment of in-vitro efficacy of 1% Virkon® against bacteria, fungi, viruses and spores by means of AFNOR guidelines. J. Hosp. Infect. 2000, 46, 203–209. [Google Scholar] [PubMed]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Van der Strate, B.; Beljaars, L.; Molema, G.; Harmsen, M.; Meijer, D. Antiviral activities of lactoferrin. Antivir. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Coutts, B.; Jones, R. Potato virus Y: Contact transmission, stability, inactivation, and infection sources. Plant Dis. 2015, 99, 387–394. [Google Scholar] [CrossRef]
- Kamenova, I.; Adkins, S. Transmission, in planta distribution, and management of Hibiscus latent Fort Pierce virus, a novel tobamovirus isolated from Florida hibiscus. Plant Dis. 2004, 88, 674–679. [Google Scholar] [CrossRef]
- Wintermantel, W. A comparison of disinfectants to prevent spread of potyviruses in greenhouse tomato production. Plant Health Prog. 2011, 12, 19. [Google Scholar] [CrossRef]
- Abdelbacki, A.M.; Taha, S.H.; Sitohy, M.Z.; Abou Dawood, A.I.; Abd-El Hamid, M.M.; Rezk, A.A. Inhibition of tomato yellow leaf curl virus (TYLCV) using whey proteins. Virol. J. 2010, 7, 26. [Google Scholar] [CrossRef]
- Sitohy, M.; Taha, S.; Osman, A.; Abdel-Hamid, M.; Hamed, A.; Abdelbacki, A. Antiviral action of native and methylated Lactoferrin and β-Lactoglobulin against Potato Virus Y (PVY) infected into potato plants grown in an open field. Antibiotics 2020, 9, 430. [Google Scholar] [CrossRef]
- Hornig, K.; Burgess, B.; Saklou, N.; Johnson, V.; Malmlov, A.; Van Metre, D.; Morley, P.; Byers, S. Evaluation of the efficacy of disinfectant footmats for the reduction of bacterial contamination on footwear in a large animal veterinary hospital. J. Vet. Intern. Med. 2016, 30, 1882–1886. [Google Scholar] [CrossRef]
- Gasparini, R.; Pozzi, T.; Magnelli, R.; Fatighenti, D.; Giotti, E.; Poliseno, G.; Pratelli, M.; Severini, R.; Bonanni, P.; De Feo, L. Evaluation of in vitro efficacy of the disinfectant Virkon. Eur. J. Epidemiol. 1995, 11, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Büttner, C.; Bandte, M. Überprüfung der viruziden Wirksamkeit von Desinfektionsmitteln am Beispiel von Menno-Florades. Gartenbauwissenschaft 1999, 64, 214–219. [Google Scholar]
- Cayanan, D.F.; Zhang, P.; Liu, W.; Dixon, M.; Zheng, Y. Efficacy of Chlorine in Controlling Five Common Plant Pathogens. HortScience 2009, 44, 157–163. [Google Scholar] [CrossRef]
- Dee, S.; Deen, J.; Pijoan, C. Evaluation of 4 intervention strategies to prevent the mechanical transmission of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2004, 68, 19. [Google Scholar] [PubMed]
- Liu, R.; Vaishnav, R.A.; Roberts, A.M.; Friedland, R.P. Humans have antibodies against a plant virus: Evidence from tobacco mosaic virus. PLoS ONE 2013, 8, e60621. [Google Scholar] [CrossRef]
- Celar, F.; Valic, N.; Kosmelj, K.; Gril, T. Evaluating the efficacy, corrosivity and phytotoxicity of some disinfectants against Erwinia amylovora (Burrill) Winslow et al. using a new statistical measure. J. Plant Dis. Prot. 2007, 114, 49–53. [Google Scholar] [CrossRef]
- Anonymous. Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products. Product Assessment Report of a Biocidal Product For National Authorisation Applications. A-Quasan B. 2021. Available online: https://echa.europa.eu/documents/10162/b725498e-9586-8f1a-e67d-2e20c57cadb6 (accessed on 6 October 2022).
- Anonymous. Registration Report Part A. National Assessment-Federal Republic of Germany. Product Code: VP-LF/5 (Menno Florades). 2017. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/04_Pflanzenschutzmittel/01_zulassungsberichte/034407-00-00.pdf?__blob=publicationFile&v=2 (accessed on 6 October 2022).




| Inoculum | ||||
|---|---|---|---|---|
| Experiment | Plant Species | Carrier Substance | Dilution | Consistency |
| (1) Suspension | N. clevelandii A. Gray | tap water | 1:5 (w:v) | liquid |
| (2) Simulation | tap water | 1:5 (w:v) | liquid | |
| (3) Practical conditions | quartz sand | 1:10 (w:w) | granular | |
| Product | Working Solution | |
|---|---|---|
| Trade Name | Active Ingredient | (%) |
| MENNO Florades (MF) | 9% (w:v) benzoic acid | 4 |
| Virkon S (VS) | 45.3% potassium Peroxymonosulfate | 1 |
| Skim milk powder (SMP) | 45–54% lactose | 10 20 |
| DanKlorix (DK) | 2.8% sodium hypochlorite | 12.5 |
| 25 | ||
| Floradix Lactoferrin (FD) | 31% (w:w) lactoferrin | 0.5 |
| Description | Experiment 1 | Experiment 2 | Experiment 3 | |
|---|---|---|---|---|
| Laboratory Conditions | Practical Conditions | |||
| Suspension | Simulation | 3.1 Shoe Sole | 3.2 Disinfection Mat | |
| Experimental factors (non-italic style) or constant experimental conditions (italic style) | ||||
| Product | Control, MF, VS, SMP (10, 20), DK (12.5, 25) | Control, MF, VS, SMP (10), DK (12.5), FD | Control, MF | Control, MF |
| Tested material/ Germ carrier | plant sap | natural rubber | natural rubber | dirt suspension |
| Contact time | 10 min | 30 s | undefined | 1d/4d |
| Mechanical cleaning | no | no | no, light, strong | no |
| Scope of experiments | ||||
| Treatments (no.) § | 7 | 6 | 6 | 4 |
| Trial repetition (no.) | 3 | 3 | 3 | 3 |
| Inoculated plants per repetition/treatment (no.) | 8 | 8 | 8 | 8 |
| Inoculated plants (total no.) | 168 | 144 | 144 | 96 |
| Inoculated leaf halves (total no.) | 504 | 432 | 432 | 288 |
| Treatment | No. of Inoculated Plants | No. of Inoculated Leaf Halves | Mean no. of Local Lesions | SEM | Reduction in Local Lesions Compared to ‘Control’ |
|---|---|---|---|---|---|
| Nc | 18 | 54 | 0 | 100 | |
| Control | 24 | 72 | 154.6 (d) | 47.4 | reference |
| MF, 4% | 24 | 72 | 13.5 (c) | 4.2 | 91.3% |
| VS, 1% | 24 | 72 | 3.1 (b) | 1.0 | 98.0% |
| SMP, 10% | 24 | 72 | 2.4 (b) | 0.8 | 98.5% |
| SMP, 20% | 24 | 72 | 1.3 (b) | 0.5 | 99.2% |
| DK, 25% | 24 | 72 | 0.8 (ab) | 0.3 | 99.5% |
| DK, 12.5% | 24 | 72 | 0.1 (a) | 0.07 | 99.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlers, J.; Nourinejhad Zarghani, S.; Kroschewski, B.; Büttner, C.; Bandte, M. Decontamination of Tomato Brown Rugose Fruit Virus-Contaminated Shoe Soles under Practical Conditions. Horticulturae 2022, 8, 1210. https://doi.org/10.3390/horticulturae8121210
Ehlers J, Nourinejhad Zarghani S, Kroschewski B, Büttner C, Bandte M. Decontamination of Tomato Brown Rugose Fruit Virus-Contaminated Shoe Soles under Practical Conditions. Horticulturae. 2022; 8(12):1210. https://doi.org/10.3390/horticulturae8121210
Chicago/Turabian StyleEhlers, Jens, Shaheen Nourinejhad Zarghani, Bärbel Kroschewski, Carmen Büttner, and Martina Bandte. 2022. "Decontamination of Tomato Brown Rugose Fruit Virus-Contaminated Shoe Soles under Practical Conditions" Horticulturae 8, no. 12: 1210. https://doi.org/10.3390/horticulturae8121210
APA StyleEhlers, J., Nourinejhad Zarghani, S., Kroschewski, B., Büttner, C., & Bandte, M. (2022). Decontamination of Tomato Brown Rugose Fruit Virus-Contaminated Shoe Soles under Practical Conditions. Horticulturae, 8(12), 1210. https://doi.org/10.3390/horticulturae8121210







