Impact of Beauveria bassiana and Metarhizium anisopliae on the Metabolic Interactions between Cucumber (Cucumis sativus L.) and Cucumber Mosaic Virus (CMV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Treatments
2.2. Confirmation of Endophytic Colonization of Plants
2.3. CMV Infection and Plant Sampling
2.4. Sample Preparation and Metabolome Profiling
2.5. Data Processing
2.6. Data Cleansing
2.7. Statistical Analysis
3. Results
3.1. Assessment of Colonization of Beauveria bassiana and Metarhizium anisopliae in Cucumber Plants
3.2. Assessment of CMV Infection
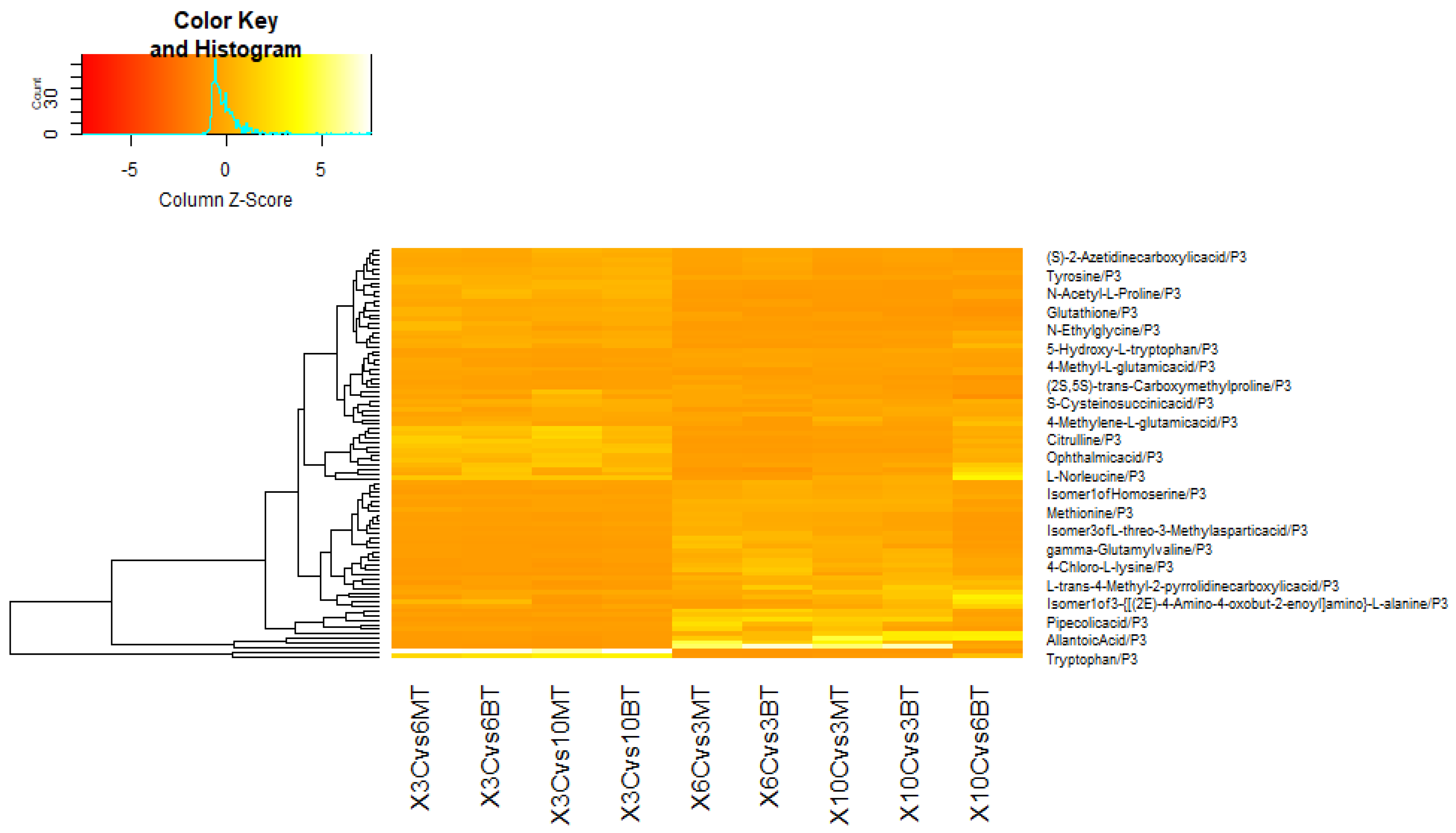
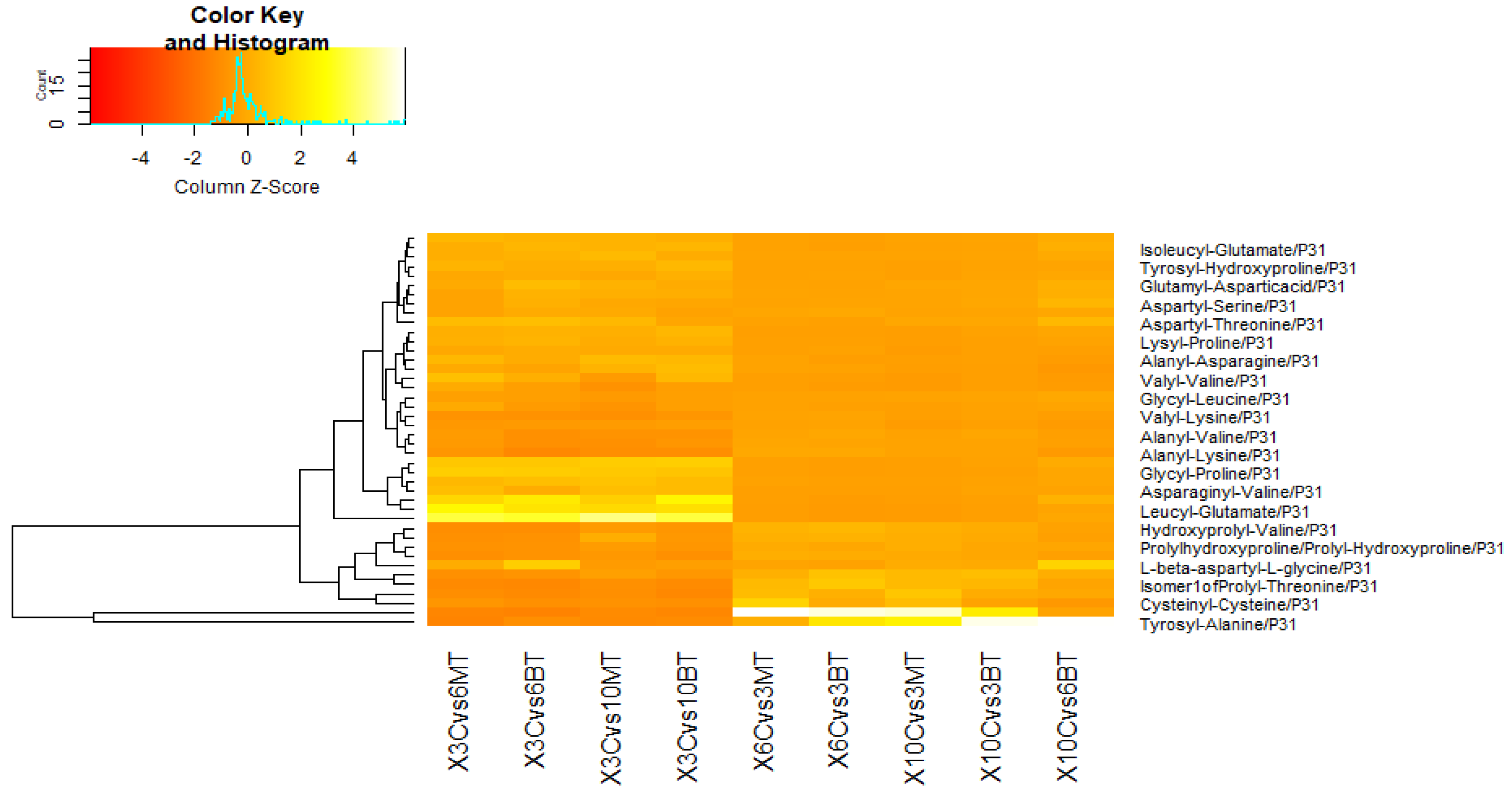
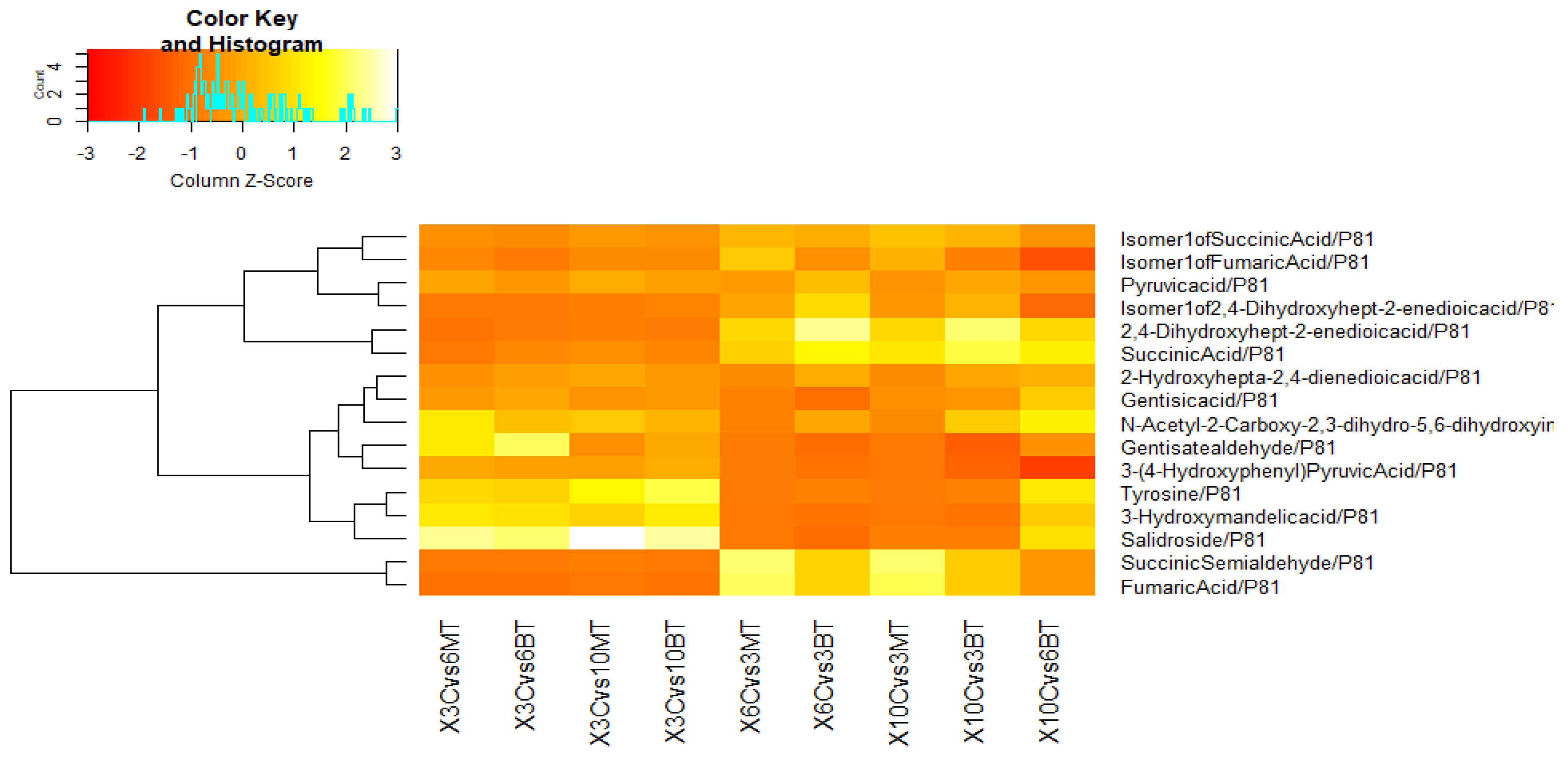
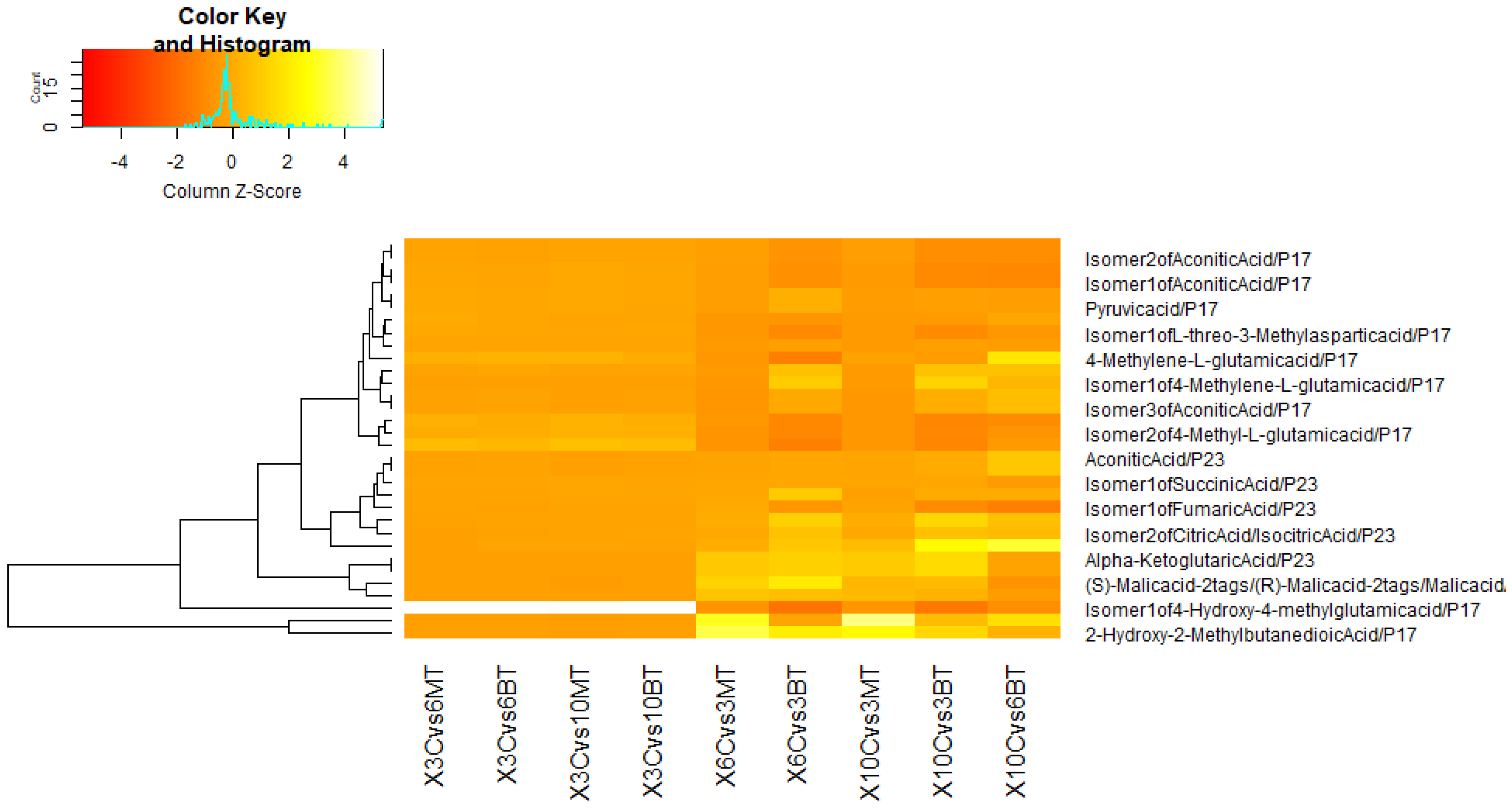
3.3. Metabolomic Adjustments Triggered by B. bassiana and M. anisopliae in CMV-Infected Cucumber Plants
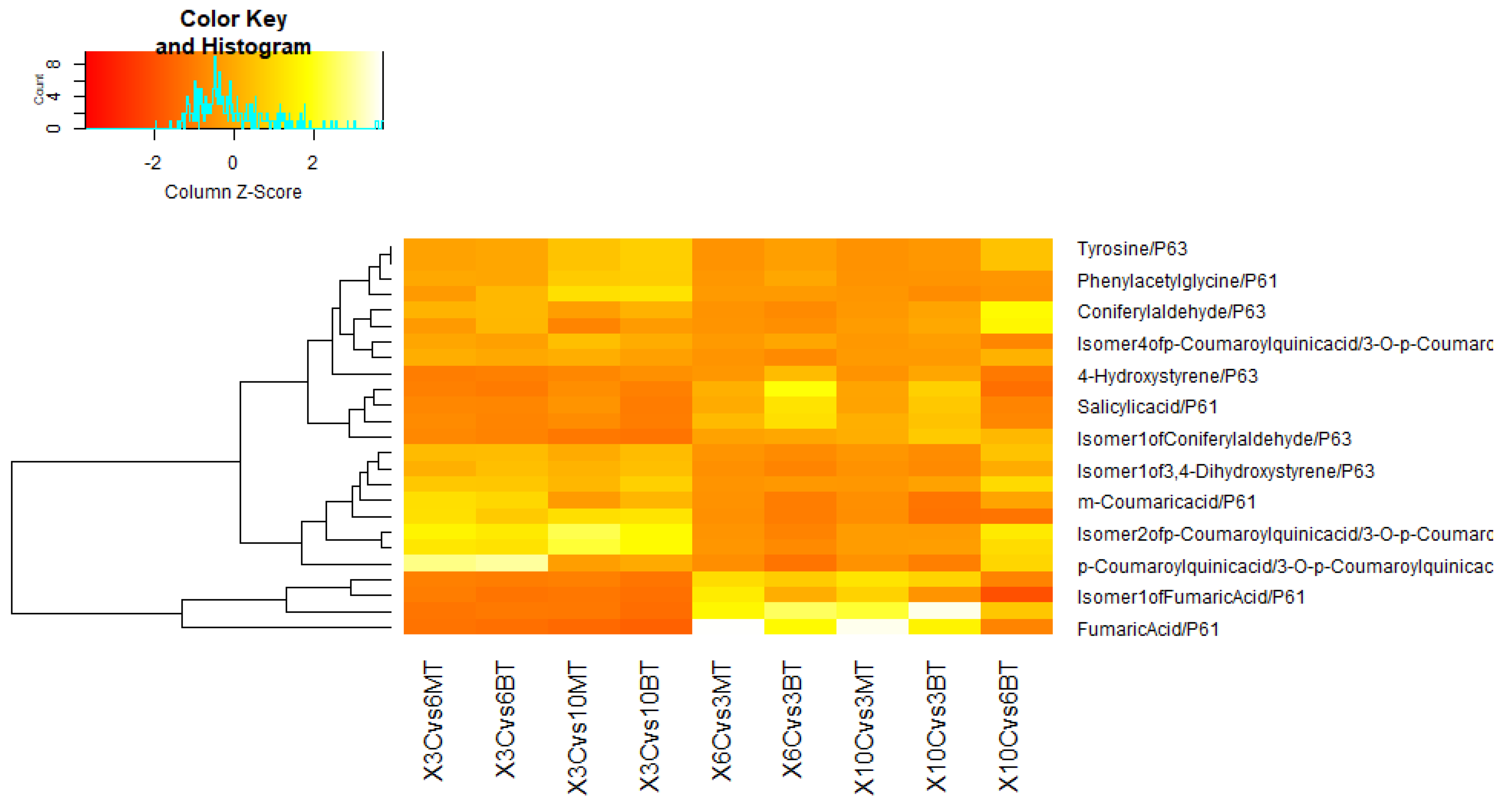
3.3.1. In Comparison with Non-Fungal-Treated Diseased Plants at Different Dpi
3.3.2. In Comparison with Same Fungal-Treated Diseased Plants at Different Dpi
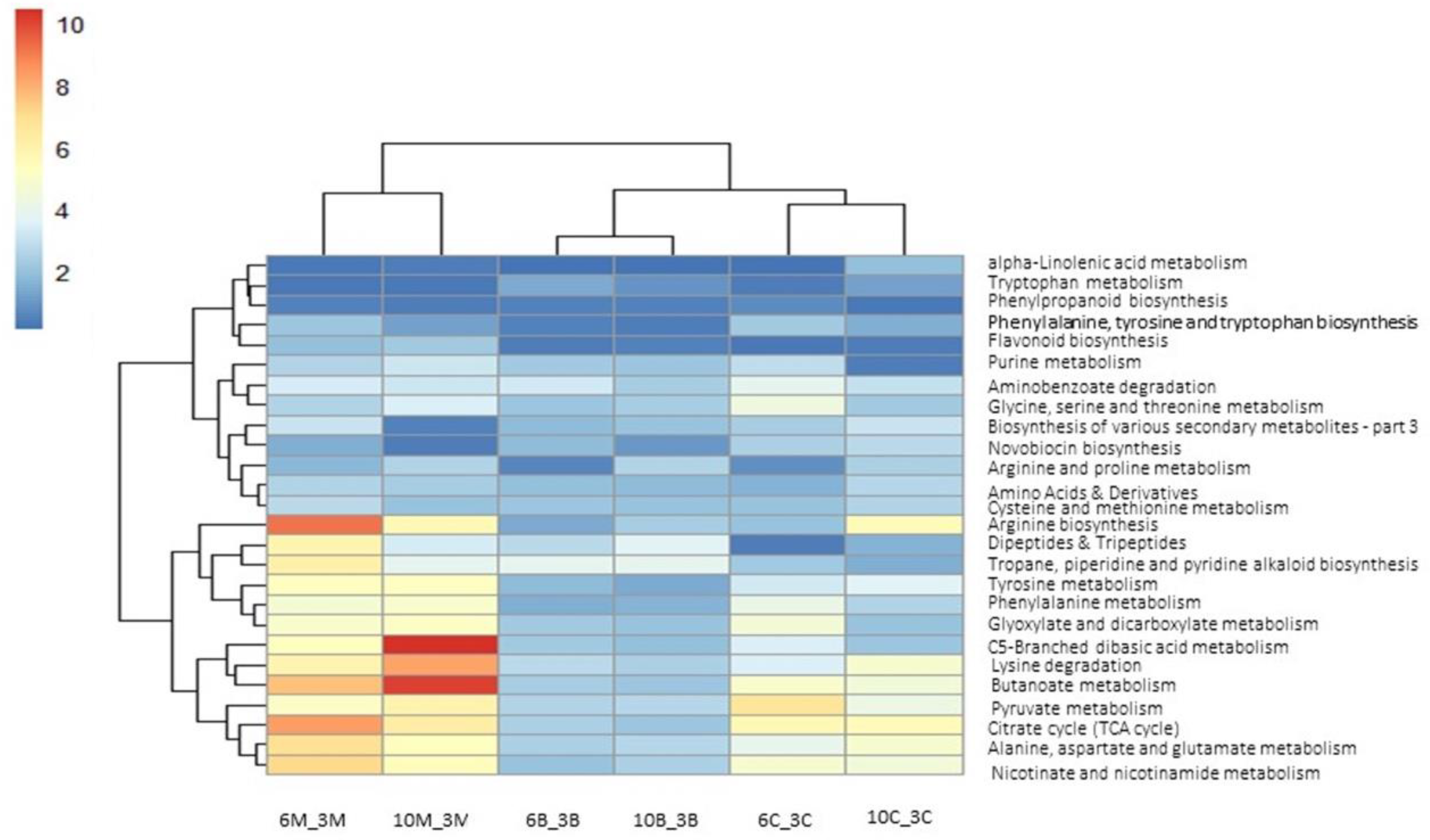
3.4. Metabolomic Adjustments Triggered by CMV in Cucumber Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, D. Endophyte: The Evolution of a Term and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Johnson, L.J.; Johnson, R.D.; Schardl, C.L.; Panaccione, D.G. Identification of differentially expressed genes in the mutualistic association of tall fescue with Neotyphodium coenophialum. Physiol. Mol. Plant Pathol. 2003, 63, 305–317. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, R.; Gerges, E.; Habib, W.; Ibrahim, L. Endophytic colonization by Beauveria bassiana and Metarhizium anisopliae induces growth promotion effect and increases the resistance of cucumber plants against Aphis gossypii. J. Plant Prot. Res. 2021, 61, 358–370. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural Products from Endophytic Microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Kaul, S.; Gupta, S.; Ahmed, M.; Dhar, M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012, 11, 487–505. [Google Scholar] [CrossRef]
- Kusari, S.; Singh, S.; Jayabaskaran, C. Biotechnological potential of plant-associated endophytic fungi: Hope versus hype. Trends Biotechnol. 2014, 32, 297–303. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Liu, L.; Xiang, M.; Wang, W.; Sun, X.; Che, Y.; Guo, L.; Liu, G.; Guo, L.; et al. Genomic and transcriptomic analysis of the endophytic fungus Pestalotiopsis fici reveals its lifestyle and high potential for synthesis of natural products. BMC Genom. 2015, 16, 28. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Chen, X.-L.; Sun, M.-C.; Chong, S.-L.; Si, J.-P.; Wu, L.-S. Transcriptomic and Metabolomic Approaches Deepen Our Knowledge of Plant–Endophyte Interactions. Front. Plant Sci. 2022, 12, 700200. [Google Scholar] [CrossRef]
- Dayalan, S.; Xia, J.; Spicer, R.A.; Salek, R.; Roessner, U. Metabolome Analysis. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 396–409. [Google Scholar] [CrossRef]
- Manchester, M.; Anand, A. Metabolomics: Strategies to define the role of metabolism in virus infection and pathogenesis. In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 98, pp. 57–81. [Google Scholar] [CrossRef]
- Skinner, M.; Parker, B.L.; Kim, J.S. Role of entomopathogenic fungi in integrated pest management. In Integrated Pest Management: Current Concepts and Ecological Perspectives; Abrol, D.P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 169–191. [Google Scholar] [CrossRef]
- Batool, R.; Umer, M.J.; Wang, Y.; He, K.; Shabbir, M.Z.; Zhang, T.; Bai, S.; Chen, J.; Wang, Z. Myco-Synergism Boosts Herbivory-Induced Maize Defense by Triggering Antioxidants and Phytohormone Signaling. Front. Plant Sci. 2022, 13, 790504. [Google Scholar] [CrossRef] [PubMed]
- Wraight, S.P.; Inglis, G.D.; Goettel, M.S. Fungi. In Field Manual of Techniques in Invertebrate Pathology: Application and Evaluation of Pathogens for Control of Insects and other Invertebrate Pest, 2nd ed.; Lacey, L.A., Kaya, H.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 223–248. [Google Scholar] [CrossRef]
- Vega, F.E.; Meyling, N.V.; Luangsa-Ard, J.J.; Blackwell, M. Fungal entomopathogens. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 171–220. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control 2010, 55, 34–41. [Google Scholar] [CrossRef]
- Castillo Lopez, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef]
- Griffin, M.R.; Ownley, B.H.; Klingeman, W.E.; Pereira, R.M. Evidence of induced systemic resistance with Beauveria bassiana against Xanthomonas in cotton. Phytopathology 2006, 96, S42. [Google Scholar]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. Biocontrol 2010, 55, 113–128. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Vega, F. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Vidal, S.; Jaber, L. Entomopathogenic fungi as endophytes: Plant–endophyte–herbivore interactions and prospects for use in biological control. Curr. Sci. 2015, 109, 46–54. [Google Scholar]
- Lehtonen, P.T.; Helander, M.; Siddiqui, S.A.; Lehto, K.; Saikkonen, K. Endophytic fungus decreases plant virus infections in meadow ryegrass (Lolium pratense). Biol. Lett. 2006, 2, 620–623. [Google Scholar] [CrossRef][Green Version]
- Jaber, L.R.; Salem, N.M. Endophytic colonisation of squash by the fungal entomopathogen, Beauveria bassiana (Ascomycota: Hypocreales) for managing Zucchini yellow mosaic virus in Cucurbits. Biocontrol. Sci. Technol. 2014, 24, 1096–1109. [Google Scholar] [CrossRef]
- Muvea, A.M.; Subramanian, S.; Maniania, N.K.; Poehling, H.M.; Ekesi, S.; Meyhöfer, R. Endophytic colonization of onions induces resistance against viruliferous thrips and virus replication. Front. Plant Sci. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Kiarie, S.; Nyasani, J.O.; Gohole, L.S.; Maniania, N.K.; Subramanian, S. Impact of Fungal Endophyte Colonization of Maize (Zea mays L.) on Induced Resistance to Thrips- and Aphid-Transmitted Viruses. Plants 2020, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.M.; Shimizu, M.; Takahashi, H.; Ozaki, K.; Hyakumachi, M. Induction of Systemic Resistance against Cucumber mosaic virus in Arabidopsis thaliana by Trichoderma asperellum SKT-1. Plant Pathol. J. 2013, 29, 193–200. [Google Scholar] [CrossRef]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 Induces Systemic Resistance in Tomato Infected by Cucumber mosaic virus. Front. Plant Sci. 2016, 7, 1520. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, N.; Quesada-Moraga, E.; Plaza, M.; Fereres, A.; Moreno, A. Changes in feeding behaviour are not related to the reduction in the transmission rate of plant viruses by Aphis gossypii (Homoptera: Aphididae) to melon plants colonized by Beauveria bassiana (Ascomycota: Hypocreales). Biol. Control 2018, 130, 95–103. [Google Scholar] [CrossRef]
- Shaalan, R.; Ibrahim, L. Entomopathogenic fungal endophytes: Can they colonize cucumber plants? In Book of Proceedings of the IX International Scientific Agriculture Symposium AGROSYM; AGROSYM: Jahorina, Bosnia and Herzegovina, 2018; pp. 853–860. [Google Scholar]
- Ibrahim, L.; Hamieh, A.; Ghanem, H.; Ibrahim, S. Pathogenicity of entomopathogenic fungi from Lebanese soils against aphids, whitefly and non-target beneficial insects. Int. J. Agric. Sci. 2011, 3, 156–164. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, L.; Laham, L.; Tomma, A.; Ibrahim, S. Mass production, yield, quality, formulation and efficacy of entomopathogenic Metarhizium anisopliae conidia. Br. J. Appl. Sci. Technol. 2015, 9, 427–440. [Google Scholar] [CrossRef]
- Humber, R.A. Fungi: Identification. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 1997; pp. 153–185. [Google Scholar] [CrossRef]
- Petrini, O.; Fisher, P.J. Fungal endophytes in Salicornia perennis. Trans. Br. Mycol. Soc. 1987, 87, 647–651. [Google Scholar] [CrossRef]
- Rizos, H.; Gunn, L.V.; Pares, R.D.; Gillings, M.R. Differentiation of cucumber mosaic virus isolates using the polymerase chain reaction. J. Gen. Virol. 1992, 73, 2099–2103. [Google Scholar] [CrossRef]
- Bos, L. Persistence of infectivity of three viruses in plant material dried over CaCl2 and stored under different conditions. Neth. J. Plant Pathol. 1977, 83, 217–220. [Google Scholar] [CrossRef]
- Sudhakar, N.; Nagendra-Prasad, D.; Mohan, N.; Murugesan, K. Induction of systemic resistance in Lycopersicon esculentum cv. PKM1 (tomato) against Cucumber mosaic virus by using ozone. J. Virol. Methods 2007, 139, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Puryear, J.; Cairney, J. A Simple and Efficient Method for Isolating RNA from Pine Trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.-S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Munir, S.; Li, Y.; He, P.; He, P.; Ahmed, A.; Wu, Y.; He, Y. Unraveling the metabolite signature of citrus showing defense response towards Candidatus Liberibacter asiaticus after application of endophyte Bacillus subtilis L1-21. Microbiol. Res. 2020, 234, 126425. [Google Scholar] [CrossRef]
- Wintermantel, W.M. Integration of omics approaches toward understanding whitefly transmission of viruses. Adv. Virus Res. 2018, 102, 199–223. [Google Scholar]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Labuschagne, N.; Dubery, I.A. Concurrent metabolic profling and quantifcation of aromatic amino acids and phytohormones in Solanum lycopersicum plants responding to Phytophthora capsici. Metabolites 2020, 10, 466. [Google Scholar] [CrossRef]
- Ting, H.M.; Cheah, B.H.; Chen, Y.C.; Yeh, P.M.; Cheng, C.P.; Yeo, F.K.S.; Vie, A.K.; Rohlof, J.; Winge, P.; Bones, A.M.; et al. The role of a glucosinolate-derived nitrile in plant immune responses. Front. Plant Sci. 2020, 11, 257. [Google Scholar] [CrossRef]
- Zhang, Y.; Bouwmeester, H.J.; Kappers, I.F. Combined transcriptome and metabolome analysis identifies defence responses in spider mite-infested pepper (Capsicum annuum). J. Exp. Bot. 2020, 71, 330–343. [Google Scholar] [CrossRef]
- Mahmood, A.; Kataoka, R. Metabolite profiling reveals a complex response of plants to application of plant growth-promoting endophytic bacteria. Microbiol. Res. 2020, 234, 126421. [Google Scholar] [CrossRef]
- Bajaj, R.; Huang, Y.; Gebrechristos, S.; Mikolajczyk, B.; Brown, H.; Prasad, R.; Varma, A.; Bushley, K.E. Transcriptional responses of soybean roots to colonization with the root endophytic fungus Piriformospora indica reveals altered phenylpropanoid and secondary metabolism. Sci. Rep. 2018, 8, 10227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, N.; Zhao, L.; Zhu, H.; Tang, C. Transcriptome analysis reveals the defense mechanism of cotton against Verticillium dahliae in the presence of the biocontrol fungus Chaetomium globosum CEF-082. BMC Plant Biol. 2020, 20, 89. [Google Scholar] [CrossRef]
- Zechmann, B. Subcellular Roles of Glutathione in Mediating Plant Defense during Biotic Stress. Plants 2020, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Zierer, W.; Hajirezaei, M.R.; Eggert, K.; Sauer, N.; von Wirén, N.; Pommerrenig, B. Phloem-Specific Methionine Recycling Fuels Polyamine Biosynthesis in a Sulfur-Dependent Manner and Promotes Flower and Seed Development. Plant Physiol. 2016, 170, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Koc, F.N.; Seckin Dinler, B. Pipecolic acid in plants: Biosynthesis, signalling, and role under stress. Botanica 2022, 28, 4–14. [Google Scholar] [CrossRef]
- Çalişkan, M. The metabolism of oxalic acid. Turk. J. Zool. 2000, 24, 103–106. [Google Scholar]
- Nakata, P.A. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci. 2003, 164, 901–909. [Google Scholar] [CrossRef]
- Smith, L. The many roles of oxalate in nature. Trans. Am. Clin. Climatol. Assoc. 2002, 113, 1–20. [Google Scholar]
- Lu, X.; Zhang, L.; Huang, W.; Zhang, S.; Zhang, S.; Li, F.; Zhang, H.; Sun, R.; Zhao, J.; Li, G. Integrated Volatile Metabolomics and Transcriptomics Analyses Reveal the Influence of Infection TuMV to Volatile Organic Compounds in Brassica rapa. Horticulturae 2022, 8, 57. [Google Scholar] [CrossRef]
- Soujanya, P.L.; Sekhar, J.C.; Ratnavathi, C.V.; Karjagi, C.G.; Shobha, E.; Suby, S.B.; Yathish, K.R.; Sunil, N.; Rakshit, S. Induction of cell wall phenolic monomers as part of direct defense response in maize to pink stem borer (Sesamia inferens Walker) and non-insect interactions. Sci. Rep. 2021, 11, 14770. [Google Scholar] [CrossRef]
- Knollenberg, B.J.; Li, G.X.; Lambert, J.D.; Maximova, S.N.; Guiltinan, M.J. Clovamide, a Hydroxycinnamic Acid Amide, Is a Resistance Factor Against Phytophthora spp. in Theobroma cacao. Front. Plant Sci. 2020, 11, 617520. [Google Scholar] [CrossRef] [PubMed]
- Farahat, A.S.; El-Morsi, A.A.; Soweha, H.E.; Sofy, A.R.; Refaey, E.E. Metabolic changes of cucumber plants due to two cmv egyptian isolates. Arab. Univ. J. Agric. Sci. 2018, 26, 2019–2028. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Király, L.; Al-Mansori, A.-N.A.; Younes, H.A.; Zeid, A.; Elsharkawy, M.M.; Behiry, S.I. Defense Responses and Metabolic Changes Involving Phenylpropanoid Pathway and PR Genes in Squash (Cucurbita pepo L.) following Cucumber mosaic virus Infection. Plants 2022, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Clemente, R.M.; Montoliu, A.; Vives-Peris, V.; Arbona, V.; Gómez-Cadenas, A. Hormonal and metabolic responses of Mexican lime plants to CTV infection. J. Plant Physiol. 2019, 238, 40–52. [Google Scholar] [CrossRef]
- Zhang, Z.; He, H.; Yan, M.; Zhao, C.; Lei, C.; Li, J.; Yan, F. Widely targeted analysis of metabolomic changes of Cucumis sativus induced by cucurbit chlorotic yellows virus. BMC Plant Biol. 2022, 22, 158. [Google Scholar] [CrossRef]
- Maluta, N.K.; Garzo, E.; Moreno, A.; Lopes, J.R.; and Fereres, A. Tomato yellow leaf curl virus benefits population growth of the Q Biotype of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Neotrop Entomol. 2014, 43, 385–392. [Google Scholar] [CrossRef]
- Cui, H.Y.; Sun, Y.C.; Zhao, Z.H.; Zhang, Y.J. The combined effect of elevated O3 levels and TYLCV infection increases the fitness of Bemisia tabaci Mediterranean on tomato plants. Environ. Entomol. 2019, 48, 1425–1433. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]







| Treatment | Leaves (% ± SE) | Stem (% ± SE) | Roots (% ± SE) |
|---|---|---|---|
| Control | 0.0 ± 0.00 a * | 0.0 ± 0.00 a | 0.0 ± 0.00 a |
| B. bassiana | 100 ± 0.00 b | 40.0 ± 0.24 bc | 60.0 ± 0.24 b |
| M. anisopliae | 80.0 ± 0.20 b | 80.0 ± 0.20 c | 80.0 ± 0.20 b |
| Treatment | Dpi 3 | Dpi 6 | Dpi 10 |
|---|---|---|---|
| Positive control plants | 2.68 ± 0.46 a,* | 2.16 ± 0.45 a | 1.74 ± 0.18 a |
| Metarhizium anisopliae | 2.44 ± 0.36 a | 1.9 ± 0.18 a | 2.04 ± 0.31 a |
| Beauveria bassiana | 1.86 ± 0.28 a | 2.03 ± 0.31 a | 2.52 ± 0.62 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, R.; Ibrahim, L.; As-sadi, F.; El Kayal, W. Impact of Beauveria bassiana and Metarhizium anisopliae on the Metabolic Interactions between Cucumber (Cucumis sativus L.) and Cucumber Mosaic Virus (CMV). Horticulturae 2022, 8, 1182. https://doi.org/10.3390/horticulturae8121182
Shaalan R, Ibrahim L, As-sadi F, El Kayal W. Impact of Beauveria bassiana and Metarhizium anisopliae on the Metabolic Interactions between Cucumber (Cucumis sativus L.) and Cucumber Mosaic Virus (CMV). Horticulturae. 2022; 8(12):1182. https://doi.org/10.3390/horticulturae8121182
Chicago/Turabian StyleShaalan, Roshan, Ludmilla Ibrahim, Falah As-sadi, and Walid El Kayal. 2022. "Impact of Beauveria bassiana and Metarhizium anisopliae on the Metabolic Interactions between Cucumber (Cucumis sativus L.) and Cucumber Mosaic Virus (CMV)" Horticulturae 8, no. 12: 1182. https://doi.org/10.3390/horticulturae8121182
APA StyleShaalan, R., Ibrahim, L., As-sadi, F., & El Kayal, W. (2022). Impact of Beauveria bassiana and Metarhizium anisopliae on the Metabolic Interactions between Cucumber (Cucumis sativus L.) and Cucumber Mosaic Virus (CMV). Horticulturae, 8(12), 1182. https://doi.org/10.3390/horticulturae8121182






