Comparative Physiochemical Mechanisms of Salt Tolerance between Cornus florida and Cornus hongkongensis subsp. elegans Based on Seed Germination and Seedling Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Seeds and Solution Preparation
2.2. Pretreatment and Germination of Seeds
2.3. Estimation of Germination Parameters
2.4. Assessment of Seedling Growth Parameters
2.5. Determination of Biochemical Parameters
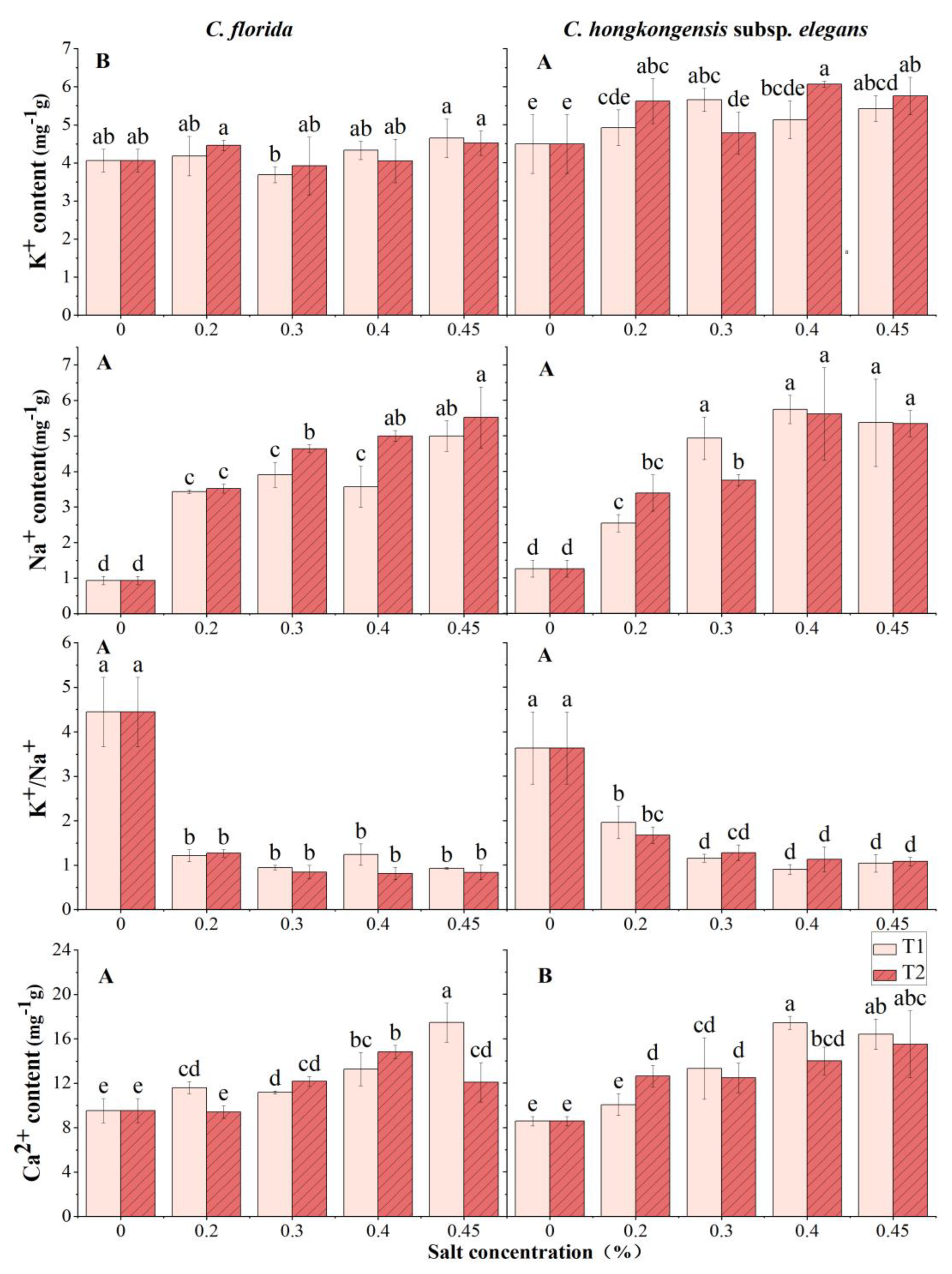
2.6. Measurement of Ion Deposition
2.7. Data Analysis
3. Results
3.1. Effect of Salt Stress on Germination Parameters
3.2. Effect of Salt Stress on Seedling Growth Traits
3.3. Effects of Salt Stress on Physiochemical Traits
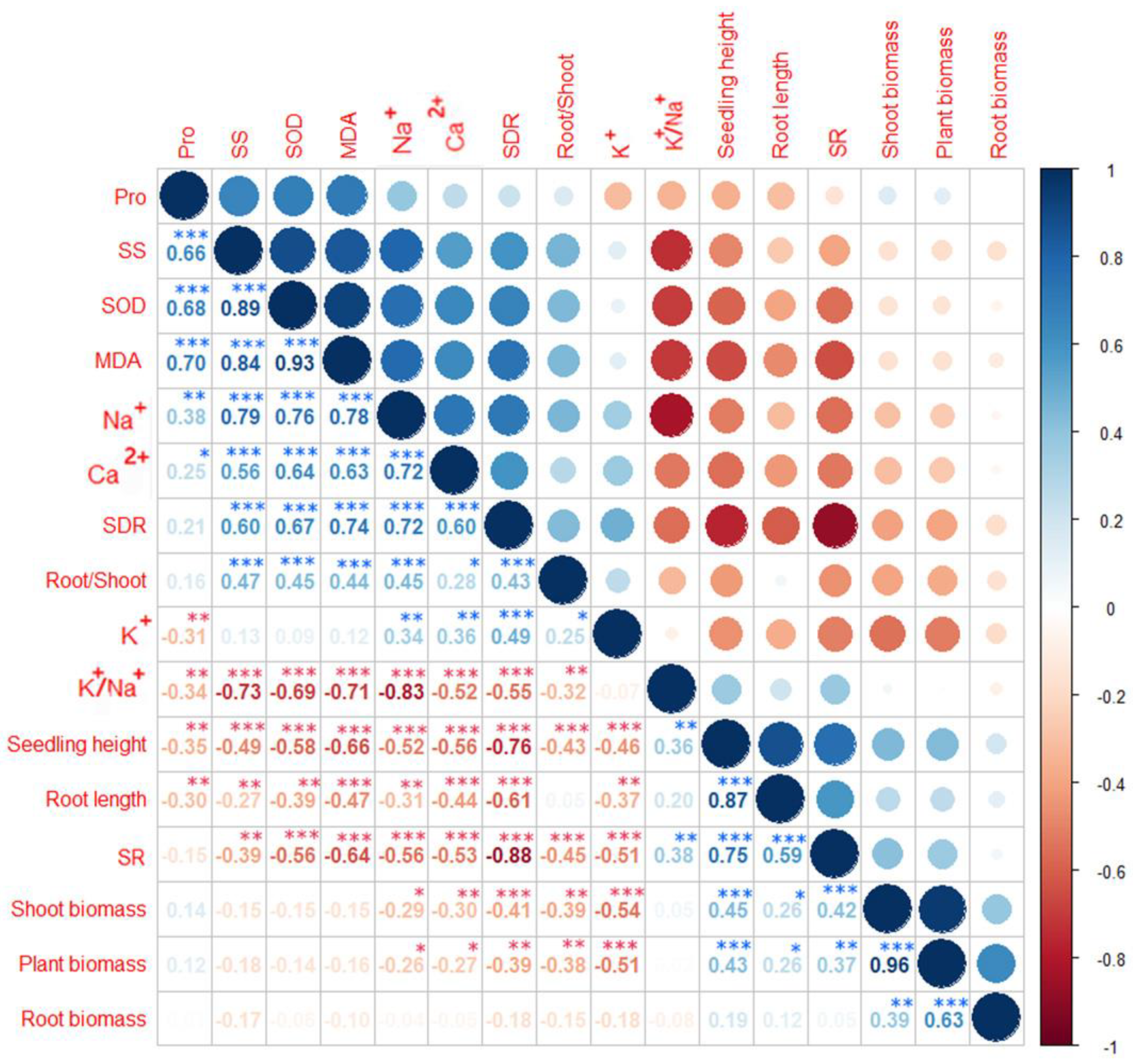
3.4. Correlations between Growth and Physiochemical Parameters
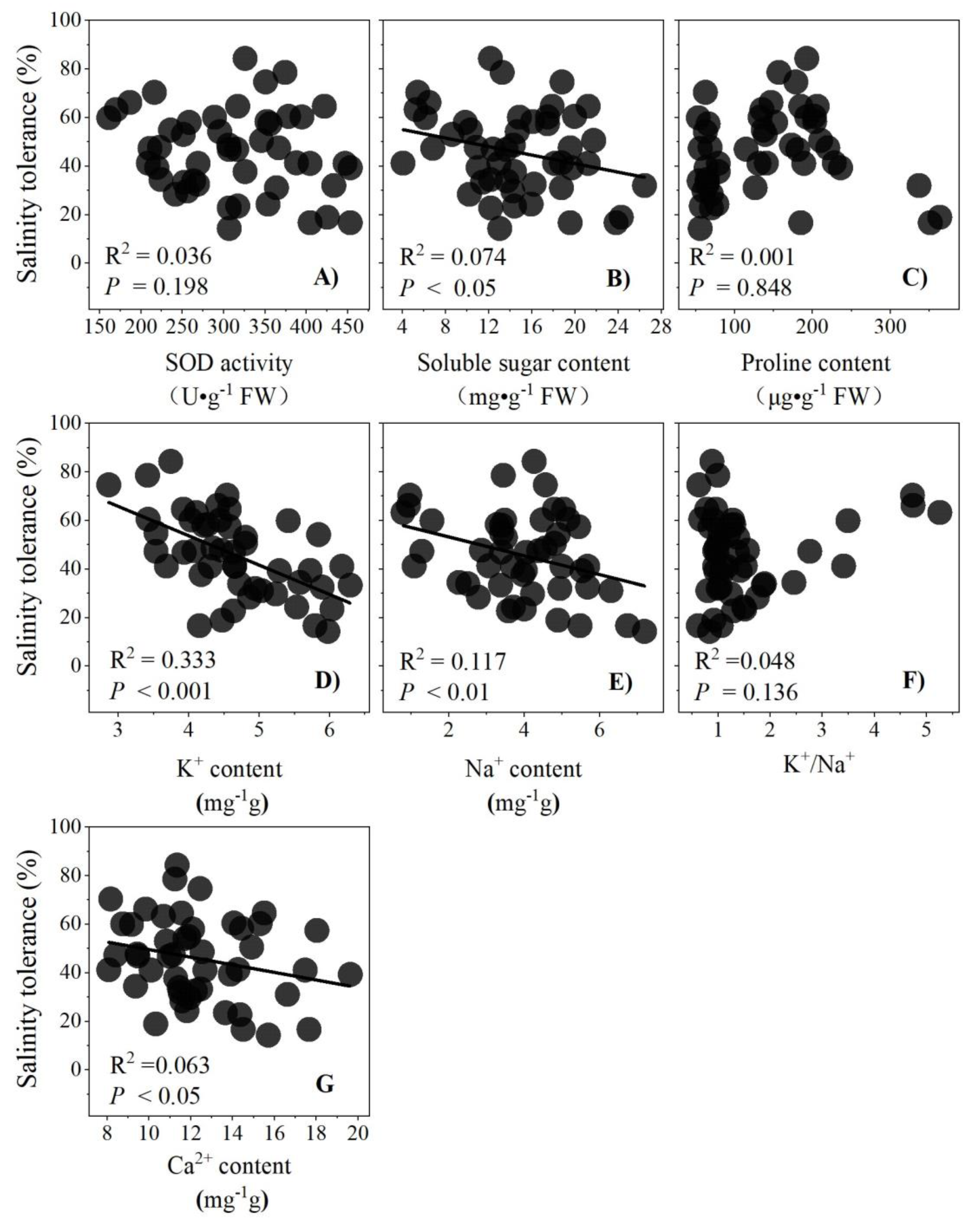
3.5. Relationships between Salinity Tolerance with Variables
4. Discussion
4.1. Salinity Inhibits Seed Germination and Seedling Growth in Two Dogwood Species, but C. florida Displays Better Salt Tolerance
4.2. Accumulation of Soluble Sugar May Be an Adaptive Indicator to Salt Stress
4.3. Ion Osmoregulation May Be Critical for Salt Tolerance in Dogwood Seedlings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yasin, N.A.; Khan, W.U.; Ahmad, S.R.; Ali, A.; Ahmad, A.; Akram, W. Imperative roles of halotolerant plant growth-promoting rhizobacteria and kinetin in improving salt tolerance and growth of black gram (Phaseolus mungo). Environ. Sci. Pollut. Res. 2018, 25, 4491–4505. [Google Scholar] [CrossRef]
- Mimouni, H.; Wasti, S.; Manaa, A.; Gharbi, E.; Chalh, A.; Vandoorne, B.; Stanley, L.; Ahmed, H.B. Does salicylic acid (SA) improve tolerance to salt stress in plants? A study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. Omics A J. Integr. Biol. 2016, 20, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, L.; Wang, H.W.; Yuan, J.Q.; Fu, X.X. Calcium Ion Richness in Cornus hongkongensis subsp. elegans (W.P. Fang et Y.T. Hsieh) Q.Y. Xiang Could Enhance Its Salinity Tolerance. Forests 2021, 12, 1522. [Google Scholar] [CrossRef]
- Wang, S.F.; Hu, Y.; Li, Z.; Sun, H.; Chen, Y. Effects of NaCl stress on growth and mineral ion uptake, transportation and distribution of Quercus Va. Acta Ecol. Sin. 2010, 30, 4609–4616. [Google Scholar]
- Jafari, S.; Garmdareh, S.E.H. Effects of salinity on morpho-physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 257, 108731. [Google Scholar] [CrossRef]
- Yadav, P.V.; Maya, K.; Zakwan, A. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Res. J. Seed Sci. 2011, 4, 125–136. [Google Scholar] [CrossRef]
- Omid, A.; Farzad, S.Z. Osmo and hydro priming improvement germination characteristics and enzyme activity of Mountain Rye (Secale montanum) seeds under drought stress. J. Stress Physiol. Biochem. 2012, 8, 253–261. [Google Scholar]
- Tiwari, J.K.; Munshi, A.D.; Kumar, R.; Pandey, R.N.; Arora, A.; Bhat, J.S.; Sureja, A.K. Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol. Plant. 2010, 32, 103–114. [Google Scholar] [CrossRef]
- Mustard, J.; Renault, S. Response of red-osier dogwood (Cornus sericea) seedlings to NaCl during the onset of bud break. Botany 2006, 84, 844–851. [Google Scholar] [CrossRef]
- Yuan, J.Q.; Sun, D.W.; Lu, Q.; Yang, L.; Wang, H.W.; Fu, X.X. Responses of Physiology, Photosynthesis, and Related Genes to Saline Stress in Cornus hongkongensis subsp. tonkinensis (W.P. Fang) Q.Y. Xiang. Plants 2022, 11, 940. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Gao, Q. Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars. BMC Plant Biol. 2020, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.C.; Grieve, C.M.; Francois, L.E. Whole-plant response to salinity. In Plant Environment Interactions; Wilkinson, R.E., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 199–244. [Google Scholar]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Blumwald, E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat. Biotechnol. 2001, 19, 765–768. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef]
- Kamran, M.; Xie, K.; Sun, J.; Wang, D.; Shi, C.; Lu, Y.; Gu, W.J.; Xu, P. Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf. 2020, 188, 109877. [Google Scholar] [CrossRef]
- Xiao, D.Q.; Lu, C.X. Germplasm Resource Distribution and Recent Research Advance in Dendrobenthamia. J. Gansu For. Sci. Technol. 2008, 33, 24–26, 68. [Google Scholar]
- Pais, A.L.; Whetten, R.W.; Xiang, Q.Y. Ecological genomics of local adaptation in Cornus florida L. by genotyping by sequencing. Ecol. Evol. 2017, 7, 441–465. [Google Scholar] [CrossRef]
- Fu, X.X.; Xu, J.; Liu, G.H. The germplasm resources and development and utilization of ornamental Cornus. J. China For. Sci. Technol. 2015, 29, 542–551. [Google Scholar] [CrossRef]
- Lakon, G. The topographical tetrazolium method for determining the germinating capacity of seeds. Plant Physiol. 1949, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Yang, L.; Lu, Q.; Fu, X.X. Effects of salt stress on seed germination and seedling growth of Cornus florida. J. Nanjing For. Univ. 2020, 44, 89–94. [Google Scholar] [CrossRef]
- Li, J.P.; Wang, X.P.; Liu, S.J.; Lu, X.L.; Wu, Z. Salt Tolerance of Maize at Seedling Stage: Identification and Evaluation Method. Chin. Agric. Sci. Bull. 2022, 38, 28–34. [Google Scholar] [CrossRef]
- Yan, K.; Cui, M.; Zhao, S.; Chen, X.; Tang, X. Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonica Thunb.). Front. Plant Sci. 2016, 7, 1563. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhou, Z.B.; Chang, Q.; Fan, J.L.; Fan, W.P. Growth, physiological and biochemical characteristics of Populus pruinose seedlings under salt-drought stress. Acta Ecol. Sin. 2018, 38, 7026–7033. [Google Scholar] [CrossRef]
- Liu, Z.X.; Zhang, H.X.; Yang, X.Y.; Liu, T.; Di, W.B. Growth, and cationic absorption, transportation and allocation of Elaeagnus angustifolia seedlings under NaCl stress. Acta Ecol. Sin. 2014, 34, 326–336. [Google Scholar] [CrossRef][Green Version]
- Fang, W.P. Notes on Dendrobenthamia. Acta Phytotaxon. Sin. 1952, 2, 89–114. [Google Scholar]
- Kao, W.Y.; Tsai, T.T.; Tsai, H.C.; Shih, C.N. Response of three Glycine species to salt stress. Environ. Exp. Bot. 2006, 56, 120–125. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Funct. Plant Biol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- El Mahi, H.; Pérez-Hormaeche, J.; De Luca, A.; Villalta, I.; Espartero, J.; Gámez-Arjona, F.; Quintero, F.J. A critical role of sodium flux via the plasma membrane Na+/H+ exchanger SOS1 in the salt tolerance of rice. Plant Physiol. 2019, 180, 1046–1065. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signaling transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, S.S.; Yang, A.; Akhtar, J.; Jacobsen, S.E. Antioxidative response of quinoa exposed to iso-osmotic, ionic and non-ionic salt stress. J. Agron. Crop Sci. 2015, 201, 452–460. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Zhang, Q.; Dai, W. Plant response to salinity stress. In Stress Physiology of Woody Plants; CRC Press: Boca Raton, FL, USA, 2019; pp. 155–173. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. Plant Growth Regul. 1996, 19, 207–218. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, V.; Takabe, T.; Rai, V.; Singh, N.K. Elucidation of salt-tolerance metabolic pathways in contrasting rice genotypes and their segregating progenies. Plant Cell Rep. 2016, 35, 1273–1286. [Google Scholar] [CrossRef]
- Jia, K.; Yan, C.; Yan, H.; Gao, J. Physiological responses of turnip (Brassica rapa L. subsp. rapa) seedlings to salt stress. HortScience 2020, 55, 1567–1574. [Google Scholar] [CrossRef]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Jose, A.M.; Maria, O.O.; Agustina, B.V.; Pedro, D.V.; Maria, S.B.; Jose, H.J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2010, 62, 185–193. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Clipson, N.J.W. Halophytes. Q. Rev. Biol. 1986, 61, 185–193. [Google Scholar] [CrossRef]
- James, R.A.; Munns, R.; Caemmerer, S.V.; Trejo, C.; Miller, C.; Condon, T.; Miller, C.; Condom, T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat. Plant Cell Environ. 2006, 29, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Jia, Y.X.; Sun, L.; He, F.; Wan, L.Q.; Yuan, Q.H.; Li, X.L. Analysis of Effects of Salt Stress on Absorption and Accumulation of Mineral Elements in Elymus Spp. Using Atomic Absorption Spectrophotometer. Spectrosc. Spectr. Anal. 2008, 28, 2984–2988. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L.; Volkenburgh, E.V.; Newman, I. Effect of divalent cations on ion fluxes and leaf photochemistry in salinized barley leaves. J. Exp. Bot. 2005, 56, 1369–1378. [Google Scholar] [CrossRef]
- Geungjoo, L.; Carrow, R.N.; Duncan, R.R.; Eiteman, M.A.; Rieger, M.W. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ. Exp. Bot. 2008, 63, 19–27. [Google Scholar] [CrossRef]
- Raven, J. Regulation of pH and generation of osmolarity in vascular plants: A cost-benefit analysis in relation to efficiency of use of energy. Nitrogen Water 1985, 101, 25–77. [Google Scholar] [CrossRef]


| CK | T1 | T2 | |
|---|---|---|---|
| Seed pretreatment | GA3 | GA3 | GA3 + Salt solution(s) * |
| Germination | Distilled water | Salt solution(s) * | Salt solution(s) * |
| Number of treatments | 1 | 4 | 4 |
| Parameter | Seedling Height (cm) | Root Length (cm) | Root/Shoot | ||||
|---|---|---|---|---|---|---|---|
| Pretreatment | Salt Concentration (%) | C. florida | C. hongkongensis subsp. elegans | C. florida | C. hongkongensis subsp. elegans | C. florida | C. hongkongensis subsp. elegans |
| T1 | 0 | 7.35 ± 0.36 Aa | 7.65 ± 0.54 Aa | 4.13 ± 0.26 Aabc | 4.67 ± 0.47 Aa | 0.56 ± 0.04 Ac | 0.61 ± 0.02 Ab |
| 0.2 | 7.33 ± 0.17 Aa | 7.00 ± 0.18 Aab | 4.23 ± 0.19 Aabc | 4.20 ± 0.14 Aa | 0.58 ± 0.03 Abc | 0.60 ± 0.01 Ab | |
| 0.3 | 7.20 ± 0.70 Aa | 6.73 ± 0.77 Bb | 4.43 ± 0.45 Aab | 4.05 ± 0.25 Aa | 0.62 ± 0.01 Aab | 0.60 ± 0.03 Ab | |
| 0.4 | 6.45 ± 0.48 Abc | 6.43 ± 0.88 Ab | 3.98 ± 0.33 Abc | 4.15 ± 0.48 Aa | 0.62 ± 0.01 Aab | 0.64 ± 0.02 Aab | |
| 0.45 | 6.48 ± 0.53 Abc | 5.55 ± 0.48 Bc | 4.00 ± 0.45 Abc | 3.38 ± 0.50 Bb | 0.62 ± 0.03 Aab | 0.61 ± 0.04 Ab | |
| T2 | 0.2 | 7.23 ± 0.33 Aa | 6.65 ± 0.19 Ab | 4.10 ± 0.14 Aabc | 4.08 ± 0.17 Aa | 0.57 ± 0.04 Ac | 0.61 ± 0.02 Ab |
| 0.3 | 7.23 ± 0.26 Aa | 6.70 ± 0.29 Ab | 4.55 ± 0.30 Aa | 4.15 ± 0.29 Aa | 0.63 ± 0.02 Aa | 0.62 ± 0.02 Aab | |
| 0.4 | 6.90 ± 0.48 Aab | 6.50 ± 0.52 Bb | 4.28 ± 0.40 Aab | 4.23 ± 0.54 Aa | 0.62 ± 0.02 Aab | 0.65 ± 0.04 Aab | |
| 0.45 | 5.93 ± 0.45 Ac | 4.43 ± 0.35 Bd | 3.73 ± 0.22 Ac | 3.00 ± 0.36 Bb | 0.63 ± 0.04 Aa | 0.68 ± 0.07 Aa | |
| Parameter | Shoot Biomass (g) | Root Biomass (g) | Plant Biomass (g) | ||||
|---|---|---|---|---|---|---|---|
| Pretreatment | Salt Concentration (%) | C. florida | C. hongkongensis subsp. elegans | C. florida | C. hongkongensis subsp. elegans | C. florida | C. hongkongensis subsp. elegans |
| T1 | 0 | 0.14 ± 0.02 Aabc | 0.12 ± 0.01 Aa | 0.04 ± 0.00 Abc | 0.03 ± 0.00 Aa | 0.18 ± 0.02 Aab | 0.16 ± 0.01 Aa |
| 0.2 | 0.12 ± 0.01 Abc | 0.10 ± 0.00 Abc | 0.05 ± 0.00 Aabc | 0.03 ± 0.00 Ba | 0.17 ± 0.01 Ab | 0.12 ± 0.01 Bb | |
| 0.3 | 0.16 ± 0.01 Aa | 0.10 ± 0.00 Bbc | 0.05 ± 0.00 Aabc | 0.03 ± 0.00 Ba | 0.22 ± 0.02 Aa | 0.12 ± 0.00 Bb | |
| 0.4 | 0.13 ± 0.01 Aabc | 0.09 ± 0.01 Bbc | 0.05 ± 0.01 Aab | 0.03 ± 0.00 Ba | 0.19 ± 0.02 Aab | 0.13 ± 0.01 Bab | |
| 0.45 | 0.15 ± 0.00 ab | 0.04 ± 0.00 abc | 0.19 ± 0.00 ab | ||||
| T2 | 0.2 | 0.11 ± 0.02 Ac | 0.09 ± 0.00 Abc | 0.05 ± 0.00 Aabc | 0.03 ± 0.00 Ba | 0.16 ± 0.00 Ab | 0.12 ± 0.01 Bb |
| 0.3 | 0.14 ± 0.01 Aabc | 0.10 ± 0.01 Bb | 0.04 ± 0.00 Ac | 0.03 ± 0.01 Aa | 0.18 ± 0.02 Ab | 0.13 ± 0.01 Bab | |
| 0.4 | 0.13 ± 0.01 Aabc | 0.08 ± 0.00 Bc | 0.05 ± 0.00 Aabc | 0.03 ± 0.01 Ba | 0.18 ± 0.01 Ab | 0.12 ± 0.00 Bb | |
| 0.45 | 0.11 ± 0.01 c | 0.04 ± 0.00 abc | 0.16 ± 0.01 b | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Yang, L.; Wang, H.; Fu, X. Comparative Physiochemical Mechanisms of Salt Tolerance between Cornus florida and Cornus hongkongensis subsp. elegans Based on Seed Germination and Seedling Growth. Horticulturae 2022, 8, 1149. https://doi.org/10.3390/horticulturae8121149
Cai M, Yang L, Wang H, Fu X. Comparative Physiochemical Mechanisms of Salt Tolerance between Cornus florida and Cornus hongkongensis subsp. elegans Based on Seed Germination and Seedling Growth. Horticulturae. 2022; 8(12):1149. https://doi.org/10.3390/horticulturae8121149
Chicago/Turabian StyleCai, Mei, Ling Yang, Haowei Wang, and Xiangxiang Fu. 2022. "Comparative Physiochemical Mechanisms of Salt Tolerance between Cornus florida and Cornus hongkongensis subsp. elegans Based on Seed Germination and Seedling Growth" Horticulturae 8, no. 12: 1149. https://doi.org/10.3390/horticulturae8121149
APA StyleCai, M., Yang, L., Wang, H., & Fu, X. (2022). Comparative Physiochemical Mechanisms of Salt Tolerance between Cornus florida and Cornus hongkongensis subsp. elegans Based on Seed Germination and Seedling Growth. Horticulturae, 8(12), 1149. https://doi.org/10.3390/horticulturae8121149





