Extraction, Quantification and Characterization Techniques for Anthocyanin Compounds in Various Food Matrices—A Review
Abstract
1. Introduction
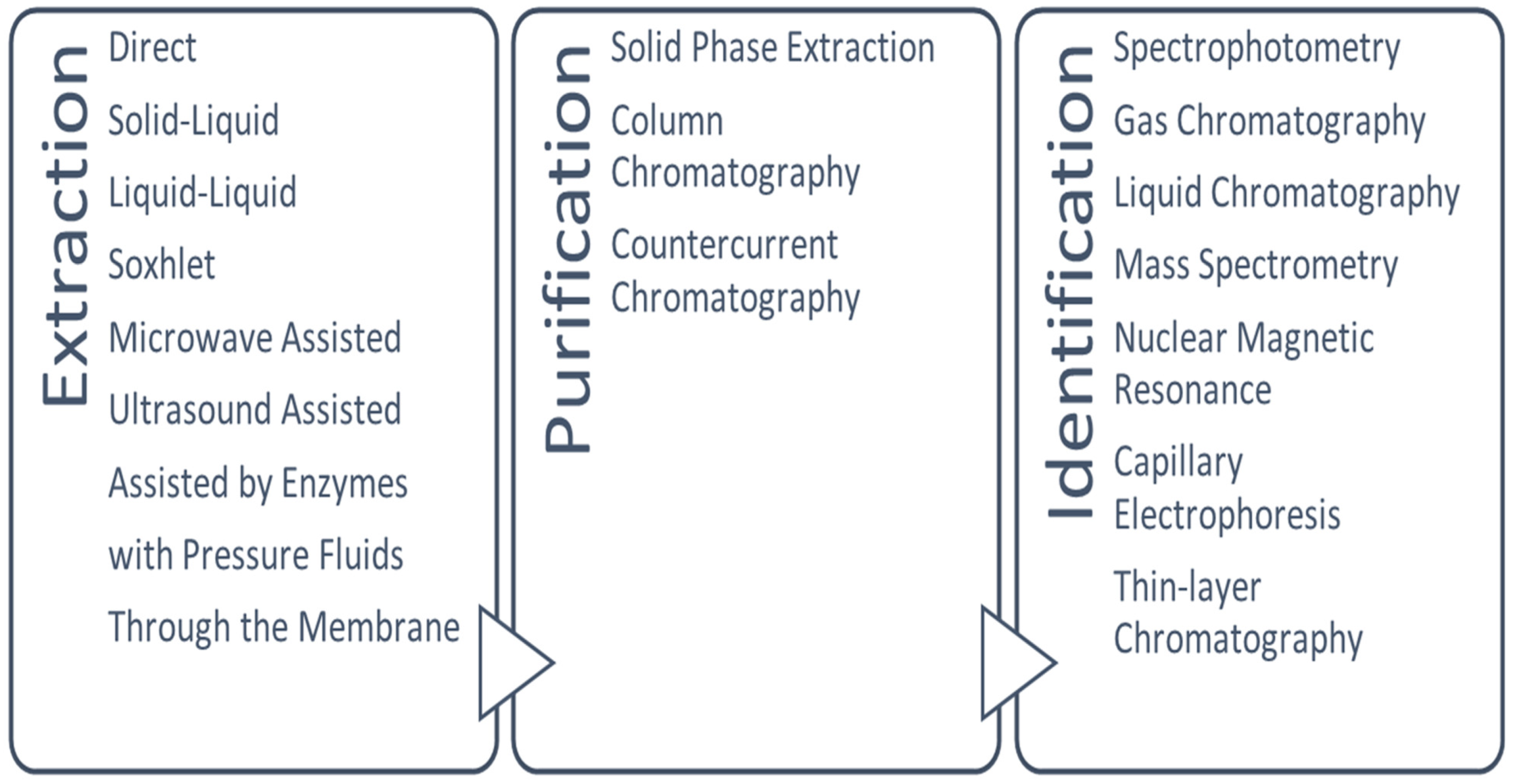
2. Procedures for the Extraction of Anthocyanins from Different Food Matrices
2.1. Preliminary Treatments
2.2. Extraction
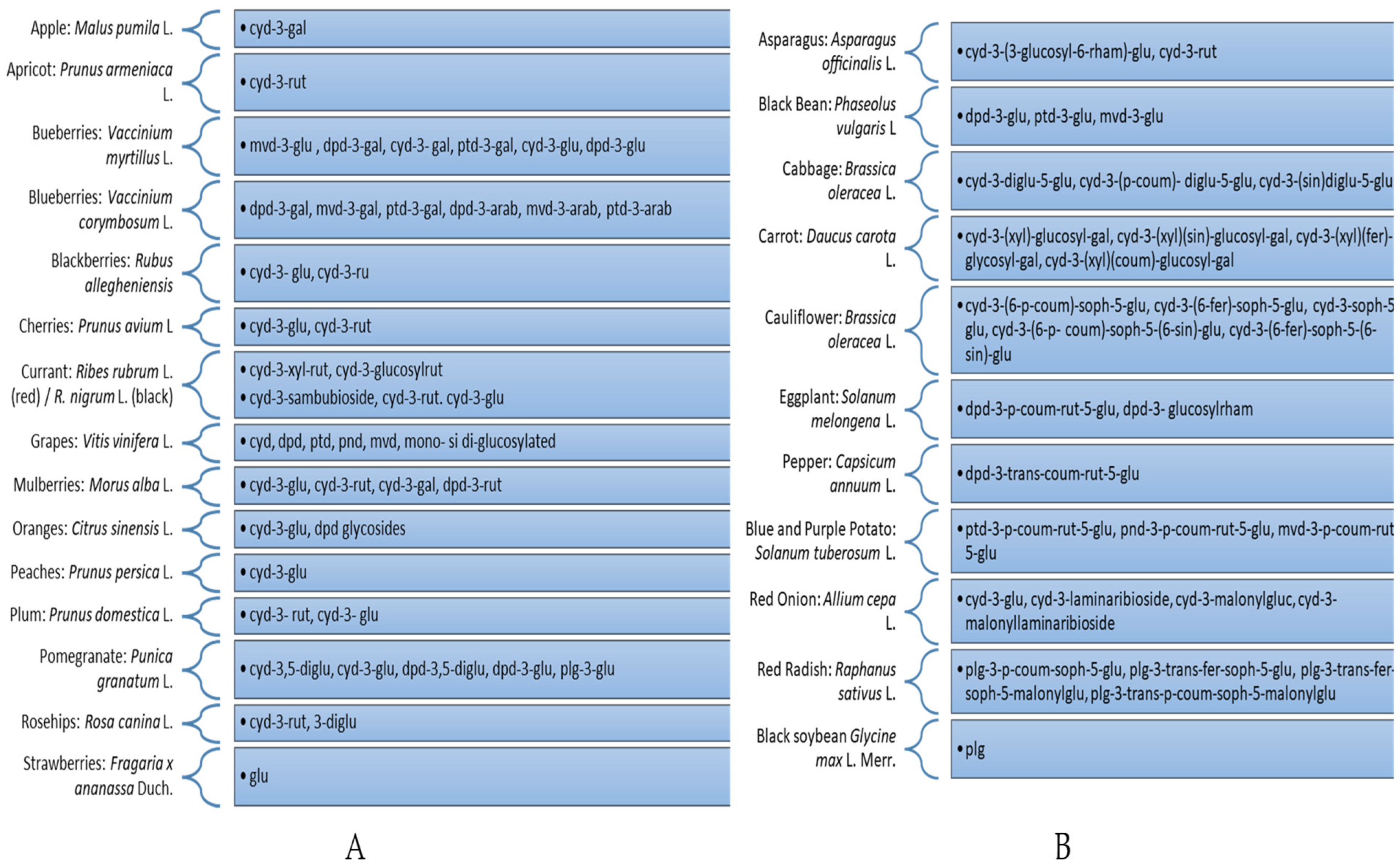
2.2.1. Procedures for the Extraction of Anthocyanins from Fruits
2.2.2. Procedures for the Extraction of Anthocyanins from Vegetables
| Matrix Genus, Species, Variety | Location | Compounds | Detection Method | Ref. |
|---|---|---|---|---|
| Anthocyanins from fruits | ||||
| Blackberries Rubus fruticosus | Iaşi, Romania | cyd-3-O-glu; dpd-3-xilozid; cyd-3-O-arab; cyd-3-malonil-glu; cyd-3-rut | HPLC-ESI-MS | [74] |
| Blackberries Rubus spp. | Samsun, Turkey | cyd-3-glu; cyd-3-rut; plg-3-glu; cyd clorid | HPLC | [75] |
| Blueberries Vaccinium coroymbosum L. | Wollongong, Australia | dpd-3-glu; ptd-3-glu; mvd-3-glu | UHPLC-PDA-ESI-MS | [23] |
| Blueberries Vaccinium cyanococcus | Corvallis, USA | dpd-3-gal; dpd-3-glu; cyd-3-gal; dpd-3-arab; cyd-3-glu; ptd-3-gal; cyd-3-arab; ptd-3-glu; ptd-3-arab; pnd-3-glu; mvd-3-gal; pnd-3-arab; mvd-3-glu; mvd-3-arab | HPLC | [57] |
| Blueberries Vaccinium corymbosum | Huelva, Spain | mvd-gal; pnd-gal; ptd-gal; cyd-gal; dpd-gal; mvd-glu; pnd-glu; cyd-glu; mvd-arb; ptd-arb; cyd-arb; dpd-arb; cyd; mvd | HPLC-DAD | [76] |
| Cherries Prunus cerasus L. Cranberries Vaccinium oxycoccos | Lazig, Turkey | dpd-3-O-glu; cyd-3-O-glu; plg-3-O-glu; mvd-3-O-glu | HPLC-ESI-MS | [38] |
| Grapes Vitis spp. Othello | Iași, Romania | cyd-3-glu-etil-coum; cyd-3-glu-etil-coum; cyd-3-O-gal; cyd-3-O-glu; dpd-3-(6-O-acetilgluc); dpd- 3-O-glu; dpd-3-O-glu; mvd-3-(6-O-acetilgluc) piruvat; mvd-3-glu-8-vinil(epi)catechina; mvd-3-O-glu | HPLC-ESI-MS | [74] |
| Grapes Vitis amurensis | Daze, China | pnd 3,5-β-do-diglu; pnd 3-O-β-d-(6″-acetil) glu 5-β-d-glu; pnd 3- (6″-op-coum)-β-d-glu; cyd 3-O-β-d-glu-5-O-β-d-glu; cyd 5-O-β-d-glu; cyd; dpd 3,5-β-do-diglu; ptd 3,5-β-do-diglu; mvd 3,5-β-do-diglu; ptd 3-(6″-O-trans-p-coum)-β-d-glu; mvd 3-(6″-O-cis-p-coum)-β-d-glu | LC-MS-MS | [47] |
| Grapes Vitis vinifera cv. Plavac mali | Dalmatia, Croatia | dpd-3-O-monoglu; cyd-3-O-monoglu; ptd-3-O-monoglu; pnd-3-O-monoglu; mvd-3-O-monoglu; mvd-3-O-acetilmonoglu; pnd- 3-(6-op coum)monoglu; mvd-3-(6-op-coum)monoglu | HPLC | [48] |
| Pomegranate Punica granatum L. | California, USA | cyd-3-O-pentozid; plg-3-O-glu; cyd-3-O-glu; dpd-3-O-glu; cyd-pentozid-hexozid; cyd-3-O-rut; cyd 3,5 -O-diglu; dpd 3,5-diglu; (epi) afzelchin-dpd-3-O-hexozid; (epi) galocatechin-plg 3-O-hexozid; (epi) afzelchin-dpd-3-O-hexozid; (epi) galocatechin-cyd-3-O-hexozid | HPLC-DAD-ESI-MS | [51] |
| Red pears Red D’Anjou Pyrus communis | Corvallis USA | cyd-3-gal; cyd-3-glu | HPLC | [57] |
| Strawberries Fragaria spp. | Lazig, Turkey | dpd-3-O-glu; cyd-3-O-glu; plg-3-O-glu; mvd-3-O-glu | HPLC-ESI-MS | [64] |
| Strawberries Fragaria × ananassa | Gwangju, Korea | cyd-3-O-glu; plg-3-O-glu; plg-3-O-rut | HPLC | [77] |
| Sweet cherries Prunus avium | Corvallis, USA | cyd-3-glu; cyd-3-rut | HPLC | [57] |
| Anthocyanins from vegetables | ||||
| Black carrot Daucus carota L. ssp. sativus var. atrorubens Alef. | Eregli, Turkey | cyd-3-xyl-glu-gal; cyd-3-xyl-gal; cyd 3-xyl-(caf-glu)-gal; cyd 3-xyl-(p-hydbenz-glu)-gal; cyd 3-xyl-sin-glu-gal; cyd 3-xyl-fer-glu-gal; cyd 3-xyl-p-coum-glu-gal; plg 3-xyl-fer-glu-gal; pnd 3-xyl-fer-glu-gal; cyd 3-xyl-gal + vinylphen; cyd 3-xyl-gal + vinylcatec | LC-DAD-ESI-MS/MS | [78] |
| Corn Zea mays L. | Commercial sources | cyd 3-glu; plg 3-glu; pnd 3-glu; cyd malonyl-glu; plg malonyl-glu; cyd dimalonyl-glu; pnd-malonylglu; plg dimalonyl-glu; pnd dimalonyl-glu | HPLC | [79] |
| Purple carrot Purple 68 Daucus carota ssp. sativus | Cottage Grove, Oregon, USA | cyd-3-(2″-xylose-gal); cyd-3-(2″-xylose-6-glucose-gal); cyd-3-(2″-xylose-6″-fer-glu-gal); cyd-3-(2″-xylose-6″-sin-glu-gal); cyd-3-(2″-xylose-6″-(4-coum)glu-gal) | UHPLC | [80] |
| Red cabbage Brassica oleracea L. var. capitata L. f. rubra | Olszty, Poland | cyd-3-diglu-5-glu; cyd-3-(p-coumaroyl)-diglu-5-glu; cyd -3-(feruloyl)-digluc-5-gluc; cyd-3-(sin)-di-glu-5-glu; cyd-3-(fer)(fer)-di-glu-5-glu; cyd-3-(fer)(sin)-di-glu-5-glu; cyd-3-(sin)(sin)-di-glu-5-glu | HPLC–DAD–MS/MS | [80] |
| Kannapolis, United States | cyd-3-diglu -5glu; cyd -3g-5glu; cyd -3diglu (sin)-5glu; cyd -3triglu(sin)-5glu; cyd -3triglu (pcoum)-5glu; cyd -3triglu(fer)-5glu; cyd -3tri glu(sin)-5glu; cyd -3triglu(pcoum)(sin)-5glu; cyd -2diglu(caf)-5 glu; cyd -3triglu(fer)(sin)-5glu; cyd -3tri glu (sin)(sin)-5 glu; cyd -3 glu (sin)-5 glu; cyd -3di glu (fer)-5 glu; cyd -3diglu (pcoum)-5glu; cyd -3glu(pcoum)-5glu; cyd -3diglu(fer)-5 glu; cyd -3diglu(sin)-5glu; cyd -3glu(fer)-5glu; cyd -3glu(sin)-5glu; peo-3diglu(pcoum)-5glu; cyd -3diglu(pcoum)(sin)-5glu; cyd -3diglu(caf)(fer)-5glu; cyd -3diglu(sin)(fer)-5glu; cyd -3diglu(sin)(caf)-5glu; cyd -3diglu(sin)(sin)-5glu; cyd -3diglu(fer)(fer)-5glu; cyd -3diglu(fer)(sin)-5glu; cyd -3glu(fer)(sin)-5glu; peo-3diglu(pcoum)(sin)-5glu | UPLC-DAD-MS | [56] | |
| Sweet potatoes with purple pulp Ipomoea batatas | Haysville, USA | cyd-3-soph-5-glu; pnd 3-soph-5-glu; cyd 3-soph-5-glu; cyd-3-soph-5-glu; pnd 3-soph-5-glu; pnd 3-soph-5-glu- 5-gluc; cyd 3-soph-5-glu; cyd 3-(6,6′-di-caf--soph)-5-glu; cyd 3-(6,6′-caf-pidroxibenzoil soph)-5-glu; cyd 3-(6, 6′-caf-fer-soph)-5-glu; pnd 3-(6,6′-di-caf-soph)-5-glu; pnd 3-(6,6′--caf-pidroxibenzoil-soph)-5-glu; pnd 3-(6,6′--caf-fer-soph)-5-glu; pnd 3-caf-p-cum-soph-5-glu | HPLC-MS/MS | [69] |
| Tomato Solanum lycopersicum L. | Viterbo, Italy | petanin; negretein | HPLC/NMR | [72] |
| Anthocyanins from cereals | ||||
| Black rice Oryza sativa L. ‘Violet Nori’ | Collobiano, Italy | cyd-3,5-diglu; cyd-3-glu; cyd-3-rut; pnd-3-glu | HPLC | [81,82] |
| Purple barley Hordeum vulgare L. | Tibet | cyd; cyd acetyl gal; cyd malonyl glu; cyd succinyl glu; cyd 3-O-glu; plg succinyl glu; pnd acetyl gal | UPLC | [83] |
| Sweet corn Zea mays L. | Guangzhou, China | cyd, plg, pnd | HPLC | [84] |
| Sweet purple/reddish-purple corn; purple/purple blue/blue corn Zea mays L. | Gatton, Australia | cyd-3-O-glu; cyd-3- malonylglu; plg-3 -O-glu, plg-3- (dimalonylglu); pnd-3 -O-gluc | UHPLC-DAD-MS | [85] |
2.2.3. Procedures for the Extraction of Anthocyanins from Cereals
2.3. Purification
3. Methods for the Quantification of Anthocyanins
- High-performance liquid chromatography (HPLC), which requires a short analysis time and a small sample, but the results must be compared with a standard.
- Liquid chromatography–mass spectrometry (HPLC-MS), which is widely used for the qualitative analysis of anthocyanins with the identification of molecular weight and the structure of anthocyanins, with effective results in the identification of anthocyanins. Therefore, due to its ability to ionize and vaporize labile and complex molecules, mass spectrometry (MS) is a significant step in analyzing anthocyanins [92].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. Bioaccess. Bioavailab. Food Compon. Contam. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Zhang, J.; Celli, G.B.; Brooks, S.L. Natural sources of anthocyanins. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health; Royal Society of Chemistry: London, UK, 2019; pp. 1–33. [Google Scholar]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Koo, I.S.; Song, O.W.; Chun, O.K. Food Matrix Affecting Anthocyanin Bioavailability: Review. Curr. Med. Chem. 2010, 18, 291–300. [Google Scholar] [CrossRef]
- Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients 2021, 13, 2831. [Google Scholar] [CrossRef]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2013; p. 362. [Google Scholar]
- Zhang, J.; Singh, R.; Quek, S. Extraction of anthocyanins from natural sources—Methods and commercial considerations. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health; Royal Society of Chemistry: London, UK, 2019; pp. 77–105. [Google Scholar]
- Nistor, M.; Pop, R.; Daescu, A.; Pintea, A.; Socaciu, C.; Rugina, D. Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview. Molecules 2022, 27, 4254. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Teng, Z.; Jiang, X.; He, F.; Bai, W. Qualitative and Quantitative Methods to Evaluate Anthocyanins. eFood 2020, 1, 339–346. [Google Scholar] [CrossRef]
- Farooq, S.; Shah, M.A.; Siddiqui, M.W.; Dar, B.N.; Mir, S.A.; Ali, A. Recent trends in extraction techniques of anthocyanins from plant materials. J. Food Meas. Charact. 2020, 14, 3508–3519. [Google Scholar] [CrossRef]
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Int. 2004, 87, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Tan, J. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Boch, K.; Schieber, A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Collaborators: Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo-Herrera, G.; Mojica, L. Black Bean Anthocyanin-Rich Extract from Supercritical and Pressurized Extraction Increased In Vitro Antidiabetic Potential, While Having Similar Storage Stability. Foods 2020, 9, 655. [Google Scholar] [CrossRef]
- Jafari, S.M.; Mahdavee Khazaei, K.; Assadpour, E. Production of a natural color through microwave-assisted extraction of saffron tepal’s anthocyanins. Food Sci. Nutr. 2019, 7, 1438–1445. [Google Scholar] [CrossRef]
- Chandra Singh, M.; Probst, Y.; Price, W.E.; Kelso, C. Relative comparisons of extraction methods and solvent composition for Australian blueberry anthocyanins. J. Food Compos. Anal. 2021, 105, 104232. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Martín, J.; Navas, M.J.; Jiménez-Moreno, A.M.; Asuero, A.G. Anthocyanin Pigments: Importance, Sample Preparation and Extraction; IntechOpen: London, UK, 2017. [Google Scholar]
- Navas, M.J.; Jiménez-Moreno, A.M.; Bueno, J.M.; Sáez-Plaza, P.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part IV: Extraction of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, L.; Bai, W.; Chen, W.; Chen, F.; Shu, C. Anthocyanins: Chemistry, Processing & Bioactivity; Springer Nature: London, UK, 2021; p. 451. [Google Scholar]
- Deng, L.Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.H.; Wang, J.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality properties and antioxidant activity of seven strawberry (Fragaria x ananassa duch) cultivars. Sci. Hortic. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.M.; Tomuta, I.; Popa, D.S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.; Yang, B.; Sainio, T. Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod. Process. 2016, 99, 136–146. [Google Scholar] [CrossRef]
- Jampani, C.; Naik, A.; Raghavarao, K.S.M.S. Purification of anthocyanins from jamun (Syzygium cumini L.) employing adsorption. Sep. Purif. Technol. 2014, 125, 170–178. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Karaaslan, N.M.; Yaman, M. Determination of anthocyanins in cherry and cranberry by high-performance liquid chromatography–electrospray ionization–mass spectrometry. Eur. Food Res. Technol. 2016, 242, 127–135. [Google Scholar] [CrossRef]
- Nankar, A.N.; Dungan, B.; Paz, N.; Sudasinghe, N.; Schaub, T.; Holguin, F.O.; Pratt, R.C. Quantitative and qualitative evaluation of kernel anthocyanins from southwestern United States blue corn. J. Sci. Food Agric. 2016, 96, 4542–4552. [Google Scholar] [CrossRef]
- Mazewski, C.; Liang, K.; Gonzalez de Mejia, E. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Li, Q.; West, L.; West, M.; Gonzalez de Mejia, E. Anthocyanin condensed forms do not affect color or chemical stability of purple corn pericarp extracts stored under different pHs. Food Chem. 2017, 232, 639–647. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The effect of different pressurized fluids on the extraction of anthocyanins and total phenolics from cranberry pomace. J. Supercrit. Fluids 2021, 175, 105279. [Google Scholar] [CrossRef]
- Gómez-García, R.; Martínez-Ávila, G.C.G.; Aguilar, C.N. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. 3 Biotech 2012, 2, 297–300. [Google Scholar] [CrossRef]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L.) byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Monrad, J.K.; Suárez, M.; Motilva, M.J.; King, J.W.; Srinivas, K.; Howard, L.R. Extraction of anthocyanins and flavan-3-ols from red grape pomace continuously by coupling hot water extraction with a modified expeller. Food Res. Int. 2014, 65, 77–87. [Google Scholar] [CrossRef]
- Oliveira, J.; Alhinho da Silva, M.; Teixeira, N.; de Freitas, V.; Salas, E. Screening of Anthocyanins and Anthocyanin-Derived Pigments in Red Wine Grape Pomace Using LC-DAD/MS and MALDI-TOF Techniques. J. Agric. Food Chem. 2015, 63, 7636–7644. [Google Scholar] [CrossRef]
- Ji, M.; Li, C.; Li, Q. Rapid separation and identification of phenolics in crude red grape skin extracts by high performance liquid chromatography coupled to diode array detection and tandem mass spectrometry. J. Chromatogr. A 2015, 1414, 138–146. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharmacal Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef]
- Zhang, S.L.; Deng, P.; Xu, Y.C.; Lü, S.W.; Wang, J.J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- Šulc, M.; Kotíková, Z.; Paznocht, L.; Pivec, V.; Hamouz, K.; Lachman, J. Changes in anthocyanidin levels during the maturation of color-fleshed potato (Solanum tuberosum L.) tubers. Food Chem. 2017, 237, 981–988. [Google Scholar] [CrossRef]
- Valencia-Arredondo, J.A.; Hernández-Bolio, G.I.; Cerón-Montes, G.I.; Castro-Muñoz, R.; Yáñez-Fernández, J. Enhanced process integration for the extraction, concentration and purification of di-acylated cyanidin from red cabbage. Sep. Purif. Technol. 2020, 238, 116492. [Google Scholar] [CrossRef]
- Canuto, G.A.B.; Oliveira, D.R.; da Conceição, L.S.M.; Farah, J.P.S.; Tavares, M.F.M. Development and validation of a liquid chromatography method for anthocyanins in strawberry (Fragaria spp.) and complementary studies on stability, kinetics and antioxidant power. Food Chem. 2016, 192, 566–574. [Google Scholar] [CrossRef]
- Fu, X.; Du, Y.; Zou, L.; Liu, X.; He, Y.; Xu, Y.; Li, L.; Luo, Z. Acidified glycerol as a one-step efficient green extraction and preservation strategy for anthocyanin from blueberry pomace: New insights into extraction and stability protection mechanism with molecular dynamic simulation. Food Chem. 2022, 390, 133226. [Google Scholar] [CrossRef]
- Silva, D.T.D.; Pauletto, R.; Cavalheiro, S.D.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Silva, C.d.B.D.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT–Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef]
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized extraction of anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ cherries. Food Chem. 2018, 256, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, C.G.; Destandau, E.; Zubrzycki, S.; Elfakir, C. Sweet cherries anthocyanins: An environmental friendly extraction and purification method. Sep. Purif. Technol. 2012, 100, 51–58. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers based on natural deep eutectic mixtures to enhance anthocyanins isolation from grape pomace by pressurized hot water extraction. LWT 2021, 149, 111889. [Google Scholar] [CrossRef]
- Fang, Z.; Lin-Wang, K.; Jiang, C.; Zhou, D.; Lin, Y.; Pan, S.; Espley, R.V.; Ye, X. Postharvest temperature and light treatments induce anthocyanin accumulation in peel of ‘Akihime’ plum (Prunus salicina Lindl.) via transcription factor PsMYB10.1. Postharvest Biol. Technol. 2021, 179, 111592. [Google Scholar] [CrossRef]
- Kalantari, S.; Roufegarinejad, L.; Pirsa, S.; Gharekhani, M.; Tabibiazar, M. β-Cyclodextrin-assisted extraction of phenolic compounds from pomegranate (Punica granatum L.) peel: A new strategy for anthocyanin copigmentation. LWT 2021, 151, 112136. [Google Scholar] [CrossRef]
- Karaaslan, N.M.; Yaman, M. Anthocyanin profile of strawberry fruit as affected by extraction conditions. Int. J. Food Prop. 2017, 20, S2313–S2322. [Google Scholar] [CrossRef]
- Farias, C.A.A.; Moraes, D.P.; Neuenfeldt, N.H.; Zabot, G.L.; Emanuelli, T.; Barin, J.S.; Ballus, C.A.; Barcia, M.T. Microwave hydrodiffusion and gravity model with a unique hydration strategy for exhaustive extraction of anthocyanins from strawberries and raspberries. Food Chem. 2022, 383, 132446. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Topolska, J.; Honke, J. Anthocyanins profile and antioxidant capacity of red cabbages are influenced by genotype and vegetation period. J. Funct. Foods 2014, 7, 201–211. [Google Scholar] [CrossRef]
- Strauch, R.C.; Mengist, M.F.; Pan, K.; Yousef, G.G.; Iorizzo, M.; Brown, A.F.; Lila, M.A. Variation in anthocyanin profiles of 27 genotypes of red cabbage over two growing seasons. Food Chem. 2019, 301, 125289. [Google Scholar] [CrossRef]
- Yigit, U.; Turabi Yolacaner, E.; Hamzalioglu, A.; Gokmen, V. Optimization of microwave-assisted extraction of anthocyanins in red cabbage by response surface methodology. J. Food Process. Preserv. 2022, 46, 16120. [Google Scholar] [CrossRef]
- Su, X.; Griffin, J.; Xu, J.; Ouyang, P.; Zhao, Z.; Wang, W. Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon 2019, 5, e01964. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato-P40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Cheng, H.; Wang, Q.; Wang, X.; Liao, X.; Xu, Z. Enhanced water extraction with high-pressure carbon dioxide on purple sweet potato pigments: Comparison to traditional aqueous and ethanolic extraction. J. CO2 Util. 2020, 40, 101188. [Google Scholar] [CrossRef]
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Mita, G.; Andersen, Ø.M. Nutraceutical Characterization of Anthocyanin-Rich Fruits Produced by “Sun Black” Tomato Line. Front. Nutr. 2019, 6, 133. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Zhou, Z.; Qiu, Z.; Cui, X. Rapid analysis of anthocyanin and its structural modifications in fresh tomato fruit. Food Chem. 2020, 333, 127439. [Google Scholar] [CrossRef]
- Paun, N.; Botoran, O.R.; Niculescu, V.C. Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study. Appl. Sci. 2022, 12, 936. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp.) using natural deep eutectic solvents. LWT 2022, 158, 113184. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; Varo, M.Á.; Mérida, J.; Serratosa, M.P. Influence of drying processes on anthocyanin profiles, total phenolic compounds and antioxidant activities of blueberry (Vaccinium corymbosum). LWT 2020, 120, 108931. [Google Scholar] [CrossRef]
- Lee, C.; Lee, J.; Lee, J. Relationship of fruit color and anthocyanin content with related gene expression differ in strawberry cultivars during shelf life. Sci. Hortic. 2022, 301, 111109. [Google Scholar] [CrossRef]
- Polat, S.; Guclu, G.; Kelebek, H.; Keskin, M.; Selli, S. Comparative elucidation of colour, volatile and phenolic profiles of black carrot (Daucus carota L.) pomace and powders prepared by five different drying methods. Food Chem. 2022, 369, 130941. [Google Scholar] [CrossRef] [PubMed]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of Anthocyanin Composition and Concentration in Diverse Maize Germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.B.; da Peña Hamparsomian, M.J.; Gonzalez, R.E.; Denoya, G.I.; Dominguez, D.L.E.; Barboza, K.; Iorizzo, M.; Simon, P.W.; Vaudagna, S.R.; Cavagnaro, P.F. Physicochemical properties, degradation kinetics, and antioxidant capacity of aqueous anthocyanin-based extracts from purple carrots compared to synthetic and natural food colorants. Food Chem. 2022, 387, 132893. [Google Scholar] [CrossRef]
- Catena, S.; Turrini, F.; Boggia, R.; Borriello, M.; Gardella, M.; Zunin, P. Effects of different cooking conditions on the anthocyanin content of a black rice (Oryza sativa L. ‘Violet Nori’). Eur. Food Res. Technol. 2019, 245, 2303–2310. [Google Scholar] [CrossRef]
- Turrini, F.; Boggia, R.; Leardi, R.; Borriello, M.; Zunin, P. Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves. Molecules 2018, 23, 844. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2021, 339, 127849. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Li, W.; Wen, T.; Li, T.; Guo, X.B.; Liu, R.H. Anthocyanin accumulation, biosynthesis and antioxidant capacity of black sweet corn (Zea mays L.) during kernel development over two growing seasons. J. Cereal Sci. 2020, 95, 103065. [Google Scholar] [CrossRef]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Optimisation of extraction procedure and development of LC–DAD–MS methodology for anthocyanin analysis in anthocyanin-pigmented corn kernels. Food Chem. 2020, 319, 126515. [Google Scholar] [CrossRef]
- Yi, J.; Qiu, M.; Zhu, Z.; Dong, X.; Andrew Decker, E.; McClements, D.J. Robust and recyclable magnetic nanobiocatalysts for extraction of anthocyanin from black rice. Food Chem. 2021, 364, 130447. [Google Scholar] [CrossRef] [PubMed]
- Constantin, O.; Skrt, M.; Ulrih, N.; Rapeanu, G. Anthocyanins profile, total phenolics and antioxidant activity of two Romanian red grape varieties: Feteasca neagra and Babeasca neagra (Vitis vinifera). Chem. Pap. 2015, 69, 1573–1581. [Google Scholar] [CrossRef]
- Ferreiro-González, M.; Carrera, C.; Ruiz-Rodríguez, A.; Barbero, G.F.; Ayuso, J.; Palma, M.; Barroso, C.G. A New Solid Phase Extraction for the Determination of Anthocyanins in Grapes. Molecules 2014, 19, 21398–21410. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, J.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod. Process. 2012, 90, 615–623. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef]
| Anthocyanidin | Abbreviation | Chemical Structure | Basic Color |
|---|---|---|---|
| Cyanidin | cyd | 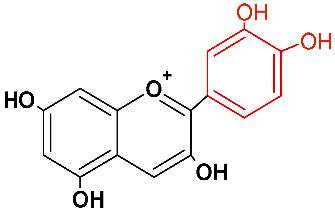 | orange red |
| Delphinidin | dpd | 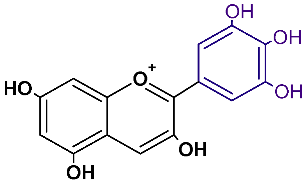 | red, blue |
| Petunidin | ptd | 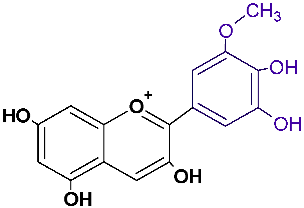 | red, blue |
| Peonidin | pnd | 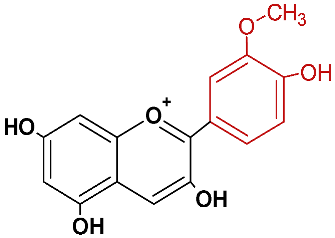 | orange red |
| Pelargonidin | plg | 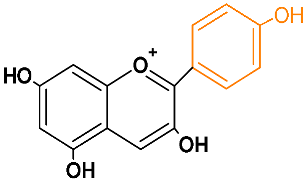 | orange |
| Malvidin | mvd | 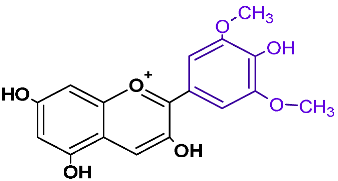 | red, blue |
| Matrix | Extraction Methods | Extraction Conditions | Solvents | Ref. |
|---|---|---|---|---|
|
Aronia Aronia melanocarpa | UAE | sonication 216W, T = 70 °C, τ = 45.6 min | 50% EtOH | [36] |
|
Beans black Phaseolus vulgaris L. | PLE | T = 40, 50 and 60 °C, P = 100, 200 and 250 bar | acidified distilled H2O, 50% EtOH, 70% EtOH | [21] |
| EFS | T = 40, 50 and 60 °C, P = 160, 200 and 300 bar | co-solvents: acidified distilled H2O, 10% EtOH, 50% EtOH | ||
|
Blueberries Vaccinium coroymbosum L. | UAE | T = 15 °C, τ = 30 min | solvent mixtures (MeOH:H2O, EtOH:H2O) at pH 2.0 or 3.0 corrected with concentrated HCl. | [23] |
| UAE + shredding | τ = 44 sec | |||
| UAE + shredding/maceration | NR | |||
| Blueberry marc Vaccinium ashei | CSE | T = 61 °C, τ = 35 min | EtOH (70%), HCl (0.01%) | [37] |
| UAE | f = 24 kHz, T = 61 °C, τ = 24 min | |||
| Cherrie Prunus cerasus L. and cranberries Vaccinium oxycoccos | CSE | room temperature | acidified ACN | [38] |
|
Corn Zea mays L. | CS | shaking for 1 min, refrigeration for 60 min | acidified MeOH | [39] |
| CS | shaking T = 4 °C, τ = 24 h in the dark | H2O | [40] | |
| T = 4 °C, τ = 20–24 h in the dark | 2% FA | |||
| extraction in water | τ = 5 min, P = 1500 psi | H2O | [41] | |
|
Cranberry marc Vaccinium macrocarpon | CSE | room temperature/τ = 15 min | 98% MeOH with 2% HCl | [42] |
| PLE | T = 120, 140, or 160 °C, P = 50 or 200 bar | 100% EtOH; ultrapure H2O:EtOH (30 or 70%); EtOH, milli-Q H2O/5% citric acid | ||
|
Grape marc Vitis vinifera | EAE | T = 40 °C, τ = 0–48 h | Acetate buffer 0.2 M (pH 3.5) | [43] |
| maceration | T = 4 °C, τ = 60 min | MeOH (0.1% HCl), solvent/solid ratio 50:1 | [44] | |
| expeller extraction | water flow (20–65 mL/min), water bath temperature (25–140 °C), expeller shaft temperature (50–100 °C), screw rotational speed, controlled by a frequency dimmer attached to the expulsor (20–60 Hz) | H2O at 100 °C | [45] | |
| CSE | T = 23.5 °C | MeOH:H2O:FA (60:37:3) | ||
| sonication | τ = 30 min/18 min in an ice bath | H2O:MeOH:acetone 3.5:1.5:5 acidified with HCl (0.01 M) | [46] | |
|
Grapes (skin) Vitis vinifera | CSE/UAE | CSE—τ = 24 h in the dark, room temperature UAE—f = 60 kHz, τ = 25 min | EtOH (70%) | [47] |
| CSE(DES)/UAE/MAE | CSE(DES)—τ = 12 h, room temperature MAE—T = 50–90 °C, τ = 15–90 min, microwave output power = 100 W UAE—T = 30–90 °C, τ = 15–90 min, f—35 kHz | H2O:MeOH (70:30) and acidified MeOH solution (MeOH:H2O:12 M HCl, 70:29:1, pH = 1.25) | [48] | |
| CSE(DES)/UAE | CSE (DES)—room temperature, τ = 45 min UAE—80% MeOH for 45 min using stirring, heating, stirring methods in the vortex and stirring + heating. 600 rpm without heating (stirring) or with heating T = 60 °C (shaking + heating). | H2O:MeOH (100%, 80%), EtOH (100%, 70%) | [49] | |
|
Onions red, yellow and white Allium cepa L. | UAE | ultrasonic power = 700 W, room temperature, τ = 30 min. | 80% EtOH (EtOH:H2O/80:20) with 0.1% HCl | [50] |
|
Pomegranate (seeds) Punica granatum L. | UAE | T = 30 °C, τ = 20 min | 70% acetone | [51] |
|
Potatoes Solanum tuberosum L. | sonication | sonication for τ = 5 min, drying in forced air furnace at T = 90 °C, τ = 3 h | HCl 2.7 M in MeOH | [52] |
|
Red cabbage Brassica oleracea | shaking in an orbital agitator | T = 24 ± 2 °C, 90 rpm, τ = 12 h | H2O:H3PO4 0.05 M (1:10) | [53] |
|
Strawberries Fragaria spp. | UAE | T = 20 °C, τ = 10min | 0.2% HCl in MeOH | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, O.E.; Istrati, D.I. Extraction, Quantification and Characterization Techniques for Anthocyanin Compounds in Various Food Matrices—A Review. Horticulturae 2022, 8, 1084. https://doi.org/10.3390/horticulturae8111084
Constantin OE, Istrati DI. Extraction, Quantification and Characterization Techniques for Anthocyanin Compounds in Various Food Matrices—A Review. Horticulturae. 2022; 8(11):1084. https://doi.org/10.3390/horticulturae8111084
Chicago/Turabian StyleConstantin, Oana Emilia, and Daniela Ionela Istrati. 2022. "Extraction, Quantification and Characterization Techniques for Anthocyanin Compounds in Various Food Matrices—A Review" Horticulturae 8, no. 11: 1084. https://doi.org/10.3390/horticulturae8111084
APA StyleConstantin, O. E., & Istrati, D. I. (2022). Extraction, Quantification and Characterization Techniques for Anthocyanin Compounds in Various Food Matrices—A Review. Horticulturae, 8(11), 1084. https://doi.org/10.3390/horticulturae8111084