An Assessment of Moringa (Moringa oleifera L.) Seed Extract on Crop Water Productivity and Physico-Biochemical Properties of Cancer Bush (Sutherlandia frutescens L.) under Deficit Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Microclimate and Photosynthetic Active Radiation (PAR) Quantifications
2.3. Irrigation Water Applied
2.4. Moringa Seed Extract (MSE) Preparation and Treatments
2.5. Experimental Design and Plant Management
2.6. Measurements of Plant Growth and Yield Attributes and Crop Water Productivity (CWP)
2.7. Measurement of Relative Water Content (RWC)
2.8. Measurement of Membrane Stability Index (MSI)
2.9. Determination of Leaf Photosynthetic Pigments
2.10. Sample Extraction
2.10.1. 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.10.2. 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Assay
2.11. Statistical Analysis
3. Results
3.1. Microclimate and Photosynthetic Active Radiation (PAR)
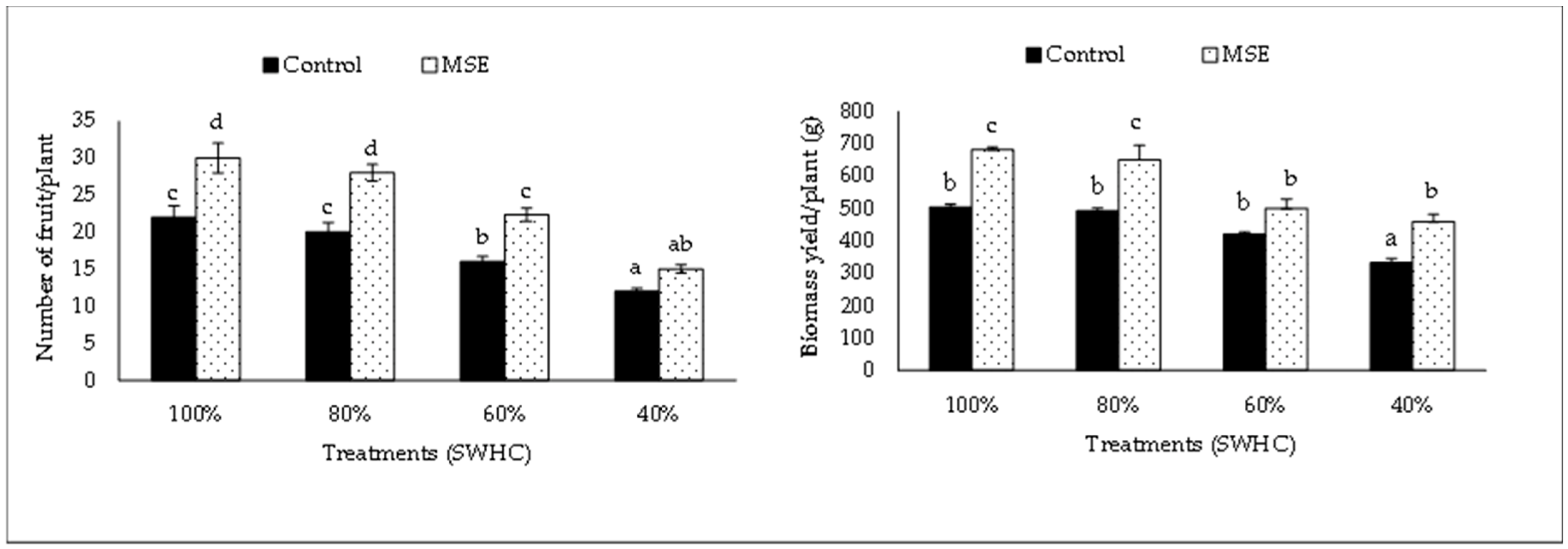
3.2. Growth and Yield Parameters
3.3. Membrane Stability Index (MSI), Relative Water Content (RWC) and Crop Water Productivity (CWP)
3.4. Photosynthetic Pigments
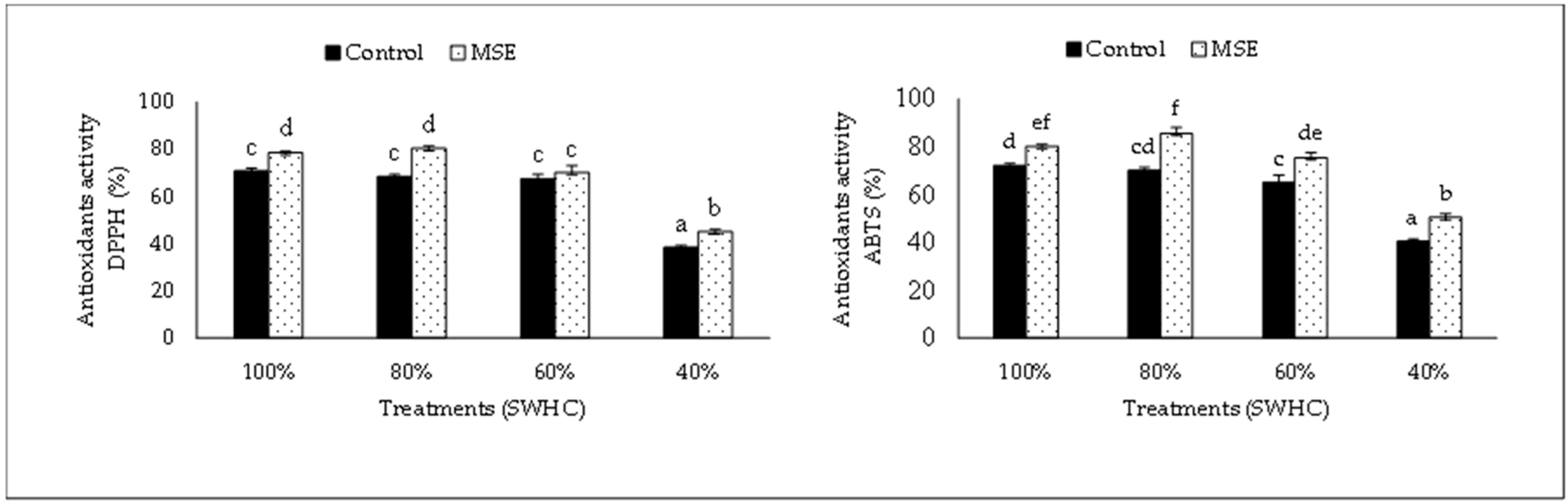
3.5. Antioxidants Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zonyane, S.; Fawole, O.A.; La Grange, C.; Stander, M.A.; Opara, U.L.; Makunga, N.P. The implication of chemotypic variation on the anti-oxidant and anti-cancer activities of Sutherlandia frutescens (L.) R. Br. (Fabaceae) from different geographic locations. Antioxidants 2020, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Aboyade, O.M.; Styger, G.; Gibson, D.; Hughes, G. Sutherlandia frutescens: The meeting of science and traditional knowledge. J. Altern. Complement. Med. 2014, 20, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.; Smit, T.; Willers, C.; Svitina, H.; Calitz, C.; Wrzesinski, K. Anticancer potential of Sutherlandia frutescens and Xysmalobium undulatum in LS180 colorectal cancer mini-tumors. Molecules 2021, 26, 605. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.L.; Waskom, R.M.; Niu, Y.; Siddique, K.H. Regulated deficit irrigation for crop production under drought stress. A review. Agron. Sustain. Dev. 2016, 36, 3. [Google Scholar] [CrossRef]
- Masih, I.; Maskey, S.; Mussá, F.E.F.; Trambauer, P. A review of droughts on the African continent: A geospatial and long-term perspective. Hydrol. Earth Syst. Sci. 2014, 18, 3635–3649. [Google Scholar] [CrossRef]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J. Plant Interact. 2014, 9, 712–721. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Pandey, V.; Patra, D.D. Crop productivity, aroma profile and antioxidant activity in Pelargonium graveolens L’Hér. under integrated supply of various organic and chemical fertilizers. Ind. Crops Prod. 2015, 67, 257–263. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Memon, S.A.; Sheikh, I.A.; Talpur, M.A.; Mangrio, M.A. Impact of deficit irrigation strategies on winter wheat in semi-arid climate of sindh. Agric. Water Manag. 2021, 243, 106389. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Xuan, T.D. Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with moringa leaf extract. Arch. Agron. Soil Sci. 2017, 63, 687–699. [Google Scholar] [CrossRef]
- Behera, B.; Supraja, K.V.; Paramasivan, B. Integrated microalgal biorefinery for the production and application of biostimulants in circular bioeconomy. Bioresour. Technol. 2021, 339, 125588. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Curá, J.A. Role of beneficial microorganisms and salicylic acid in improving rainfed agriculture and future food safety. Microorganisms 2020, 8, 1018. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Elrys, A.S.; Rady, M.M. Integrative moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals-contaminated saline soil. Ecotoxico. Envirn. Saf. 2019, 169, 50–60. [Google Scholar] [CrossRef]
- Saleem, A.; Saleem, M.; Akhtar, M.F. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. S. Afri. J. Bot. 2020, 128, 246–256. [Google Scholar] [CrossRef]
- Khan, S.; Basit, A.; Hafeez, M.B.; Irshad, S.; Bashir, S.; Bashir, S.; Maqbool, M.M.; Saddiq, M.S.; Hasnain, Z.; Aljuaid, B.S.; et al. Moringa leaf extract improves biochemical attributes, yield and grain quality of rice (Oryza sativa L.) under drought stress. PLoS ONE 2021, 16, e0254452. [Google Scholar] [CrossRef]
- Abdalla, M.M. Boosting the growth of rocket plants in response to the application of Moringa oleifera extracts as a biostimulant. Life Sci. J. 2014, 11, 1113–1121. [Google Scholar]
- Al Taweel, S.K.; Al-Anbari, I.H. Moringa olifera: A review on the phytochemical screening, proximate analysis, medicinal, nutritional, and plant biostimulants values of its leaves, pods, seeds and roots. Plant Arch. 2019, 19, 1612–1622. [Google Scholar]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive investigation of Moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Selahle, K.M.; Sivakumar, D.; Jifon, J.; Soundy, P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. 2015, 173, 951–956. [Google Scholar] [CrossRef]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Shu, L.Z.; Liu, R.; Min, W.; Wang, Y.S.; Hong-mei, Y.; Zhu, P.F.; Zhu, J.R. Regulation of soil water threshold on tomato plant growth and fruit quality under alternate partial root-zone drip irrigation. Agric. Water Manag. 2020, 238, 106200. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.W.; Checchio, M.V.; dos Reis, A.R.; de Cássia Alves, R.; Tezzoto, T.; Gratão, P. Selenium restricts cadmium uptake and improve micronutrients and proline concentration in tomato fruits. Biocatal. Agric. Biotechnol. 2019, 18, 101057. [Google Scholar] [CrossRef]
- Khatri, D.; Panigrahi, J.; Prajapati, A.; Bariya, H. Attributes of Aloe vera gel and chitosan treatments on the quality and biochemical traits of post-harvest tomatoes. Sci. Hortic. 2020, 259, 2–8. [Google Scholar] [CrossRef]
- Ehteshami, S.; Abdollahi, F.; Ramezanian, A.; Dastjerdi, A.M.; Rahimzadeh, M. Enhanced chilling tolerance of pomegranate fruit by edible coatings combined with malic and oxalic acid treatments. Sci. Hortic. 2019, 250, 388–398. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Odindo, A.O.; Workneh, T.S.; Mathaba, N. Levels of anti-oxidants in different parts of moringa (Moringa oleifera) seedling. Afri. J. Agric. Res. 2011, 6, 5123–5132. [Google Scholar]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Akladious, S.A.; El-Beltagi, H.S. Mitigation the harmful effect of salt stress on physiological, biochemical and anatomical traits by foliar spray with trehalose on wheat cultivars. Fresenius Environ. Bull. 2018, 27, 7054–7065. [Google Scholar]
- Zulfiqar, F.; Younis, A.; Finnegan, P.M.; Ferrante, A. Comparison of soaking corms with moringa leaf extract alone or in combination with synthetic plant growth regulators on the growth, physiology and vase life of sword lily. Plants 2020, 9, 1590. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. Combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Hai, N.N.; Chuong, N.N.; Tu, N.H.C.; Kisiala, A.; Hoang, X.L.T.; Thao, N.P. Role and regulation of cytokinins in plant response to drought stress. Plants 2020, 9, 422. [Google Scholar] [CrossRef]
- Darvishan, M.; Tohidi-Moghadam, H.R.; Zahedi, H. The effects of foliar application of ascorbic acid (vitamin C) on physiological and biochemical changes of corn (Zea mays L.) under irrigation withholding in different growth stages. Maydica 2013, 58, 195–200. [Google Scholar]
- Khan, N.; Bano, A.; Ali, S.; Babar, M. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Jiang, H.; Shui, Z.; Xu, L.; Yang, Y.; Li, Y.; Yuan, X.; Shang, J.; Asghar, M.A.; Wu, X.; Yu, L.; et al. Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol. Biochem. 2020, 150, 209–221. [Google Scholar] [CrossRef]
- Falk, J.; Munne-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [PubMed]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential role for phytol kinase and tocopherol in tolerance to combined light and temperature stress in tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef]
- De Vargas, F.S.; Almeida, P.; de Boleti, A.P.A.; Pereira, M.M.; de Souza, T.P.; de Vasconcellos, M.C.; Nunez, C.V.; Pohlit, A.M.; Lima, E.S. Antioxidant activity and peroxidase inhibition of Amazonian plants extracts traditionally used as anti-inflammatory. BMC Complement. Altern. Med. 2016, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Stasolla, C.; Katahira, R.; Thorpe, T.A.; Ashihara, H. Purine and pyrimidine nucleotide metabolism in higher plants. J. Plant Physiol. 2003, 160, 1271–1295. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.S. Antiviral roles of abscisic acid in plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Del Buono, D. Use of a biostimulant to mitigate salt stress in maize plants. Agronomy 2021, 11, 1755. [Google Scholar] [CrossRef]
| Air Temperature (°C) | Relative Humidity (%) | Photosynthetic Active Radiation (μmol m−2 s−1) |
|---|---|---|
| 21.08 ± 0.60 | 43.01 ± 0.57 | 234.07 ± 0.07 |
| Component | Unit | Value |
|---|---|---|
| Protein | g 100 g−1 | 30.44 ± 1.11 |
| Total free amino acids | 28.11 ± 0.56 | |
| Free proline | 0.25 ± 0.03 | |
| Soluble sugars | 10.11 ± 0.37 | |
| Vitamin B1 | mg 100 g−1 | 0.12 ± 0.04 |
| Vitamin B2 | 0.04 ± 0.01 | |
| Vitamin B3 | 0.12 ± 0.04 | |
| Vitamin C | 2.92 ± 0.16 | |
| Vitamin E | 490.35 ± 5.47 | |
| Calcium (Ca) | 35.08 ± 0.13 | |
| Magnesium (Mg) | 509.12 ± 0.64 | |
| Phosphorus (P) | 59.96 ± 0.27 | |
| Copper (Cu) | 4.19 ± 0.59 | |
| Sulphur (S) | 0.02 ± 0.00 | |
| Cytokinins (CKs) | µg g−1 | 0.94 ± 0.04 |
| Gibberellins (GAs) | 0.85 ± 0.01 |
| Treatments | Plant Height (cm) | Stem Diameter (cm) | Leaf Area Index | Number of Branches | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| 100% of SWHC (C) | 35.05 ± 0.61 bc | 3.61 ± 0.36 bc | 52.12 ± 1.57 d | 24.00 ± 2.65 c | 12.33 ± 0.88 b | 2.02 ± 0.06 b |
| 100% of SWHC (MSE) | 50.12 ± 0.99 e | 4.80 ± 0.33 d | 60.38 ± 1.43 e | 35.33 ±1.45 d | 18.33 ± 1.45 c | 3.01 ± 0.26 c |
| 80% of SWHC (C) | 33.19 ± 1.44 b | 3.08 ± 0.25 abc | 40.11 ± 1.07 c | 26.00 ± 1.73 c | 13.04 ± 0.20 b | 1.81 ± 0.11 b |
| 80% of SWHC (MSE) | 49.04 ± 2.53 e | 4.68 ± 0.32 d | 54.16 ± 1.07 d | 34.00 ±0.58 d | 17.16 ± 0.54 c | 2.89 ± 0.19 c |
| 60% of SWHC (C) | 25.03 ± 1.15 a | 2.92 ± 0.43 ab | 34.07 ± 0.49 b | 18.00 ± 2.08 b | 8.05 ± 0.30 a | 1.61 ± 0.31 ab |
| 60% of SWHC (MSE) | 40.35 ± 0.89 d | 4.02 ± 0.29 cd | 40.34 ± 2.67 c | 23.00 ± 1.15 c | 11.14 ± 0.19 b | 1.80 ± 0.06 b |
| 40% of SWHC (C) | 20.38 ± 2.63 a | 2.52 ± 0.45 a | 29.37 ± 0.89 a | 13.00 ± 1.15 a | 6.36 ± 0.64 a | 1.03 ± 0.24 a |
| 40% of SWHC (MSE) | 39.24 ± 0.68 cd | 3.52 ± 0.08 abc | 34.08 ± 1.75 b | 18.00 ± 0.58 b | 8.26 ± 0.39 a | 1.62 ± 0.16 ab |
| Treatment | CWP (g DW/L of Irrigated Water) | RWC (%) | MSI (%) |
|---|---|---|---|
| 100% of SWHC (C) | 1.17 ± 0.44 ab | 76.11 ± 2.21 d | 58.33 ± 3.84 bcd |
| 100% of SWHC (MSE) | 1.39 ± 0.15 b | 80.03 ± 0.93 d | 67.33 ± 4.91 d |
| 80% of SWHC (C) | 1.087 ± 0.06 ab | 64.06 ± 3.55 bc | 55.33 ± 3.28 bc |
| 80% of SWHC (MSE) | 1.483 ± 0.02 b | 78.39 ± 0.89 d | 63.33 ± 1.45 cd |
| 60% of SWHC (C) | 0.957 ± 0.03 ab | 60.40 ± 0.98 b | 50.33 ± 1.76 ab |
| 60% of SWHC (MSE) | 1.603 ± 0.21 b | 69.35 ± 0.52 c | 56.33 ± 3.76 bc |
| 40% of SWHC (C) | 0.617 ± 0.13 a | 44.94 ± 3.02 a | 44.33 ± 0.88 a |
| 40% of SWHC (MSE) | 1.623 ± 0.38 b | 58.05 ± 1.57 b | 49.33 ± 0.88 ab |
| Treatment | Chlorophyll “a” (mg g−1 FW) | Chlorophyll “b” (mg g−1 FW) | Total Chlorophylls (mg g−1 FW) | Total Carotenoids (mg g−1 FW) |
|---|---|---|---|---|
| 100% of SWHC (C) | 1.13 ± 0.12 ab | 0.50 ± 0.12 abc | 1.46 ± 0.03 abcd | 0.42 ± 0.05 bc |
| 100% of SWHC (MSE) | 1.45 ± 0.26 b | 0.80 ± 0.12 d | 1.82 ± 0.05 df | 0.69 ± 0.05 d |
| 80% of SWHC (C) | 1.04 ± 0.12 ab | 0.47 ± 0.03 abc | 1.43 ± 0.12 abc | 0.39 ± 0.01 abc |
| 80% of SWHC (MSE) | 1.32 ± 0.16 b | 0.73 ± 0.13 cd | 1.72 ± 0.07 cdef | 0.64 ± 0.11 d |
| 60% of SWHC (C) | 0.90 ± 0.23 ab | 0.43 ± 0.04 ab | 1.28 ± 0.08 ab | 0.33 ± 0.02 ab |
| 60% of SWHC (MSE) | 1.20 ± 0.12 ab | 0.61 ± 0.06 bcd | 1.59 ± 0.24 bcdef | 0.54 ± 0.04 cd |
| 40% of SWHC (C) | 0.70 ± 0.15 a | 0.32 ± 0.07 a | 1.11 ± 0.12 a | 0.23 ± 0.05 a |
| 40% of SWHC (MSE) | 1.11 ± 0.06 ab | 0.51 ± 0.01 abc | 1.46 ± 0.05 abcde | 0.41 ± 0.01 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buthelezi, N.M.D.; Gololo, S.S.; Mugivhisa, L.L. An Assessment of Moringa (Moringa oleifera L.) Seed Extract on Crop Water Productivity and Physico-Biochemical Properties of Cancer Bush (Sutherlandia frutescens L.) under Deficit Irrigation. Horticulturae 2022, 8, 938. https://doi.org/10.3390/horticulturae8100938
Buthelezi NMD, Gololo SS, Mugivhisa LL. An Assessment of Moringa (Moringa oleifera L.) Seed Extract on Crop Water Productivity and Physico-Biochemical Properties of Cancer Bush (Sutherlandia frutescens L.) under Deficit Irrigation. Horticulturae. 2022; 8(10):938. https://doi.org/10.3390/horticulturae8100938
Chicago/Turabian StyleButhelezi, Nana Millicent Duduzile, Sechene Stanley Gololo, and Liziwe Lizbeth Mugivhisa. 2022. "An Assessment of Moringa (Moringa oleifera L.) Seed Extract on Crop Water Productivity and Physico-Biochemical Properties of Cancer Bush (Sutherlandia frutescens L.) under Deficit Irrigation" Horticulturae 8, no. 10: 938. https://doi.org/10.3390/horticulturae8100938
APA StyleButhelezi, N. M. D., Gololo, S. S., & Mugivhisa, L. L. (2022). An Assessment of Moringa (Moringa oleifera L.) Seed Extract on Crop Water Productivity and Physico-Biochemical Properties of Cancer Bush (Sutherlandia frutescens L.) under Deficit Irrigation. Horticulturae, 8(10), 938. https://doi.org/10.3390/horticulturae8100938






