Abstract
Microbial contamination commonly occurs in microgreens due to contaminated seeds. This study investigated the decontamination effects of water wash (control), 5% hydrogen peroxide (H2O2), UV-C (36 watts), advanced oxidation process (AOP; H2O2 + UV-C), and improved AOP by combination with microbubbles (MBs; H2O2 + MBs and H2O2 + UV-C + MBs) on microbial loads, seeds’ viability, and physio-biochemical properties of microgreens from corresponding roselle seeds. Results showed that H2O2 and AOP, with and without MBs, significantly reduced total aerobic bacteria, coliforms, Escherichia coli (E. coli), and molds and yeast log count in seeds as compared to the control. Improved AOP treatment of H2O2 + UV-C + MBs significantly augmented antimicrobial activity against total bacteria and E. coli (not detected,) as compared to control and other treatments due to the formation of the highest hydroxy radicals (5.25 × 10−13 M). Additionally, H2O2 and combined treatments promoted seed germination, improved microbiological quality, total phenolic, flavonoids, and 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) activity of the grown microgreens. Ascorbic acid content was induced only in microgreens developed from H2O2-treated seeds. Single UV-C treatment was ineffective to inactivate the detected microorganism population in seeds. These findings demonstrated that improved AOP treatment (H2O2 + UV-C + MBs) could potentially be used as a new disinfection technology for seed treatment in microgreens production.
1. Introduction
Over the past decade, the demand for plant-based diets has been on the rise, compelled by the growing awareness of physical and environmental health in society [1]. Therefore, the quest for wholesome nutrient-dense foods that support environment, health, and longevity, combined with gastronomic delight, is crucial. Microgreens, miniature tender leafy greens produced from seeds of vegetables, herbs, or grains, having two fully developed cotyledon leaves with or without the emergence of a rudimentary pair of first true leaves [2,3], has recently attracted many as novel culinary ingredients for both sweet and savory dishes. The popularity of microgreens is accelerated worldwide, and this has been ascribed to their high nutrient content with unique flavors, as compared to their seeds and mature plants, along with the fast and convenient production in space-efficient controlled environments [4]. They are commonly consumed raw, slightly cooked, or as decorative appetizers.
Sprouts and microgreens production is mostly focused on alfalfa, soybean, mung bean, and members of the Brassicaceae family grown predominantly in temperate regions, but scarce information is available on those in tropical regions [4,5]. Roselle (Hibiscus sabdariffa L.) is extensively cultivated locally, and Thailand is known to be one of the world’s largest producers of the species [6]. Research has documented that among the 10 best-studied culinary microgreens, roselle scores the highest for ascorbic acid content, antioxidant activity, and phenolic content, and is the most nutritious after radish and French basil microgreens [4]. The possible direct health benefits and abundant availability of seeds make this herbal plant ideal to be developed as a nutritional microgreen, thus, expanding its utilization further than the calyx.
Microgreens are germinated from seeds, hence, there is a systemic risk posed by contaminated seeds specifically related to food-borne pathogens that could lead to illnesses [2,7]. If the seeds are contaminated, pathogens can become internalized from the beginning of the growing process, and once incorporated, they are very difficult to remove [8,9]. Furthermore, postharvest washings appear so far to be ineffective and may instead increase contamination risk due to tissue damage that may induce pathogen growth. Therefore, seed decontamination plays a critical role in a production by assuring the quality and safety of microgreens [8]. The United States Food and Drug Administration cites 20,000 ppm calcium hypochlorite as the standard method of chemical disinfection of seeds [10]. Nonetheless, chlorine produces toxic byproducts that are carcinogenic and mutagenic, and the efficiency of this technique is varied and inconsistent which raises the need to look for better alternative techniques [11].
Advanced oxidation processes (AOPs) have attracted considerable attention as one of the promising advanced technologies for disinfection. Its strength lies in the in-situ generation of hydroxyl radicals (OH•), owing to its oxidation potent and non-selective properties [12]. The AOPs include ozonation, hydrogen peroxide (H2O2), ultrasonic, UV radiation, Fenton, and photocatalytic treatments [13]. H2O2 is categorized as safe and is allowed to be used in sanitizing organic products [14]. The H2O2, at varying concentrations (3–10%), has been employed as a seed decontamination treatment agent for sprouted-seeds production, and 8% H2O2 is reported to be able to reduce food-borne pathogens up to 4 log CFU g−1 [5]. Several studies also have documented that H2O2 has a beneficial effect on seed germination [15,16]. Apart from that, a shortwave, non-ionizing artificial UV-C light (180–280 nm) has been considered as an alternative to chemical fungicide to control postharvest diseases. The UV-C’s high germicidal effects have also been described to reduce microbial growth in fresh-cut juice products [17]. Exposure of groundnut and mung bean to UV-C for 15 min reduced root infecting fungi and at the same time increased total chlorophyll and carbohydrate contents [18]. Thus, treatment with UV-C light offers several advantages as it does not leave any residue, is easy to use, is lethal to most types of microorganisms, and does not require extensive safety equipment to be implemented [19]. Despite the advantages of both H2O2 and UV-C, there are limited findings on the application of AOPs through UV-C/H2O2 combination as a seed decontamination technique. The combination of UV-C/H2O2 has been developed mostly for the inactivation of postharvest food-borne pathogens for fresh produces [20] and degradation of micropollutants in water treatments [21] with limited efficiency.
Micro-nano bubbles (MBs) technology has become of interest in recent years due to its wide applications in many fields of science and technology, such as water treatment, biomedical engineering, and agriculture [22]. MBs are tiny bubbles with diameters of less than 50 µm that are known to generate free radicals when collapsed [23]. Nevertheless, the amount of OH• radicals formed during the collapse of these air MBs is minimal.
Consequently, the MBs have been combined with other oxidation techniques such as ozone to generate large quantities of OH• radicals to improve water treatment and fresh produce decontamination [24,25]. Hence, this compelled the present study of using the combination of MBs technology with H2O2 and AOP treatment (UV-C/H2O2) to augment the biocidal effects in seed decontamination.
To the best of our knowledge, there are scanted reports available regarding the utilization of H2O2 and AOP (UV-C/H2O2) in combination with or without MBs to decontaminate seeds for microgreens production. Therefore, this study aims to evaluate the effect of AOP (UV-C/H2O2) and MBs combination treatments on microbial contamination reduction, seeds’ viability, and microgreens’ physio-biochemical performance upon being grown from their corresponding seeds.
2. Materials and Methods
2.1. Seeds and Decontamination Treatments
Dry roselle (Hibiscus sabdariffa L.) seeds were purchased from a local market. The seeds were then selected to ensure that they were defect-free in appearance (in terms of molds, damages, and discoloration), weighed, and divided into six main groups. Then, the seeds of each group were rinsed with reversed osmosis (RO) water three times to remove dirt and debris. The rinsed seeds were immersed in RO water at a ratio of 1:10 (g/v) for 5 h at 24 ± 1 °C. The water was then disposed of and the seeds were rinsed under running RO water for 30 s prior to seed decontamination treatments as described below.
The seeds were immersed in various decontamination treatments which contained 0.005% Tween 20 for 15 min at 24 ± 1 °C in continuous agitation (using the same seed to water ratio as above). All the treatments were conducted in four replicates. The treatments included a control (RO water), 5% H2O2, UV-C (36 watts), 5% H2O2 + UV-C, 5% H2O2 + MBs, and 5% H2O2 + UV-C + MBs. The 5% H2O2 treatment was selected based on a preliminary study that resulted in the optimum microbial reduction without interfering with the viability and growth of the seeds. UV-C treatment contained 4 bulbs (submersible UV-C lamp, 0.25 m long, 9 W, Philips, Shanghai, China) which emits UV-C at λ-254 nm. MBs were generated in 5% H2O2 and 5% H2O2 + UV-C solutions using a swiveling type MBs generator (Model BT-50; Thai Isekyu Co., Ltd., Bangkok, Thailand; Figure 1).
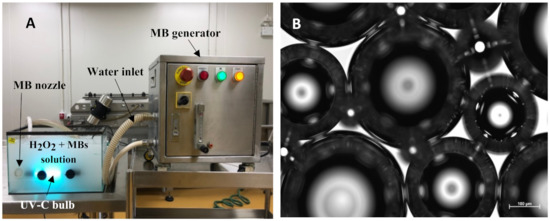
Figure 1.
(A) The experimental setup for the combined treatment of hydrogen peroxide, UV-C, and MBs. (B) The image of MBs (30 to 110 µm) was taken by the Axiocam 503 mono microscope camera (Carl Zeiss Microscopy GmbH, Berlin, Germany). MB = microbubble; H2O2 = hydrogen peroxide. Bar = 100 µm.
The pH and temperature of each decontamination solution before and after treatment were recorded using a pH meter (ExStik® pH meter; Extech Instruments Corporation, Nashua, NH, USA). The decontamination solutions were then discarded, and the seeds were rinsed with RO water. The excess water was discarded prior to microbiological analysis, seed viability test, and microgreens production.
2.2. Seed Germination
One hundred seeds from each untreated and treated seed were randomly selected to be evaluated for their viability using the top of a paper method with four replications for each sample. The seeds were spread on two-layered sterile distilled water saturated blotter paper and placed in a transparent box that was then stored in an incubator at 25 ± 2 °C. The paper was moistened every day with sterile water. The seeds were observed daily to check for germination percentage and index, days to emergence (DTE), the number of fungal infected seeds, and abnormal seedlings [26]. When the seed coat is ruptured due to protruding hypocotyl, the seeds were considered germinated.
2.3. Seed Sprouting and Microgreen Growth
The soaked and treated seeds described in Section 2.1 were weighed at 5 g each and placed in a box (6 cm × 6 cm) containing a wetted sponge (soilless culture) and sprouted under dark conditions at 23 ± 1 °C, 70–80% RH for 3 d. Subsequently, the sprouted seeds were exposed to white LED light (18 W, Philips, Shanghai, China). The light intensity was controlled at 100 μmol m−2s−1(16 h photoperiod) for 4 d at 23 ± 1 °C, 70–80% RH. The carbon dioxide concentration was controlled at 800 ± 100 mg L−1 using RAD-0501 CO2 Controller (CO2Meter Inc., Ormond Beach, FL, USA). The sprouted seeds or microgreens were manually sprayed with RO water twice (morning and evening) daily, old excess water was discarded before spraying, and with no watering on the harvest day. The microgreens were harvested on the fourth day after the light exposure for microbial analysis and physio-biochemical properties.
2.4. Microbiological Analysis
General and selective growth media were used to detect microbial contamination of roselle seeds and microgreens, and the standard plate count method was referred to as viable count enumeration. The seeds (12.5 g) and microgreens (25 g) were mixed separately with 0.85% sterile saline (NaCl; UNIVAR®, Ajax Finechem, 5 Caribbean, Australia) in a sterile stomacher bag (Stomacher®, Lab-Blender, West Sussex, UK) and homogenized using a stomacher (BagMixer® 400, Interscience International, Saint Nom la Brétèche, France) for 2 min. Then, under sterile conditions, 1 mL of the obtained suspension was taken and serially diluted with 0.85% sterile saline. An aliquot (100 µL) of proper dilution was pipetted into prepared media agar and incubated at 37 °C for 24–48 h. The general media used in this study were plate count agar (PCA; HiMedia Laboratories Pvt. Ltd., Mumbai, India) for total aerobic bacteria and potato dextrose agar (PDA; HiMedia Laboratories Pvt. Ltd., India) for molds and yeast. The selective media of eosin methylene blue (EMB; HiMedia Laboratories Pvt. Ltd., Mumbai, India) agar was used for total coliform and Escherichia coli (E. coli). The agar was weighed (the weight is agar-specific) and mixed with 1000 mL distilled water. Then, the mixture was autoclaved for 15 min and cooled to 45–50 °C prior to pouring into a plate. For PDA, streptomycin (Strepto, General Drugs House Co., Ltd., Bangkok, Thailand) was added into the agar before pouring into a petri dish.
2.5. Measurement of Hydroxyl Radical Concentration
The measurement of OH• radicals in the treatments including single MBs was performed using para-chlorobenzoic acid (ρ-CBA; Merck, Darmstadt, Germany) [27]. The treatments were carried out as described in Section 2.1 with the addition of ρ-CBA (5 mM as the stock solution) to the solution. The reaction solution was sampled after every 3 min interval for 21 min and analyzed using high-performance liquid chromatography (HPLC, Shimadzu, Japan) using the C-18 Symmetry C-18 (4.6 × 250 mm, 5 µm) column, Waters, USA as described by Park et al. (2004) [27]. The elution was performed by pumping acetonitrile and water (60:40 v/v) isocratically at a flow rate of 1.0 mL min−1, where the absorbance was measured at 234 nm using a photodiode array detector [27]. The column temperature was maintained at 35 °C (±0.1 °C). The concentration of OH• radical was calculated using the equation below:
where: C0 = initial ρ-CBA concentration; C = ρ-CBA concentration after H2O2 contractor; t = time; kOH•/ρ-CBA = 5 × 109 M−1s−1.
2.6. Microgreens Physio-Biochemical Properties
2.6.1. Fresh Weight, Color, and Total Chlorophyll Content
For fresh weight, 100 plants of microgreens were randomly harvested from each seedling tray and weighed. For color, the cotyledons surface color (L*, a*, b*, and hue angle values) was determined using a colorimeter (Chromameter Model CR-400, Minolta Corp., Ramsey, NJ, USA). The total chlorophyll content was measured by using N,N-dimethylformamide (ChemSupply Pty. Ltd., Gillman, SA, Australia) [28]. The freshly chopped microgreens weighed 1.5 g were mixed with 20 mL of N,N-dimethylformamide (ChemSupply Pty. Ltd., Gillman, SA, Australia) and incubated in the dark for 24 h at 4 °C. The and the absorbance was read at 440, 647, and 664 nm. Total chlorophyll was calculated as the sum of chlorophyll a (Chla) and chlorophyll b (Chlb) using the following formula:
Chla (mg L−1) = 12.64OD664 − 2.99OD647
Chlb (mg L−1) = −0.56OD664 + 23.26OD647
The result was expressed as a fresh weight basis (FW) in g kg−1.
2.6.2. Total Ascorbic Acid Content
The total ascorbic acid content was estimated according to the method of Roe et al. (1948) [29]. Frozen microgreens weighing 2.5 g were homogenized with 10 mL of cold 5% metaphosphoric acid (Loba Chemie Pvt. Ltd., Mumbai, India) and filtered through Whatman no.1 filter paper. The filtrate of 0.4 mL was mixed with 0.2 mL of 0.02% di-indophenol (Loba Chemie Pvt. Ltd., Mumbai, India) and let to rest at room temperature (RT) for 3 min for the reaction to start. Then, the mixture was mixed with 0.4 mL of 2% thiourea (Loba Chemie Pvt. Ltd., Mumbai, India) and 0.2 mL of 1% dinitrophenol hydrazine (Loba Chemie Pvt. Ltd., Mumbai, India), and incubated at 50 °C for 1 h. The reaction was stopped by adding 1 mL of 85% sulfuric acid (EMSURE®, Merck, Kenilworth, NJ, USA) to the mixture. Absorbance at 540 nm was recorded using a spectrophotometer (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan) and the result was expressed as FW basis of g kg−1.
2.6.3. Total Flavonoid and Phenolic Content, and Antioxidant Activity
Sample extraction for total antioxidant activity (TAA) and total phenolic content (TPC) was carried out by homogenizing 1.5 g of frozen microgreens with 10 mL of 80% (v/v) ethanol (EMSURE®, Merck, Kenilworth, NJ, USA) in ice water. The homogenate was centrifuged at 12,000× g for 15 min at 4 °C. The obtained crude extract was used to determine TAA and TPC. Measurement of TAA was estimated using 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich, Darmstadt, Germany) radical scavenging activity according to Rajurkar and Hande’s (2011) [30] method with some modifications. The extract of 50 µL was mixed with 200 µL ethanolic DPPH (0.8 mM) and 2.25 mL 80% (v/v) ethanol (EMSURE®, Merck, Kenilworth, NJ, USA). The mixture was incubated for 30 min in the dark at RT. Absorbance was read at 515 nm using a spectrophotometer (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan) and the antioxidant activity was expressed as percentage (%) inhibition.
TPC was determined according to the method of Toor and Savage (2005) [31] with modifications. An aliquot of crude extract was mixed with 5 mL of Folin-Ciocalteu reagent (Loba Chemie Pvt. Ltd., India) and 4 mL of 7.5% (w/v) sodium carbonate (UNIVAR®, Ajax Finechem, 5 Caribbean, Australia). The mixture was incubated at 30 °C for 1 h. Absorbance was read using a spectrophotometer (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan) at 765 nm and the results were compared with the standard curve of gallic acid (Sigma-Aldrich, Darmstadt, Germany) and expressed on an FW basis as g kg−1 gallic acid equivalent (GAE).
For total flavonoid content (TFC) extraction, 0.5 g frozen sample was homogenized with 14.5 mL absolute ethanol (EMSURE®, Merck, Kenilworth, NJ, USA). Then, the homogenate was incubated at 78 °C for 1 h and centrifuged at 12,000× g for 15 min. The TFC was determined using Lin and Tang’s (2007) [32] method and expressed on an FW basis as g kg−1 quercetin equivalent (QE).
2.7. Statistical Analysis
The experiment was arranged in a completely randomized design where all the data were presented as means ± standard error (SE) with four replications. The data were analyzed using analysis of variance (ANOVA). The obtained means were compared using Duncan’s multiple range test (DMRT) when F-test was significant.
3. Results
3.1. Effects of Decontamination Treatments on Microbial Reduction of Roselle Seeds and OH• Radicals’ Generation
The roselle seeds were contaminated with different microorganisms mainly related to coliforms and aerobic bacteria, which recorded total viable counts of 7.22 log CFU g−1 and 7.15 log CFU g−1, respectively, for unwashed seeds (Figure 2). This is followed by the counts of molds and yeast at 6.16 log CFU g−1 and E. coli at 5.13 log CFU g−1. Washed seeds with RO water (control) had slightly reduced microbial loads (<1 log) but no significant impact in reducing the total counts of coliforms and E. coli except for total aerobic bacteria, molds, and yeast, as compared to unwashed seeds. On the contrary, pronounced log counts reduction in all types of detected microorganisms were observed upon washing the seeds with H2O2, H2O2 + UV-C, H2O2 + MBs, and H2O2 + UV-C + MBs. The H2O2 has been used as an alternative to chlorine due to its broad-ranged antimicrobial efficacy against bacteria, fungi, and yeast, where the biocidal mechanism is based on the oxidation of protein and lipids that leads to cell membrane injury [33]. The presence of a physical force of effervescence of H2O2 could also contribute to the better removal of microorganisms on the surface of the seeds [34].
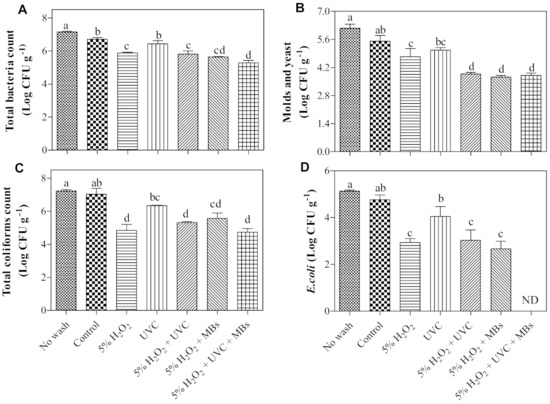
Figure 2.
Effects of seed decontamination treatments on (A) total bacteria count, (B) molds and yeast count, (C) total coliforms count, and (D) Escherichia coli count in roselle seeds for microgreen production. Data are means of four replicates ± SE. Means with the same letter are not significantly different at p ≤ 0.05 by the DMRT test. ND = not detected.
All the AOPs (with and without MBs) treated seeds showed a notable reduction in molds and yeast compared to H2O2 alone and control (about 1 and 2 log reductions, respectively), as shown in Figure 2B. This is probably due to the significant formation of OH• radicals, which were obtained from the combined treatments (Figure 3). The lowest concentration was recorded in the AOP treatment (H2O2 + UV-C; Table 1). Our study confirmed that the addition of MBs improved the formation of OH• radicals in both H2O2 and AOP treatments. The application of MBs technology is found to induce oxidation when combined with H2O2 for decolorization of indigo carmine dye wastewater [35] or ozone for surface decontamination of sweet basil [36].
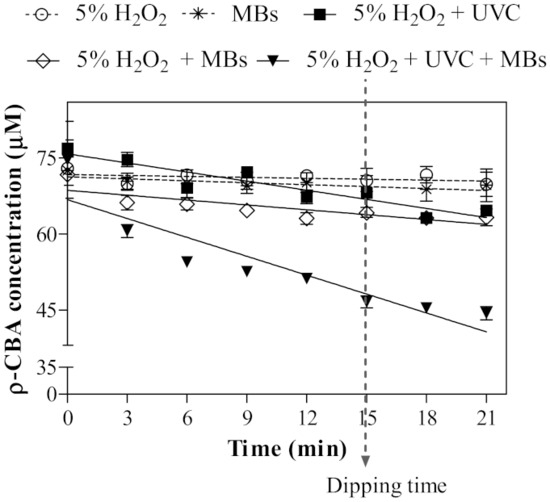
Figure 3.
Changes in para-chlorobenzoic acid (ρ-CBA) concentration over time in different treatments. The dotted line indicated non-significant different regression relationship. (5% H2O2: y = 71.73, R2 = 0.04; MBs: y = 71.27, R2 = 0.06; 5% H2O2 + UV-C: y = −0.60x + 75.83, R2 = 0.61: 5% H2O2 + MBs: y = −0.32x + 68.63, R2 = 0.51; 5% H2O2 + UV-C + MBs: y = −1.24x + 71.27, R2 = 0.70). H2O2 = hydrogen peroxide, MBs = microbubbles.

Table 1.
Hydroxyl radicals (OH•) concentration in advanced oxidation process treatments generated at 15 min.
However, there was no significant difference in log reduction in molds and yeast for the combined treatment. Additionally, no significant difference in log reduction in total coliforms by single H2O2 or the combined treatments (Figure 2C). The limited efficacy of these combined treatments is possibly explained by the insufficient contact between the radicals formed and targeted microorganisms on the seed’s surface, as the radicals can be sequestered away, attributed to their highly reactive but short-lived nature [37]. Additionally, the adhesion and internalization of the microorganism on the seeds would minimize the interaction between antimicrobials and microorganisms [38]. The rough, uneven, and hairy surface of roselle seeds would provide protective sites for the microorganisms and cause minimal penetration of the antimicrobial agent.
In the case of E. coli, a reduction of 2 logs was achieved by using H2O2, while the addition of UV-C (H2O2 + UV-C) or MBs (H2O2 + MBs) were not able to reduce the E. coli log count further. Nevertheless, the greater reduction was observed with the enhanced AOP treatment with MBs (H2O2 + UV-C + MBs), in which the E. coli population was notably reduced to a non-detectable level (Figure 2D). A similar trend was also observed for the total bacteria count (Figure 2A). These results demonstrated that there is a variation in susceptibility among the native microorganism towards the decontamination treatments. The enhanced lethality of the combined treatment of H2O2 + UV-C + MBs towards total bacteria, molds and yeast, and E. coli is most likely contributed by the high concentration of OH• radicals formed (Table 1) caused by the elevation of the water temperature from 24 ± 1 °C to 45 ± 1 °C through the generation of MBs. Although the generation of MBs alone did not significantly produce OH• radicals (Figure 3), there is evidence that MBs helped in removing attached microorganisms on surfaces by enhancing the agitation force to ease their removal [36]. Besides, their ability to decrease in size (Figure 1B) and dispersibility would create a wide surface area for both H2O2 and the OH• radicals to have better contact with E. coli.
The UV-C exposure resulted in a significant reduction in total coliforms, E. coli, and molds, and yeast count with respect to unwashed seeds, but not to control. The efficacy of UV-C depends on the areas being exposed directly to UV-C light. The rough and uneven seed surface would cause a shadowing effect and consequently minimize the effect of UV-C, albeit the constant stirring of the seeds during treatment, owing to insufficient length of exposure to inactivate the microorganisms. This shortcoming that resulted in an inadequacy of the biocidal effect of UV was also reported for wild blueberry processing [20].
3.2. Effects of Decontamination Treatments on Seed Germination
Soaking the seeds for 5 h significantly reduced the DTE of the seeds, regardless of the decontamination treatments used (Table 2).

Table 2.
Effects of seed decontamination treatments on percentage germination, germination index, days to emergence (DTE), the number of fungal-infected seeds, and abnormal roselle seedlings.
Besides, soaking has a pronounced effect on seed viability even without using an antimicrobial agent, exhibiting 18% and 56% higher germination rate and index than unwashed seeds, respectively. The H2O2, H2O2 + UV-C, H2O2 + MBs, and H2O2 + UV-C + MBs treatments further significantly improved the seed viability, showing an average of 10% and 21% higher germination rate and index than the control, respectively, although the results between treatments were not significantly different. During sprouting, a significant reduction in the number of fungal-infected seeds was observed in all combined treatments, exhibiting an average of 66% less infection than the control. Compared to the control, no significant adverse treatment effect was observed in the number of abnormal seedlings. Instead, 5% H2O2 remarkably reduced the number by 44%. Studies have suggested that H2O2 promotes seed germination by causing the oxidation of germination inhibitors [39], and also that it could act as a signaling molecule in regulating redox state and plant hormones such as by decreasing abscisic acid and zeatin ribosides, which are plant hormones that inhibit seed germination [40]. On the other hand, another study demonstrated that the improvement of seed germination and vigor is mainly due to the antimicrobial activity of the treatment [16].
3.3. Physio-Biochemicals Properties of Roselle Microgreens Grown from Treated Seeds
As shown in Table 3, the combined treatment of H2O2 + MBs resulted in a significantly heavier microgreen than the control. The other treatments did not have any adverse effect on the microgreens’ FW when compared to the control. Likewise, no significant differences were observed in total chlorophyll content and color (L, a*, and h° values) among the treatments or between the control. Nevertheless, the TPC in the microgreens was enhanced by H2O2, H2O2 + MBs, and H2O2 + UV-C + MBs treatments, recording an average of 27% higher than control; while no effects were observed from the UV-C- and H2O2 + UV-C-treated seeds (Figure 4A). Similarly, TAA by DPPH scavenging activity was remarkably elevated in microgreens produced from seeds treated with H2O2, H2O2 + MBs, and H2O2 + UV-C + MBs, resulting in an average of 68% higher than the control (Figure 4C). The TAA has also stimulated in microgreens of H2O2 + UV-C-treated seeds, but the rise of the activity was lesser than H2O2 and the combined treatments with MBs. There was no difference in the TAA between UV-C treatment and control. On the contrary, TFC increased significantly in microgreens grown from all treated seeds as compared to the control; whereby the highest TFC was obtained in microgreens grown from seeds treated with H2O2 + UV-C + MBs, exhibiting 41% higher than the control (Figure 4B). Apart from that, the elevation of ascorbic acid content was only observed in microgreens produced from seeds treated with H2O2, while the other treatments showed no significant changes when compared to the control (Figure 4D). Exogenous H2O2 treatment has been proven to induce the synthesis of phenolics and flavonoids content in quinoa sprouts [41]. Furthermore, H2O2 plays an important role as signaling molecules in acclimatization by inducing antioxidant defense mechanisms [16]. There are also studies suggesting that OH• radicals could play a signaling role in stress adaption in plants [42,43]. However, the role of OH• radicals or MBs of combined treatment, H2O2 + UV-C + MBs, in the induction of flavonoids in roselle microgreens developed from their corresponding seeds needs to be studied further.

Table 3.
Effects of seed decontamination treatments on fresh weight, total chlorophyll content, L*, a*, b*, and hue angle values of their corresponding roselle microgreens.
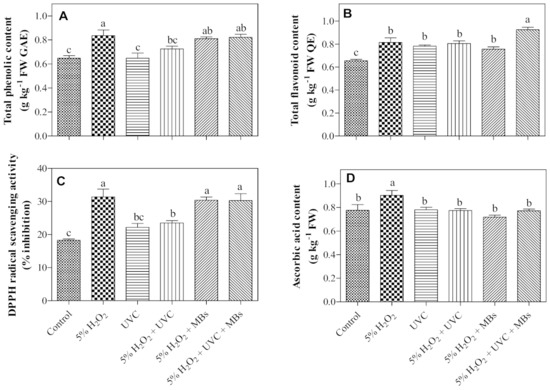
Figure 4.
Effects on seed decontamination treatments on (A) total phenolic content, (B) total flavonoid content, (C) DPPH radical scavenging activity, and (D) ascorbic acid content of their corresponding roselle microgreens. Data are means of four replications ± SE. Means with the same letter are not significantly different at p ≤ 0.05 by the DMRT test.
4. Conclusions
The synergistic microbial inactivation efficacy of H2O2 and UV-C (H2O2 + UV-C) or MBs (H2O2 + MBs) is marginally effective towards total aerobic bacteria, coliforms, E. coli, and molds and yeast populations. However, the improved AOP treatment using MBs (H2O2 + UV-C + MBs) enhanced antimicrobial lethality against E. coli through the induction of OH• radicals. Furthermore, this improved treatment accelerated seed germination, improved microbiological quality, and phytonutrient of microgreens without negatively affecting the overall microgreens growth as compared to the control. Therefore, the combined AOP treatment with MBs (H2O2 + UV-C + MBs) could be a promising technology for seed treatment in microgreens industrial production.
Author Contributions
Conceptualization, S.P., N.P. and V.S.; methodology, S.P. and N.P.; investigation, S.P., S.Y. and J.O.; resources, N.P. and V.S.; writing—original draft preparation, S.P.; writing— review and editing, N.P. visualization, S.P. and N.P.; supervision, V.S.; project administration, N.P.; funding acquisition, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by King Mongkut’s University of Technology Thonburi Post-Doctoral Fellowship through Surisa Phornvillay, National Research Council Thailand, Agricultural Research Development Agency (No. CRP6305031930), and United Graduate School of Agricultural Science (UGSAS), Gifu University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Darmanin, M.; Kozak, D.; Mallia, J.O.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Generation of plasma functionalized water: Antimicrobial assessment and impact on seed germination. Food Control 2020, 113, 107168. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Gioia, F.D.; Kyratzis, A.; Serio, F.; Renna, M.; Pascale, S.D.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content ant nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Holliday, S.L.; Scouten, A.J.; Beuchat, L.R. Efficacy of chemical treatments in eliminating Salmonella and Escherichia coli O157:H7 on sacrified and polished alfalfa seeds. J. Food Prot. 2001, 64, 1489–1495. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Jamini, T.S.; Islam, A.K.M.M.; Yeasmin, S. Roselle: A functional food with high nutritional and medicinal values. Fundam. Appl. Agric. 2016, 1, 44–49. [Google Scholar]
- Xiao, Z.; Nou, X.; Luo, Y.; Wang, Q. Comparison of the growth of Escherichia coli O157: H7 and O104: H4 during sprouting and microgreen production from contaminated radish seeds. Food Microbiol. 2014, 44, 60–63. [Google Scholar] [CrossRef]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens-a review of food safety consideration along the farm to fork continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef]
- Wang, Q.; Kniel, K.E. Survival and transfer of murin norovirus within a hydroponic system during kale and mustard microgreen harvesting. Appl. Environ. Microbiol. 2016, 82, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration. Guidance for Industry: Reducing Microbial Food Safety Hazards for Sprouted Seeds; U.S. Food and Drug Administration: Maryland, MD, USA, 1999. [Google Scholar]
- McDonald, T.; Komulainen, H. Carcinogenicity of the chlorination disinfection by-product MX. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2005, 23, 163–214. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wu, Q.; Du, Y.; Hu, H. Degradation of natural organic matter by UV/chlorine oxidation: Molecular decomposition, formation of oxidation byproducts and cytotoxicity. Water Res. 2017, 124, 251–258. [Google Scholar] [CrossRef]
- Fan, X.; Song, Y. Advanced oxidation process as a postharvest decontamination technology to improve microbial safety of fresh produce. J. Agric. Food Chem. 2020, 68, 12916–12926. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, J.; Wakeling, C.; Bach, S.; Orban, S.; Delaquis, P. Disinfection of alfalfa and radish sprouting seed using oxidizing agents and treatments compliant with organic food production principles. J. Food Prot. 2020, 83, 779–787. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Hernandez, A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal. Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szopinska, D. Effects of hydrogen peroxide treatment on the germination, vigour and health of Zizinnia elegans seeds. Folia Hortic. 2014, 26, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Santhirasegaram, V.; Razali, Z.; George, D.S.; Somasundram, C. Comparison of UV-C treatment and thermal pasteurization on quality of Chokanan mango (Mangifera indica L.) juice. Food Bioprod. Processing 2015, 94, 313–321. [Google Scholar] [CrossRef]
- Siddiqui, A.; Dawar, S.; Zaki, M.J.; Hamid, N. Role of ultra violet (UV-C) radiation in the control of root infecting fungi on groundnut and mung bean. Pak. J. Bot. 2011, 43, 2221–2224. [Google Scholar]
- Turtoi, M. Ultraviolet light treatment of fresh fruits and vegetables surface: A review. J. Agroaliment. Processes Technol. 2013, 19, 325–337. [Google Scholar]
- Crowe, K.M.; Bushway, A.A.; Bushway, R.J.; Davis-Dentici, K.; Hazen, R.A. A comparison of single oxidants versus advanced oxidation processes as chlorine-alternaltives for wild strawberry processing (Vaccinium angustifolium). Int. J. Food Microbiol. 2007, 116, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.Y.; Fang, J.Y.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation processes. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.G.; Liu, Y.Y. Principal and application of microbuble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Chiba, K.; Li, P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Klintham, P.; Tongchitpakdee, S.; Chinsirikul, W.; Mahakarnchanakul, W. Combination of microbubbles with oxidizing sanitizers to eliminate Escherichia coli and Salmonella Typhimurium on Thai leafy vegetables. Food Control 2017, 77, 260–269. [Google Scholar] [CrossRef]
- Takahashi, M.; Ishikawa, H.; Asano, T.; Horibe, H. Effect of microbubbles on ozonized water for photoresist removal. J. Phys. Chem. C 2012, 116, 12578–12583. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association (ISTA): Zurich, Switzerland, 2022. [Google Scholar]
- Park, J.; Choi, H.; Cho, J. Kinetic decomposition of ozone and para-chlorobenzoic zcid (pCBA) during catalytic ozonation. Water Res. 2004, 38, 2285–2292. [Google Scholar] [CrossRef]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Roe, J.H.; Milles, M.B.; Oesterling, M.J.; Damron, C.M. The determination of diketo-l-gulonic acid, dehydro-l-ascorbic acid and l-ascorbic acid in the same tissue extract by the 2,4-dinitrophenylhydrazine method. J. Biol. Chem. 1948, 174, 201–208. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, C. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.; Fan, X.; Yan, R. Effect of combination of ultraviolet light and hydrogen peroxide on inactivation of Escherichia coli O157:H7, native microbial loads, and quality of button mushrooms. Food Control 2013, 34, 554–559. [Google Scholar] [CrossRef]
- Wang, J.; Li, D. Enhancing advanced oxidation process by microbubbles technology and the analysis of its degradation process. IOP Conf. Ser. Earth Environ. Sci. 2018, 146, 012048. [Google Scholar] [CrossRef]
- Phaephiphat, A.; Mahakarnchanakul, W. Surface decontamination of Salmonella Typhimurium and Escherichia coli on sweet basil by ozone microbubbles. Cogent Food Agric. 2018, 4, 1558496. [Google Scholar] [CrossRef]
- Xie, Y.; Hajdok, C.; Mittal, G.S.; Warriner, K. Inactivation of MS2 F(+) coliphage on lettuce by a combionation of UV light and hydrogen peroxide. J. Food Prot. 2008, 71, 903–907. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, S. Review: Comparison of the effectiveness of decontaminating strategies for fresh fruits and vegetables and related limitations. Crit. Rev. Food Sci. Nutr. 2019, 58, 3189–3208. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Iwabuchi, M.A. Mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 2001, 42, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Barba-Espín, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Faize, M.; Albacete, A.; Perez-Alfocea, F.; Hernandez, J.A. Hydrogen peroxide as an inducer of seed germination: Its effects on antioxidative metabolism and plant hormone contents in pea seedlings. Acta Hortic. 2011, 898, 229–236. [Google Scholar] [CrossRef]
- Swieca, M. Hydrogen peroxide treatment and the phenylpropanoid pathway precursors feeding improve phenolics and antioxidant capacity of quinoa sprouts via an induction of L-tyrosine and L-phenylalanine ammonia-lases activities. J. Chem. 2016, 2016, 1936516. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.H.; Wang, W.X.; Chen, C.; Wang, Y.F.; Liu, T.; Li, X.; Shang, Z.L. Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein a subunit and reactive oxygen species. Mol. Plant 2012, 5, 852–864. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.X.; Steudel, E. Gating of aquaporins by light and reactive oxygen species in leaf parenchyma cells of the midrib of Zea mays. J. Exp. Bot. 2009, 60, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).