A New Leaf Blight Disease Caused by Alternaria jacinthicola on Banana in China
Abstract
:1. Introduction
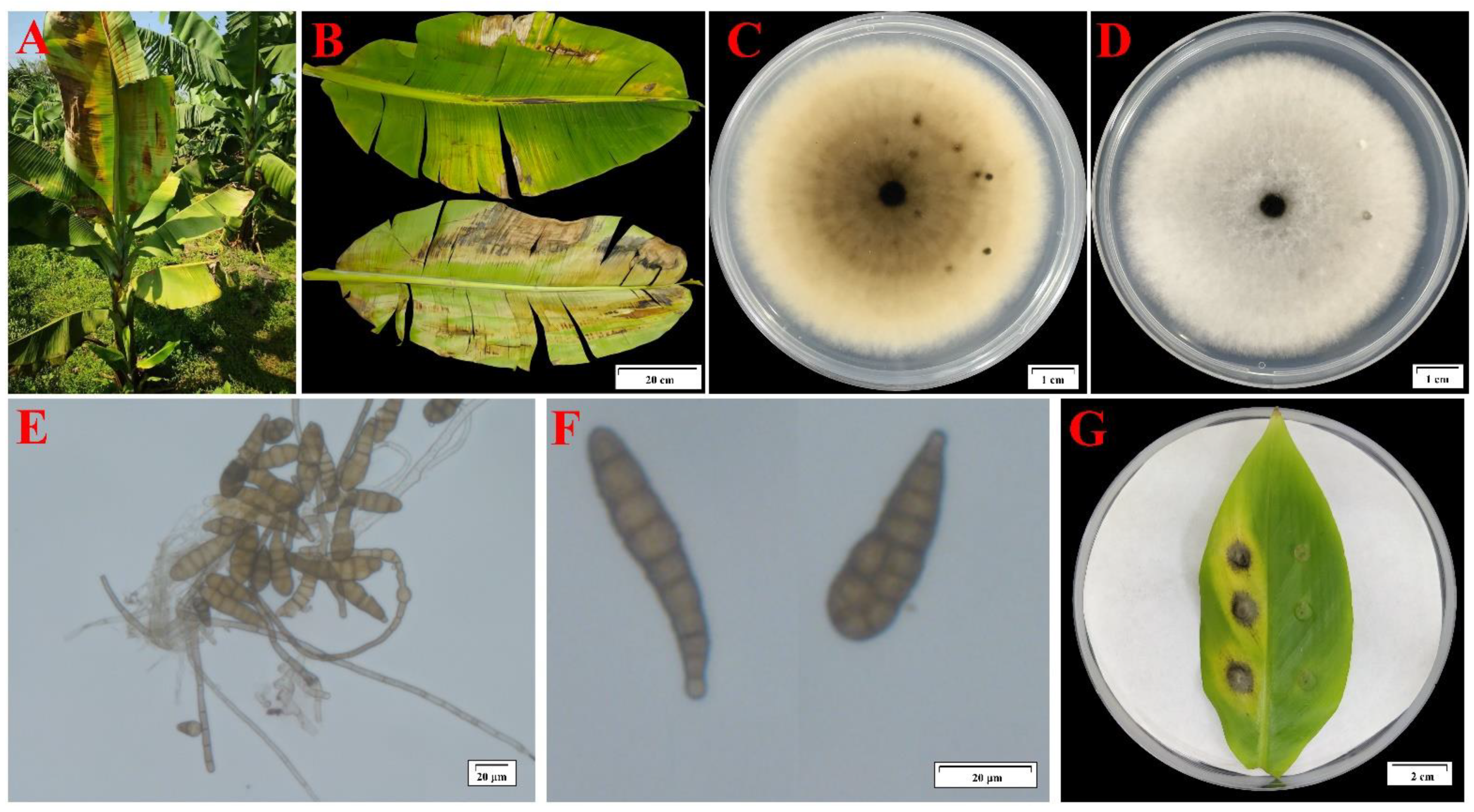
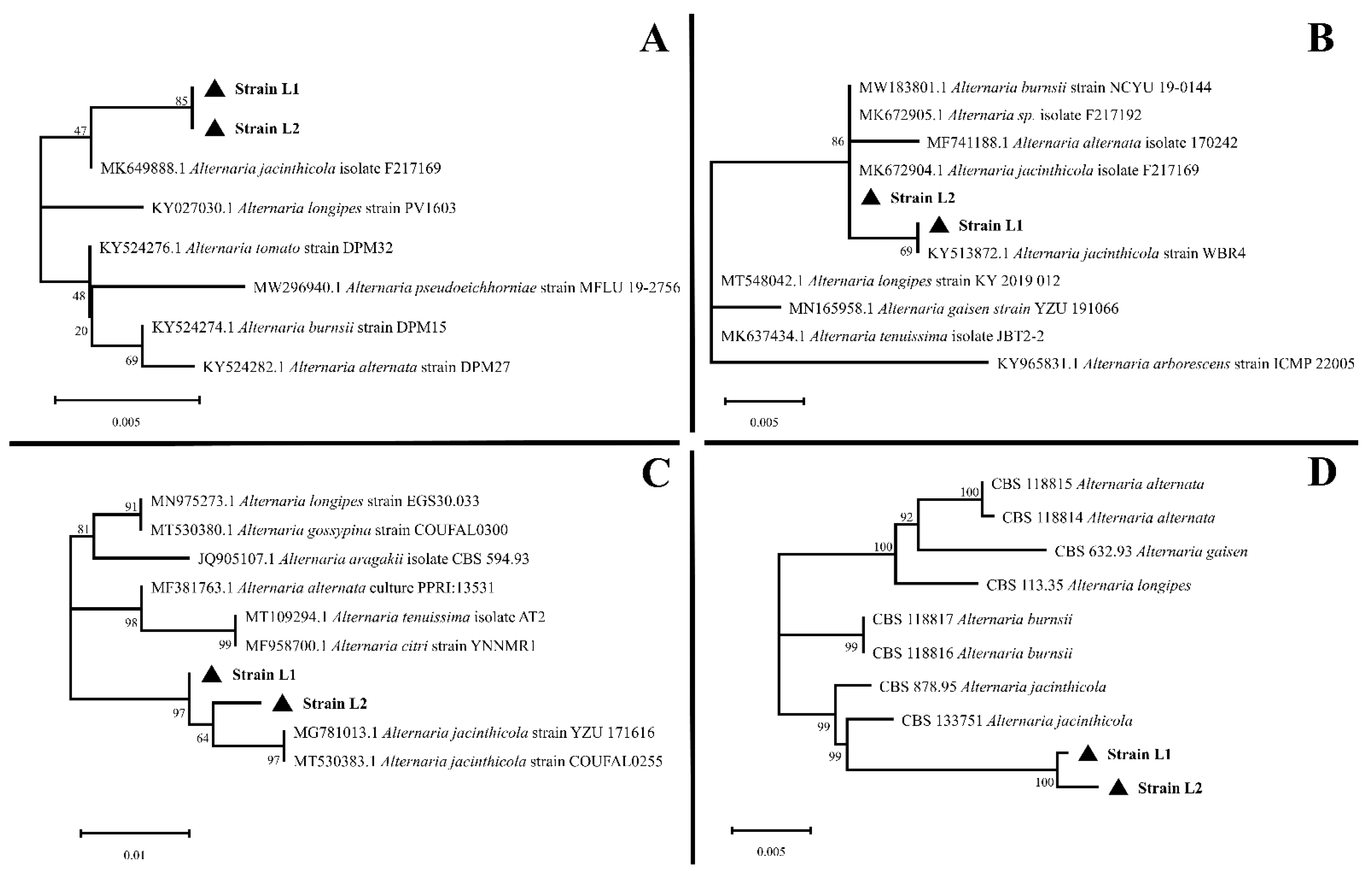
2. Results
3. Discussion
4. Materials and Methods
4.1. Disease Investigation and Sample Collection
4.2. Isolation and Purification of Pathogenic Fungi
4.3. Morphological Observation and Molecular Identification of Pathogenic Fungi
4.4. Pathogenicity Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kema, G.; Drenth, A.; Dita, M.; Jansen, K.; Vellema, S.; Stoorvogel, J.J. Editorial: Fusarium wilt of banana, a recurring threat to global banana production. Front. Plant Sci. 2020, 11, 628888. [Google Scholar] [CrossRef] [PubMed]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [Green Version]
- Thammaiah, N.; Kanamadi, V.C.; Shirol, A.M.; Kulkarni, M.S.; Reddy, B.S. Management of freckle leaf spot disease of banana. Int. J. Plant Prot. 2009, 2, 42–44. [Google Scholar]
- Stover, R.H. Sigatoka leaf spots of bananas and plantains. Plant Dis. 1980, 64, 751. [Google Scholar] [CrossRef]
- Fu, G.; Huang, S.; Ye, Y.; Wu, Y.; Cen, Z.; Lin, S. Characterization of a bacterial biocontrol strain B106 and its efficacies on controlling banana leaf spot and post-harvest anthracnose diseases. Biol. Control 2010, 55, 1–10. [Google Scholar] [CrossRef]
- Zimmermann, A. Über einige an tropischen kulturepflanzen beobachtete pilze. II. Zbl. Bakt. 1902, 8, 216–221. [Google Scholar]
- Hayden, H.L.; Carlier, J.; Aitken, E.A.B. Population differentiation in the banana leaf spot pathogen Mycosphaerella musicola, examined at a global scale. Plant Pathol. 2010, 52, 713–719. [Google Scholar] [CrossRef]
- Wardlaw, C.W. Cercospora leaf spot disease of bananas. Nature 1939, 144, 11–14. [Google Scholar] [CrossRef]
- Lin, S.H.; Liang, S.; Huang, Q.Q. Characterization of Exserohilum rostratum, a new causal agent of banana leaf spot disease in China. Australas. Plant Pathol. 2011, 40, 246–259. [Google Scholar] [CrossRef]
- Arzanlou, M.; Abeln, E.; Kema, G.; Waalwijk, C.; Crous, P.W. Molecular diagnostics for the Sigatoka disease complex of banana. Phytopathology 2007, 97, 1112–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amani, M.; Avagyan, G. First report of banana Septoria leaf spot disease caused by Septoria eumusae in Iran. Int. J. Farming Allied Sci. 2014, 3, 1140–1144. [Google Scholar]
- Chang, T.C.; Salvucci, A.; Crous, P.W.; Stergiopoulos, I. Comparative genomics of the Sigatoka disease complex on banana suggests a link between parallel evolutionary changes in Pseudocercospora fijiensis and Pseudocercospora eumusae and increased virulence on the banana host. PLoS Genet. 2016, 12, e1005904. [Google Scholar] [CrossRef]
- George, M.; Anita, C.K.; Mathew, D. Symptomatology of Sigatoka leaf spot disease in banana landraces and identification of its pathogen as Mycosphaerella eumusae. J. Saudi Soc. Agric. Sci. 2021. [Google Scholar] [CrossRef]
- Takaoka, S.; Kurata, M.; Harimoto, Y.; Hatta, R.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. Complex regulation of secondary metabolism controlling pathogenicity in the phytopathogenic fungus Alternaria alternata. New Phytol. 2014, 202, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gautam, S.; Kumar, S. Encyclopedia of Food Microbiology, 1st ed.; Academic Press: London, UK, 1999; pp. 2398–2399. [Google Scholar]
- Corazza, L.; Luongo, L.; Parisi, M. First report of leaf spot caused by Alternaria alternata on kiwifruit in Italy. Plant Dis. 1999, 83, 487. [Google Scholar] [CrossRef]
- Vicent, A.; Armengol, J.; Sales, R.; García-Jiménez, J.; Alfaro-Lassala, F. First report of Alternaria brown spot of citrus in Spain. Plant Dis. 2000, 84, 1044. [Google Scholar] [CrossRef] [PubMed]
- Harteveld, D.; Akinsanmi, O.A.; Chandra, K.; Drenth, A. Timing of infection and development of Alternaria diseases in the canopy of apple trees. Plant Dis. 2014, 98, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Ma, X.; Wan, W.; Long, N.; Dong, Y. Comparative genomics of pathogens causing brown spot disease of tobacco: Alternaria longipes and Alternaria alternata. PLoS ONE 2016, 11, e155258. [Google Scholar]
- Jones, D.R. Diseases of Banana, Abacá and Enset, 1st ed.; CAB International: Wallingford, UK, 2000; pp. 1–36. [Google Scholar]
- Pragya, A.; Yeonyee, O.; Dilip, P. Current status of early blight resistance in tomato: An update. Int. J. Mol. Sci. 2017, 18, 2019. [Google Scholar]
- Kant, R.; Joshi, P.; Bhandari, M.S.; Pandey, A.; Pandey, S. Identification and pathogenicity of Alternaria alternata causing leaf spot and blight disease of Ailanthus excelsa in India. For. Pathol. 2020, 50, e12584. [Google Scholar] [CrossRef]
- George, M.; Cherian, K.A.; Beena, S. A new leaf spot disease of banana cultivar Nendran caused by Alternaria alternata. Indian Phytopathol. 2020, 73, 797–798. [Google Scholar] [CrossRef]
- Dagno, K.; Crovadore, J.; Lefort, F.; Lahlali, R.; Jijakli, M.H. Alternaria jacinthicola, a new fungal species causing blight leaf disease on water hyacinth [Eichhornia crassipes (Martius) Solms-Laubach]. J. Yeast Fungal Res. 2011, 2, 99–105. [Google Scholar]
- Luo, H.; Jia, G.G.; Ma, D.F.; Sun, Z.X.; Deng, J.X. First report of leaf spot disease caused by Alternaria jacinthicola on Tagetes erecta in China. Plant Dis. 2018, 102, 2375. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Paul, N.C.; Deng, J.X.; Lee, H.B.; Yu, S.H. Characterization and pathogenicity of Alternaria burnsii from seeds of Cucurbita maxima (Cucurbitaceae) in Bangladesh. Mycobiology 2015, 43, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Poudel, B.; Velázquez-del Valle, M.G.; Hernández-Lauzardo, A.N.; Zhang, S. First report of Alternaria tomato causing leaf spot on sunflower in Mexico. Plant Dis. 2019, 103, 2917. [Google Scholar] [CrossRef]
- Kusaba, M.; Tsuge, T. Phylogeny of Alternaria fungi known to produce host-specific toxins on the basis of variation in internal transcribed spacers of ribosomal DNA. Curr. Genet. 1995, 28, 491–498. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Berbee, M.L.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Hong, S.G.; Cramer, R.A.; Lawrence, C.B.; Pryor, B.M. Alt a 1 allergen homologs from Alternaria and related taxa: Analysis of phylogenetic content and secondary structure. Fungal Genet. Biol. 2005, 42, 119–129. [Google Scholar] [CrossRef]
- Li, H.; Bian, R.; Liu, Q.; Sun, L. Identification of a novel hypovirulence-inducing hypovirus from Alternaria alternata. Front. Microbiol. 2019, 10, 1076. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, T.; Berbee, M.L.; Simmons, E.G.; Cardoso, C.R.; Mizubuti, E. First report of Alternaria tomatophila and A. grandis causing early blight on tomato and potato in Brazil. New Dis. Rep. 2010, 22, 28. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Fu, Y.; Nie, D.; Stewart, J.E.; Peever, T.L.; Li, H. Identification of a novel phylogenetic lineage of Alternaria alternata causing citrus brown spot in China. Fungal Biol. 2015, 119, 320–330. [Google Scholar] [CrossRef]
- Kwon, O.K.; Jeong, A.R.; Jeong, Y.J.; Kim, Y.A.; Park, C.J. Incidence of Alternaria species associated with watermelon leaf blight in Korea. Plant Pathol. J. 2021, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, S.P. Danish species of Alternaria and Stemphylium taxonomy, parasitism, economical significance. Q. Rev. Biol. 1945, 160, 313–314. [Google Scholar]
- Parkunan, V.; Li, S.; Fonsah, E.G.; Ji, P. First report of Alternaria leaf spot of banana caused by Alternaria alternata in the United States. Plant Dis. 2013, 97, 1116. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.Z.; Zhang, Z.H.; Wang, L.H.; Li, G.Y.; Zhang, J.Z.; Zhang, Y. First report of leaf spot caused by Alternaria alternata on Chinese dwarf banana in China. Plant Dis. 2014, 98, 691. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, Y.; Liu, J.; Sheng, O.; Liu, F.; Qiu, D.; Lü, P.; Deng, G.; Cheng, C. A New Leaf Blight Disease Caused by Alternaria jacinthicola on Banana in China. Horticulturae 2022, 8, 12. https://doi.org/10.3390/horticulturae8010012
Wang B, Zhang Y, Liu J, Sheng O, Liu F, Qiu D, Lü P, Deng G, Cheng C. A New Leaf Blight Disease Caused by Alternaria jacinthicola on Banana in China. Horticulturae. 2022; 8(1):12. https://doi.org/10.3390/horticulturae8010012
Chicago/Turabian StyleWang, Bin, Yongyan Zhang, Jiapeng Liu, Ou Sheng, Fan Liu, Dongliang Qiu, Peitao Lü, Guiming Deng, and Chunzhen Cheng. 2022. "A New Leaf Blight Disease Caused by Alternaria jacinthicola on Banana in China" Horticulturae 8, no. 1: 12. https://doi.org/10.3390/horticulturae8010012
APA StyleWang, B., Zhang, Y., Liu, J., Sheng, O., Liu, F., Qiu, D., Lü, P., Deng, G., & Cheng, C. (2022). A New Leaf Blight Disease Caused by Alternaria jacinthicola on Banana in China. Horticulturae, 8(1), 12. https://doi.org/10.3390/horticulturae8010012







