Impact of Salinity on the Growth and Chemical Composition of Two Underutilized Wild Edible Greens: Taraxacum officinale and Reichardia picroides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Treatments and Growing Conditions
2.2. Growth Parameters
2.3. Leaf and Root Minerals and Nitrate Content
2.4. Chemical Composition Analyses in Leaves
2.4.1. Total Phenolic Compounds Content
2.4.2. Proline Content
2.5. Statistical Analysis
3. Results and Discussion
3.1. Plant Growth
3.2. Leaf and Root Minerals Concentrations
3.2.1. Total Leaf Nitrogen Content
3.2.2. Leaf and Root Phosphorus Content
3.2.3. Leaf and Root Potassium Content
3.2.4. Leaf and Root Calcium and Magnesium Content
3.2.5. Leaf and Root Sodium and Leaf Chlorine Content
3.2.6. Leaf K/Na and Ca/Na Ratios
3.2.7. Leaf and Root Micronutrients Content
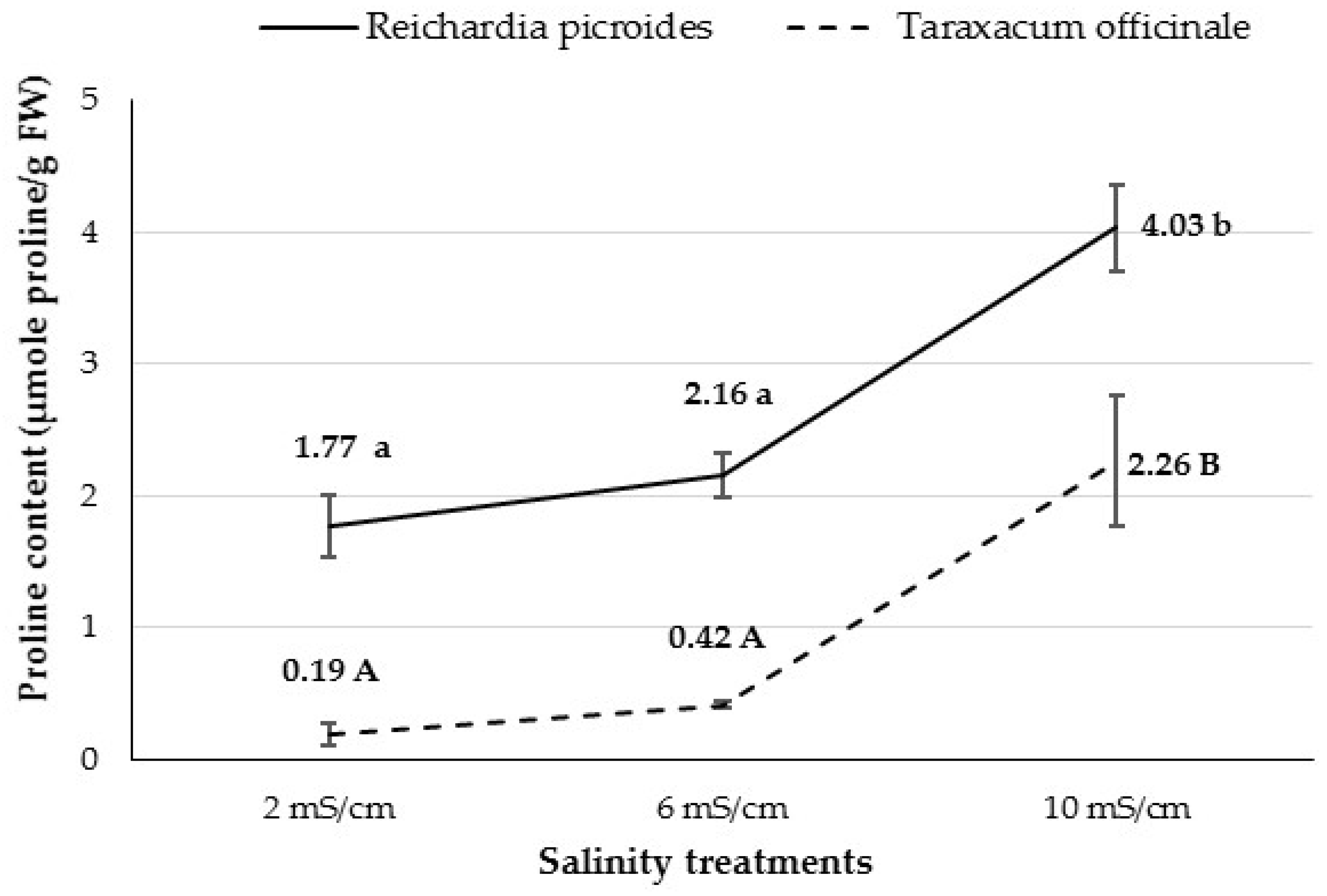
3.2.8. Proline Content
3.3. Chemical Composition
3.4. Correlations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments—A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Paranychianakis, N.V.; Chartzoulakis, K.S. Irrigation of Mediterranean crops with saline water: From physiology to management practices. Agric. Ecosyst. Environ. 2005, 106, 171–187. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriti, M.; Vitalini, S. Sustainable crop protection, global climate change, food security and safety—Plant immunity at the crossroads. Vaccines 2020, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A. Practical applications of plant biostimulants in greenhouse vegetable crop production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Voutsela, S.; Yarsi, G.; Petropoulos, S.A.; Khan, E.M. The effect of grafting of five different rootstocks on plant growth and yield of tomato plants cultivated outdoors and indoors under salinity stress. Afr. J. Agric. Res. 2012, 7, 5553–5557. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Khah, E.M.; Passam, H.C. Evaluation of rootstocks for watermelon grafting with reference to plant development, yield and fruit quality. Int. J. Plant Prod. 2012, 6, 481–492. [Google Scholar]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Biodiversity for Food and Agriculture. In The State of the World’s Biodiversity for Food and Agriculture; Pilling, D., Bélanger, J., Eds.; FAO Commission on Genetic Resources for Food and Agriculture Assessments: Rome, Italy, 2019; p. 572. ISBN 9789251312704. [Google Scholar]
- Corrêa, R.C.G.; Gioia, F.D.; Ferreira, I.C.F.R.; Petropoulos, S.A. Wild greens used in the Mediterranean diet. In The Mediterranean Diet: An Evidence-based Approach; Preedy, V., Watson, R., Eds.; Academic Press: London, UK, 2020; pp. 209–228. ISBN 9788578110796. [Google Scholar]
- Chatzopoulou, E.; Carocho, M.; Di Gioia, F.; Petropoulos, S.A. The beneficial health effects of vegetables and wild edible greens: The case of the mediterranean diet and its sustainability. Appl. Sci. 2020, 10, 9144. [Google Scholar] [CrossRef]
- Capurso, C.; Vendemiale, G. The Mediterranean Diet Reduces the Risk and Mortality of the Prostate Cancer: A Narrative Review. Front. Nutr. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Maggini, R.; Pardossi, A. Agronomic, Nutraceutical, and Organoleptic Performances of Wild Herbs of Ethnobotanical Tradition. Int. J. Veg. Sci. 2017, 23, 270–281. [Google Scholar] [CrossRef]
- Johns, T.; Powell, B.; Maundu, P.; Eyzaguirre, P.B. Agricultural biodiversity as a link between traditional food systems and contemporary development, social integrity and ecological health. J. Sci. Food Agric. 2013, 93, 3433–3442. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT-Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Zimmerer, K.S.; Vanek, S.J. Toward the integrated framework analysis of linkages among agrobiodiversity, livelihood diversification, ecological systems, and sustainability amid global change. Land 2016, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Conversa, G.; Elia, A. Effect of seed age, stratification, and soaking on germination of wild asparagus (Asparagus acutifolius L.). Sci. Hortic. 2009, 119, 241–245. [Google Scholar] [CrossRef]
- Cros, V.; Martínez-Sánchez, J.J.; Franco, J.A. Good yields of common purslane with a high fatty acid content can be obtained in a peat-based floating system. Horttechnology 2007, 17, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Ntatsi, G.; Barros, L.; Ferreira, I. Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 2017, 237, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Antoniadis, V.; Barros, L.; Ferreira, I. Nutrient solution composition and growing season affect yield and chemical composition of Cichorium spinosum plants. Sci. Hortic. 2018, 231, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finimundy, T.C.; Karkanis, A.; Fernandes, Â.; Petropoulos, S.A.; Calhelha, R.; Petrović, J.; Soković, M.; Rosa, E.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of Sanguisorba minor L. cultivated in central Greece under different fertilization regimes. Food Chem. 2020, 327. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Ivanov, M.; Sokovic, M.D.; Ferreira, I.C.F.R.; Barros, L. The Effect of Nitrogen Fertigation and Harvesting Time on Plant Growth and Chemical Composition of Centaurea raphanina subsp. mixta (DC.) Runemark. Molecules 2020, 25, 1–21. [Google Scholar]
- Petropoulos, S.; Karkanis, A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G.; Petrotos, K.; Lykas, C.; Khah, E. Chemical composition and yield of six genotypes of common purslane (Portulaca oleracea L.): An alternative source of omega-3 fatty acids. Plant Foods Hum. Nutr. 2015, 70, 420–426. [Google Scholar] [CrossRef]
- González, J.A.; García-Barriuso, M.; Amich, F. The consumption of wild and semi-domesticated edible plants in the Arribes del Duero (Salamanca-Zamora, Spain): An analysis of traditional knowledge. Genet. Resour. Crop. Evol. 2011, 58, 991–1006. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Marandos, E.; Assimakopoulou, A.; Vidalis, N.; Petropoulos, S.A.; Karapanos, I.C. Effect of Nutrient Solution pH on the Growth, Yield and Quality of Taraxacum officinale and Reichardia picroides in a Floating Hydroponic System. Agronomy 2021, 11, 1118. [Google Scholar] [CrossRef]
- Rao, N.S.; Shivashankara, K.S.; Laxman, R.H. Abiotic Stress Physiology of Horticultural Crops; Rao, N.S., Shivashankara, K.S., Laxman, R.H., Eds.; Springer India: New Delhi, India, 2016; ISBN 9788132227236. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [Green Version]
- Giordano, M.; Petropoulos, S.A. Response and Defence Mechanisms of Vegetable Crops against Drought, Heat and Salinity Stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C. Biochemical, Physiological, and Molecular Aspects of Ornamental Plants Adaptation to Deficit Irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J. Food Security in the Face of Salinity, Drought, Climate Change, and Population Growth. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 111–123. ISBN 9780128018545. [Google Scholar]
- Correa, R.C.G.; Di Gioia, F.; Ferreira, I.; SA, P. Halophytes for Future Horticulture: The Case of Small-Scale Farming in the Mediterranean Basin. In Halophytes for Future Horticulture: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 1–28. ISBN 9783030178543. [Google Scholar]
- Alleva, K.; Niemietz, C.M.; Sutka, M.; Maurel, C.; Parisi, M.; Tyerman, S.D.; Amodeo, G. Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J. Exp. Bot. 2006, 57, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, Z.; Li, H.; Zhang, X.; Wang, X.; Lu, X. Cultivation of dandelion (Taraxacum erythropodium) on coastal saline land based on the control of salinity and fertilizer. Folia Hortic. 2019, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Salonikioti, A.; Petropoulos, S.; Antoniadis, V.; Levizou, E.; Alexopoulos, A. Wild Edible Species with Phytoremediation Properties. Procedia Environ. Sci. 2015, 29, 98–99. [Google Scholar] [CrossRef] [Green Version]
- Bosiacka, B.; Mysliwy, M.; Bosiacki, M. Habitat conditions strongly affect macroand microelement concentrations in Taraxacum microspecies growing on coastal meadows along a soil salinity gradient. PeerJ 2020, 8. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Chrysargyris, A.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.D.; Barros, L.; et al. Chemical composition and plant growth of Centaurea raphanina subsp. mixta plants cultivated under saline conditions. Molecules 2020, 25, 2204. [Google Scholar] [CrossRef]
- Kalra, Y. Handbook of Reference Methods for Plant. Analysis; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Boltz, D.F.; Lueck, C.H. Colorimetric Determination of Non-Metals; Interscience Publishers: New York, NY, USA, 1958. [Google Scholar]
- Allen, S. Chemical Analysis of Ecological Materials, 2nd ed.; Blackwell Scientific Publications: Oxford and London, UK, 1989. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Karapanos, I.; Papandreou, A.; Skouloudi, M.; Makrogianni, D.; Fernández, J.A.; Rosa, E.; Ntatsi, G.; Bebeli, P.J.; Savvas, D. Cowpea fresh pods—a new legume for the market: Assessment of their quality and dietary characteristics of 37 cowpea accessions grown in southern Europe. J. Sci. Food Agric. 2017, 97, 4343–4352. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bates, L.S. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Uddin, M.N.; Tariqul Islam, M.; Karim, M.A. Salinity tolerance of three mustard/rapeseed cultivars. J. Bangladesh Agril. Univ. 2005, 3, 203–208. [Google Scholar]
- Wang, Y.; Nii, N. Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hortic. Sci. Biotechnol. 2000, 75, 623–627. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hu, Y.; Schmidhalter, U. Growth, ion content, gas exchange, and water relations of wheat genotypes differing in salt tolerances. Aust. J. Agric. Res. 2005, 56, 123–134. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Rangaiah, D. V High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust. J. Crop. Sci. 2008, 2, 40–48. [Google Scholar]
- Turhan, A.; Eniz, V.; Kuşçu, H. Genotypic variation in the response of tomato to salinity. Afr. J. Biotechnol. 2009, 8, 1062–1068. [Google Scholar] [CrossRef]
- Ziaf, K.; Amjad, M.; Pervez, M.A.; Iqbal, Q.; Rajwana, I.A.; Ayyub, M. Evaluation of different growth and physiological traits as indices of salt tolerance in hot pepper (Capsicum annuum L.). Pak. J. Bot. 2009, 41, 1797–1809. [Google Scholar]
- Fageria, N.K.; Gheyi, H.R.; Moreira, A. Nutrient bioavailability in salt affected soils. J. Plant Nutr. 2011, 34, 945–962. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. HortScience 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Sergio, L.; de Paola, A.; Cantore, V.; Pieralice, M.; Cascarano, N.A.; Bianco, V.V.; Di Venere, D. Effect of salt stress on growth parameters, enzymatic antioxidant system, and lipid peroxidation in wild chicory (Cichorium intybus L.). Acta Physiol. Plant 2012, 34, 2349–2358. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulou, A.; Salmas, I.; Roussos, P.A.; Nifakos, K.; Kalogeropoulos, P.; Kostelenos, G. Salt tolerance evaluation of nine indigenous Greek olive cultivars. J. Plant Nutr. 2017, 40, 1099–1110. [Google Scholar] [CrossRef]
- Pérez-Alfocea, F.; Balibrea, M.E.; Santa Cruz, A.; Estañ, M.T. Agronomical and physiological characterization of salinity tolerance in a commercial tomato hybrid. Plant Soil 1996, 180, 251–257. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Xiao, G.; Zhai, M.; Pan, X.; Huang, R.; Zhang, H. CYP71D8L is a key regulator involved in growth and stress responses by mediating gibberellin homeostasis in rice. J. Exp. Bot. 2020, 71, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Akladious, S.A.; Ashry, N.A. Evaluation of Water Stress Tolerance of Soybean Using Physiological Parameters and Retrotransposon-Based Markers. Gesunde Pflanz. 2018, 70, 205–215. [Google Scholar] [CrossRef]
- Maggini, R.; Benvenuti, S.; Leoni, F.; Pardossi, A. Terracrepolo (Reichardia picroides (L.) Roth.): Wild food or new horticultural crop? Sci. Hortic. 2018, 240, 224–231. [Google Scholar] [CrossRef]
- Pessarakli, M.; Tucker, T.C. Dry Matter Yield and Nitrogen-15 Uptake by Tomatoes under Sodium Chloride Stress. Soil Sci. Soc. Am. J. 1988, 52, 698–700. [Google Scholar] [CrossRef]
- Camalle, M.; Standing, D.; Jitan, M.; Muhaisen, R.; Bader, N.; Bsoul, M.; Ventura, Y.; Soltabayeva, A.; Sagi, M. Effect of salinity and nitrogen sources on the leaf quality, biomass, and metabolic responses of two ecotypes of Portulaca oleracea. Agronomy 2020, 10, 656. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants–focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Pizzorno, L. 203-Osteoporosis, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2020; pp. 1633–1658.e17. ISBN 978-0-323-52342-4. [Google Scholar]
- Assimakopoulou, A.; Nifakos, K.; Salmas, I.; Kalogeropoulos, P. Growth, Ion Uptake, and Yield Responses of Three Indigenous Small-Sized Greek Tomato (Lycopersicon esculentum L.) Cultivars and Four Hybrids of Cherry Tomato under NaCl Salinity Stress. Commun. Soil Sci. Plant Anal. 2015, 46, 2357–2377. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Villora, G.; Moreno, D.A.; Pulgar, G.; Romero, L. Salinity affects phosphorus uptake and partitioning in zucchini. Commun. Soil Sci. Plant Anal. 2000, 31, 501–507. [Google Scholar] [CrossRef]
- Tang, H.; Niu, L.; Wei, J.; Chen, X.; Chen, Y. Phosphorus limitation improved salt tolerance in maize through tissue mass density increase, osmolytes accumulation, and Na+ uptake inhibition. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rus, A.; Lee, B.H.; Muñoz-Mayor, A.; Sharkhuu, A.; Miura, K.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol. 2004, 136, 2500–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, M.; Van Hasselt, P.; Kuiper, P. NaCl effects on root plasma membrane ATPase of salt tolerant wheat. Biol. Plant. 2000, 43, 61–66. [Google Scholar] [CrossRef]
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Shibli, R.A.; Sawwan, J.; Swaidat, I.; Tahat, M. Increased phosphorus mitigates the adverse effects of salinity in tissue culture. Commun. Soil Sci. Plant Anal. 2001, 32, 429–440. [Google Scholar] [CrossRef]
- Essa, T.A. Effect of Salinity Stress on Growth and Nutrient Composition of Three Soybean (Glycine max L. Merrill) Cultivars. J. Agron. Crop. Sci. 2002, 188, 86–93. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Badakhshan, H. Effects of Zinc Application on Growth, Absorption and Distribution of Mineral Nutrients under Salinity Stress in Soybean (Glycine max L.). J. Plant Nutr. 2014, 37, 2255–2269. [Google Scholar] [CrossRef]
- Assimakopoulou, A.; Salmas, I.; Nifakos, K.; Kalogeropoulos, P. Effect of salt stress on Three Green Bean (Phaseolus vulgaris L.) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Semiz, G.D.; Suarez, D.L.; Ünlükara, A.; Yurtseven, E. Interactive Effects of Salinity and N on Pepper (Capsicum annuum L.) Yield, Water Use Efficiency and Root Zone and Drainage Salinity. J. Plant Nutr. 2014, 37, 595–610. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Stallings, V.A.; Harrison, M.; Oria, M. Dietary Reference Intakes for Sodium and Potassium; Stallings, V.A., Harrison, M., Oria, M., Eds.; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bolarín, M.C.; Fernández, F.G.; Cruz, V.; Cuartero, J. Salinity Tolerance in Four Wild Tomato Species using Vegetative Yield-Salinity Response Curves. J. Am. Soc. Hortic. Sci. 2019, 116, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Subbarao, G.V.; Johansen, C.; Jana, M.K.; Kumar Rao, J.V.D.K. Effects of the Sodium/Calcium Ratio in Modifying Salinity Response of Pigeonpea (Cajanus cajan). J. Plant Physiol. 1990, 136, 439–443. [Google Scholar] [CrossRef]
- Hunt, C.D.; Johnson, L.A.K. Calcium requirements: New estimations for men and women by cross-sectional statistical analyses of calcium balance data from metabolic studies. Am. J. Clin. Nutr. 2007, 86, 1054–1063. [Google Scholar] [CrossRef]
- Onodera, M.; Nakajima, T.; Nanzyo, M.; Takahashi, T.; Xu, D.; Homma, K.; Kokubun, M. Regulation of root-to-leaf Na and Cl transport and its association with photosynthetic activity in salt-tolerant soybean genotypes. Plant Prod. Sci. 2019, 22, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.Q.; Gao, Q. Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars. BMC Plant Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Dietary Reference Values for chloride. EFSA J. 2019, 17, e05779. [Google Scholar]
- Almeida, D.M.; Margarida Oliveira, M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingham, F.T.; Strong, J.E.; Rhoades, J.D.; Keren, R. Effects of salinity and varying boron concentrations on boron uptake and growth of wheat. Plant Soil 1987, 351, 345–351. [Google Scholar] [CrossRef]
- Hasana, R.; Miyake, H. Salinity Stress Alters Nutrient Uptake and Causes the Damage of Root and Leaf Anatomy in Maize. KnE Life Sci. 2017, 3, 219. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Vance, G.F.; Munn, L.C. Salinity Induced Effects on the Nutrient Status of Soil, Corn Leaves and Kernels. Commun. Soil Sci. Plant Anal. 1993, 24, 2251–2269. [Google Scholar] [CrossRef]
- Izzo, R.; Navari-Izzo, F.; Quartacci, M.F. Growth and mineral absorption in maize seedlings as affected by increasing NaCl concentrations. J. Plant Nutr. 1991, 14, 687–699. [Google Scholar] [CrossRef]
- Shibli, R.A.; Kushad, M.; Yousef, G.G.; Lila, M.A. Physiological and biochemical responses of tomato microshoots to induced salinity stress with associated ethylene accumulation. Plant Growth Regul. 2007, 51, 159–169. [Google Scholar] [CrossRef]
- Neocleous, D.; Koukounaras, A.; Siomos, A.S.; Vasilakakis, M. Assessing the Salinity Effects on Mineral Composition and Nutritional Quality of Green and Red “Baby” Lettuce. J. Food Qual. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Fatemi, H.; Carvajal, M.; Rios, J.J. Foliar application of Zn alleviates salt stress symptoms of pak choi plants by activating water relations and glucosinolate synthesis. Agronomy 2020, 10, 1528. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111 Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development BT-Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., A. Khan, N., Eds.; Springer India: New Delhi, India, 2016; pp. 155–166. ISBN 978-81-322-2616-1. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Liu, A.; Hua, X. Proline accumulation and transcriptional regulation of proline biothesynthesis and degradation in Brassica napus. BMB Rep. 2009, 42, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Chou, I.T.; Chen, C.T.; Kao, C.H. Characteristics of the induction of the accumulation of proline by abscisic acid and isobutyric acid in detached rice leaves. Plant Cell Physiol. 1991, 32, 269–272. [Google Scholar] [CrossRef]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.A.H. Amelioration of Chilling Injuries in Mung Bean (Vigna radiata L.) Seedlings by Paclobutrazol, Abscisic Acid and Hydrogen Peroxide. Am. J. Plant Physiol. 2007, 2, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Mou, B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J. Am. Soc. Hortic. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Chatzigianni, M.; Alkhaled, B.; Livieratos, I.; Stamatakis, A.; Ntatsi, G.; Savvas, D. Impact of nitrogen source and supply level on growth, yield and nutritional value of two contrasting ecotypes of Cichorium spinosum L. grown hydroponically. J. Sci. Food Agric. 2017, 98, 1615–1624. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Singh, H.; Dunn, B.; Payton, M.; Brandenberger, L. Fertilizer and cultivar selection of lettuce, Basil, and Swiss chard for hydroponic production. Horttechnology 2019, 29, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar] [CrossRef]
- Petersen, K.K.; Willumsen, J.; Kaack, K. Composition and taste of tomatoes as affected by increased salinity and different salinity sources. J. Hortic. Sci. Biotechnol. 1998, 73, 205–215. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Klados, E.; Tzortzakis, N. Effects of substrate and salinity in hydroponically grown Cichorium spinosum. J. Soil Sci. Plant Nutr. 2014, 14, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Garrido, Y.; Tudela, J.A.; Marín, A.; Mestre, T.; Martínez, V.; Gil, M.I. Physiological, phytochemical and structural changes of multi-leaf lettuce caused by salt stress. J. Sci. Food Agric. 2014, 94, 1592–1599. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Dae, Y.K. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Gent, M.P.N. Composition of hydroponic lettuce: Effect of time of day, plant size, and season. J. Sci. Food Agric. 2012, 92, 542–550. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cantabella, D.; Piqueras, A.; Acosta-Motos, J.R.; Bernal-Vicente, A.; Hernández, J.A.; Díaz-Vivancos, P. Salt-tolerance mechanisms induced in Stevia rebaudiana Bertoni: Effects on mineral nutrition, antioxidative metabolism and steviol glycoside content. Plant Physiol. Biochem. 2017, 115, 484–496. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Kafkafi, U.; Yaeesh Siddiqi, M.; Ritchie, R.J.; Glass, A.D.M.; Ruth, T.J. Reduction of nitrate (13no3) influx and nitrogen (13n) translocation by tomato and melon varieties after short exposure to calcium and potassium chloride salts. J. Plant Nutr. 1992, 15, 959–975. [Google Scholar] [CrossRef]
- Abdelgadir, E.M.; Oka, M.; Fujiyama, H. Characteristics of nitrate uptake by plants under salinity. J. Plant Nutr. 2005, 28, 33–46. [Google Scholar] [CrossRef]
- Rameeh, V.; Rezai, A.; Saeidi, G. Study of salinity tolerance in rapeseed. Commun. Soil Sci. Plant Anal. 2004, 35, 2849–2866. [Google Scholar] [CrossRef]
| EC (mS cm−1) | Leaf Number Plant−1 | Rosette Diameter (cm) | Nonmarketable Leaf Number Plant−1 | SPAD Index | Maximum Leaf Length (cm) | Maximum Leaf Width (cm) |
| 2.0 | 32.28 b * | 35.56 b | 2.18 a | 49.94 a | 18.41 b | 2.79 b |
| 6.0 | 40.65 c | 28.60 b | 2.60 a | 54.61 a | 14.63 b | 2.48 b |
| 10.0 | 24.75 a | 17.32 a | 1.93 a | 52.11 a | 8.97 a | 1.86 a |
| EC (mS cm−1) | Total Plant FW (kg m−2) | Upper Plant Part FW (kg m−2) | Root FW (kg m−2) | Root/Shoot Ratio | Marketable Leaves FW (kg m−2) | Leaf DMC (%) |
| 2.0 | 1.40 b | 1.12 b | 0.27 a | 0.25 a | 0.99 b | 9.23 b |
| 6.0 | 1.68 b | 1.30 b | 0.37 a | 0.29 a | 1.13 b | 7.89 a |
| 10.0 | 0.96 a | 0.72 a | 0.23 a | 0.32 a | 0.62 a | 8.63 ab |
| EC (mS cm−1) | Leaf Number Plant−1 | Rosette Diameter (cm) | Nonmarketable Leaf Number Plant−1 | SPAD Index | Maximum Leaf Length (cm) | Maximum Leaf Width (cm) |
| 2.0 | 31.00 b * | 37.60 b | 1.80 a | 42.40 a | 19.15 b | 6.28 b |
| 6.0 | 13.96 a | 18.73 a | 1.78 a | 42.59 a | 9.62 a | 3.41 a |
| 10.0 | 16.09 a | 19.98 a | 1.62 a | 39.34 a | 10.41 a | 3.81 a |
| EC (mS cm−1) | Total Plant FW (kg m−2) | Upper Plant Part FW (kg m−2) | Root FW (kg m−2) | Root/Shoot Ratio | Marketable Leaves FW (kg m−2) | Leaf DMC (%) |
| 2.0 | 1.37 b | 1.01 b | 0.35 b | 0.35 a | 0.90 b | 11.59 a |
| 6.0 | 0.35 a | 0.23 a | 0.12 a | 0.52 b | 0.20 a | 15.16 b |
| 10.0 | 0.44 a | 0.29 a | 0.15 a | 0.53 b | 0.21 a | 15.14 b |
| Leaf | N | P | K | Ca | Mg | Na | Cl | Fe | Mn | Zn | Cu | B | K/Na | Ca/Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % leaf DW | mg kg−1 leaf DW | |||||||||||||
| EC (mS cm−1) | Reichardia picroides | |||||||||||||
| 2.0 | 4.25 a * | 0.77 a | 7.04 c | 0.90 b | 0.20 b | 0.55 a | 0.82 a | 60.0 a | 42.9 a | 37.5 a | 4.8 a | 143.2 b | 12.7 b | 1.63 b |
| 6.0 | 4.37 a | 1.04 b | 4.49 b | 0.72 a | 0.16 a | 3.68 b | 1.09 a | 57.2 a | 46.2 a | 65.7 b | 3.5 a | 138.2 ab | 1.23 a | 0.19 a |
| 10.0 | 5.12 b | 1.29 c | 3.66 a | 0.63 a | 0.16 a | 4.02 c | 1.79 b | 55.6 a | 71.6 b | 75.4 c | 4.5 a | 129.1 a | 0.91 a | 0.16 a |
| Taraxacum officinale | ||||||||||||||
| 2.0 | 4.77 b | 1.16 a | 5.48 b | 0.92 b | 0.33 c | 0.05 a | 0.53 a | 77.2 ab | 30.7 a | 39.1 a | 6.8 a | 46.8 a | 137.4 b | 23.18 b |
| 6.0 | 4.81 b | 1.37 b | 3.02 a | 0.75 a | 0.26 a | 2.00 b | 0.99 b | 68.5 a | 23.7 a | 45.8 ab | 6.1 a | 35.0 a | 1.51 a | 0.38 a |
| 10.0 | 4.17 a | 1.69 c | 2.99 a | 0.83 ab | 0.29 b | 3.84 c | 1.58 c | 90.0 b | 31.9 a | 50.3 b | 5.7 a | 38.8 a | 0.78 a | 0.22 a |
| Root | P | K | Ca | Mg | Na | Fe | Mn | Zn | Cu | B | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % leaf DW | mg kg−1 leaf DW | ||||||||||
| EC (dS m−1) | Reichardia picroides | ||||||||||
| 2.0 | 1.04 a * | 5.98 b | 0.37 a | 0.17 b | 0.19 a | 221.8 a | 60.3 a | 84.2 b | 15.1 a | 14.1 a | |
| 6.0 | 1.46 b | 5.35 a | 0.33 a | 0.15 ab | 1.02 b | 385.9 b | 46.2 a | 98.8 c | 16.7 a | 15.4 a | |
| 10.0 | 1.67 c | 5.00 a | 0.41 a | 0.14 a | 1.22 b | 257.8 a | 55.5 a | 62.8 a | 14.9 a | 17.4 a | |
| Taraxacum officinale | |||||||||||
| 2.0 | 1.47 b | 5.27 b | 0.46 a | 0.18 a | 0.11 a | 162.1 a | 16.3 a | 50.8 a | 15.5 b | 16.7 a | |
| 6.0 | 1.18 a | 4.01 a | 0.43 a | 0.17 a | 0.71 b | 158.5 a | 16.7 a | 44.6 a | 10.7 a | 21.7 b | |
| 10.0 | 1.28 ab | 3.62 a | 0.51 a | 0.17 a | 1.20 c | 157.3 a | 20.0 a | 44.6 a | 9.4 a | 15.6 a | |
| Nutrient Solution EC | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids+ Xanthophylls | Total Phenolics | TSSC | TA | Nitrate Content |
|---|---|---|---|---|---|---|---|---|
| EC (dS m−1) | mg/100 g leaf FW | mg GAE/100 g leaf FW | 0 Brix | g malic acid/100 g leaf FW | mg/kg leaf FW | |||
| Reichardia picroides | ||||||||
| 2 | 41.0 a * | 18.7 a | 59.6 a | 7.08 a | 113.6 b | 5.00 a | 0.16 a | 5509.8 ab |
| 6 | 41.6 a | 19.0 a | 60.6 a | 7.21 a | 100.9 b | 4.58 a | 0.17 a | 6877.6 b |
| 10 | 41.4 a | 18.3 a | 59.7 a | 7.01 a | 62.2 a | 4.73 a | 0.22 b | 4586.9 a |
| Taraxacum officinale | ||||||||
| 2 | 77.7 a | 38.2 a | 115.9 a | 12.3 a | 79.6 a | 5.50 a | 0.16 a | 2410.5 b |
| 6 | 82.4 a | 39.6 a | 122.0 a | 13.2 a | 91.6 a | 6.10 a | 0.20 ab | 885.7 a |
| 10 | 87.0 a | 43.0 a | 130.0 a | 13.5 a | 95.8 a | 5.85 a | 0.22 b | 597.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexopoulos, A.A.; Assimakopoulou, A.; Panagopoulos, P.; Bakea, M.; Vidalis, N.; Karapanos, I.C.; Petropoulos, S.A. Impact of Salinity on the Growth and Chemical Composition of Two Underutilized Wild Edible Greens: Taraxacum officinale and Reichardia picroides. Horticulturae 2021, 7, 160. https://doi.org/10.3390/horticulturae7070160
Alexopoulos AA, Assimakopoulou A, Panagopoulos P, Bakea M, Vidalis N, Karapanos IC, Petropoulos SA. Impact of Salinity on the Growth and Chemical Composition of Two Underutilized Wild Edible Greens: Taraxacum officinale and Reichardia picroides. Horticulturae. 2021; 7(7):160. https://doi.org/10.3390/horticulturae7070160
Chicago/Turabian StyleAlexopoulos, Alexios A., Anna Assimakopoulou, Panagiotis Panagopoulos, Maria Bakea, Nikolina Vidalis, Ioannis C. Karapanos, and Spyridon A. Petropoulos. 2021. "Impact of Salinity on the Growth and Chemical Composition of Two Underutilized Wild Edible Greens: Taraxacum officinale and Reichardia picroides" Horticulturae 7, no. 7: 160. https://doi.org/10.3390/horticulturae7070160
APA StyleAlexopoulos, A. A., Assimakopoulou, A., Panagopoulos, P., Bakea, M., Vidalis, N., Karapanos, I. C., & Petropoulos, S. A. (2021). Impact of Salinity on the Growth and Chemical Composition of Two Underutilized Wild Edible Greens: Taraxacum officinale and Reichardia picroides. Horticulturae, 7(7), 160. https://doi.org/10.3390/horticulturae7070160








