An Optimized Protocol for In Vitro Indirect Shoot Organogenesis of Impala Bronzovaya and Zanzibar Green Ricinus communis L. Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Obtaining of Aseptic Donor Plants
2.2. The Preparation of Explants and their Cultivation
2.3. Statistical Analysis
3. Results
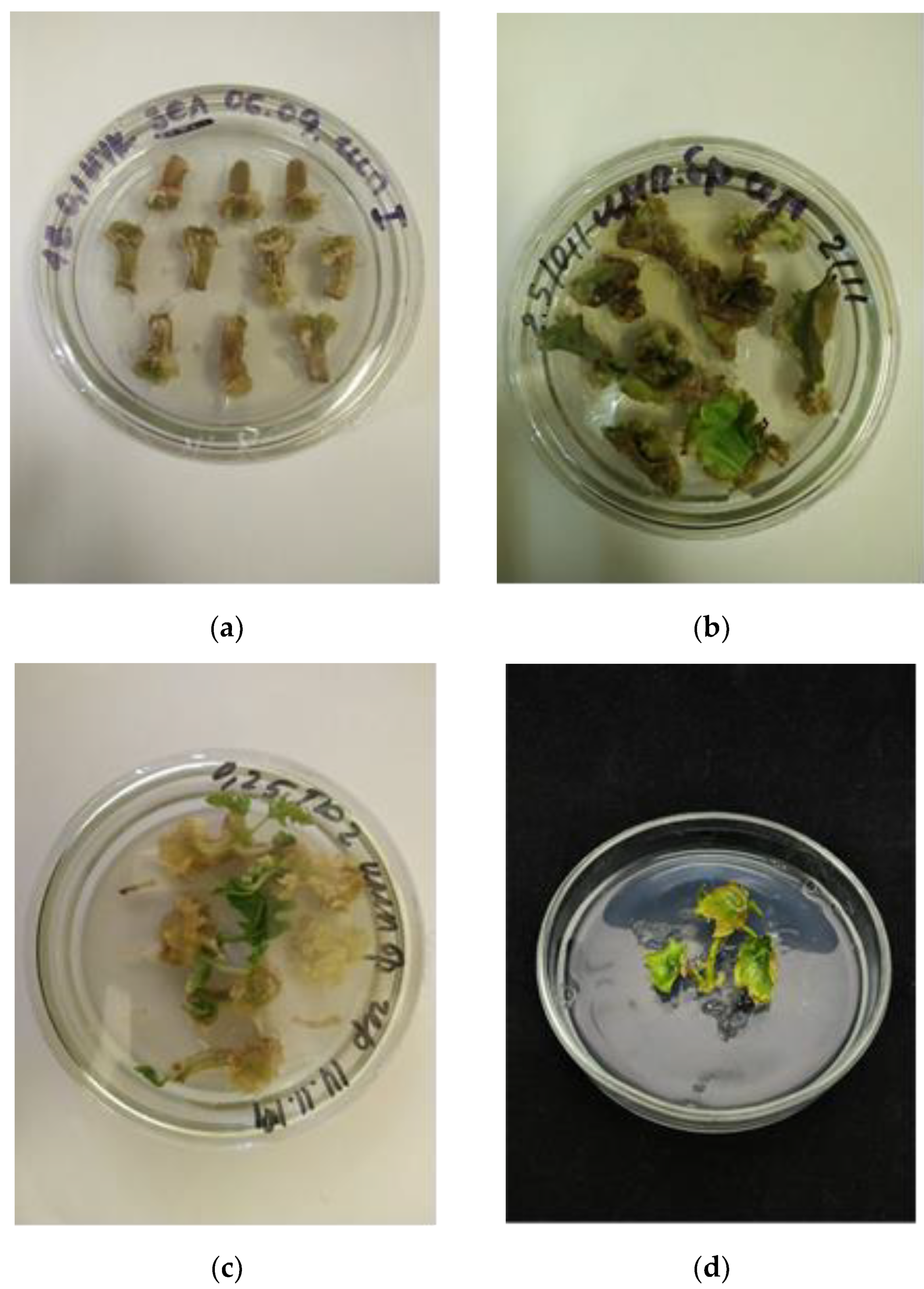
3.1. Callus Formation and Characteristics
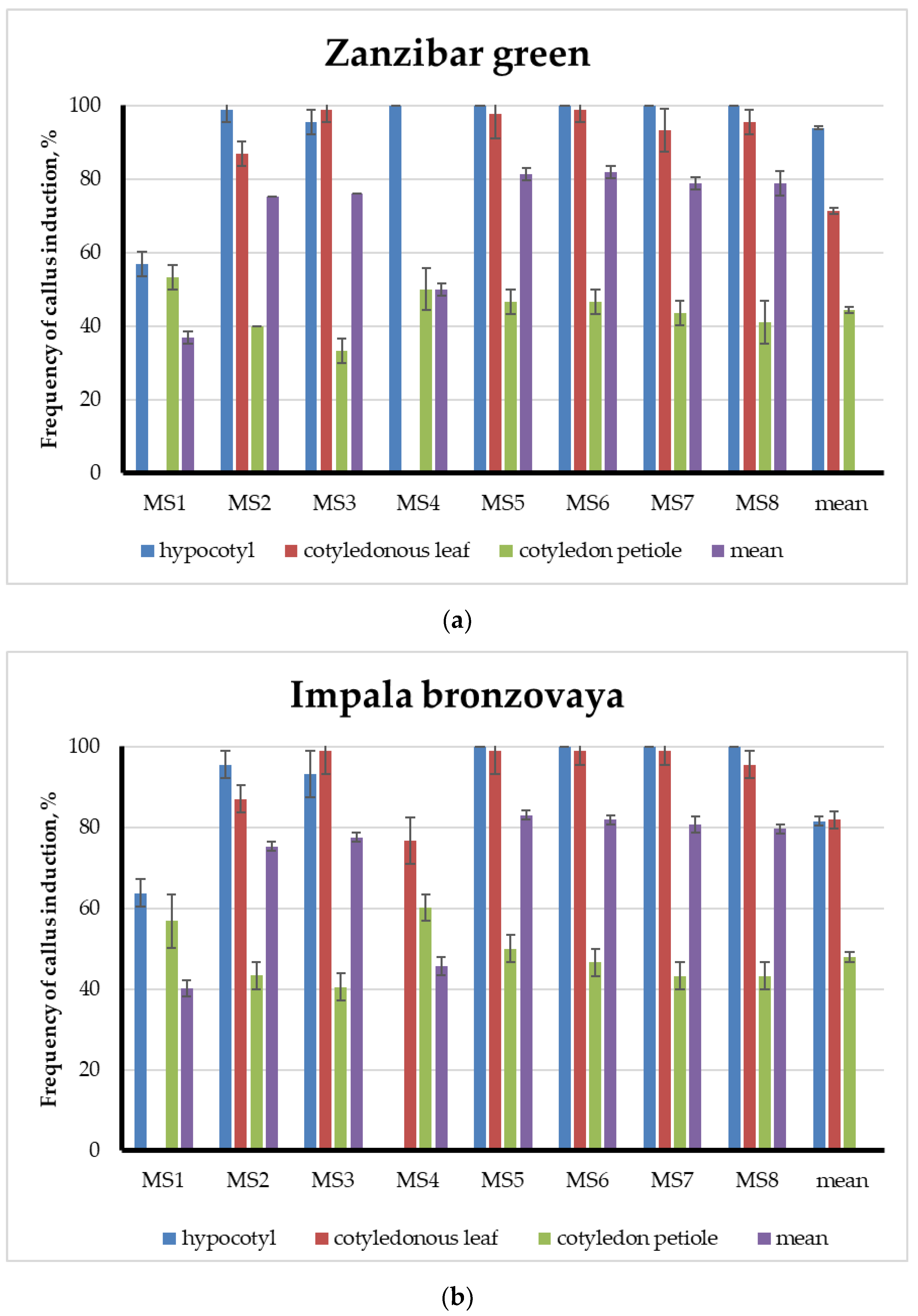
3.2. A Comparative Analysis of Callus Formation Frequencies
3.3. Somatic Organogenesis of Shoots and Comparative Analysis of Frequencies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Perry, B.A. Chromosome number and phylogenetic relationships in the Euphorbiaceae. Am. J. Bot. 1943, 30, 527–543. [Google Scholar] [CrossRef]
- Fayed, A.A.; Soliman, M.; Faried, A.; Hassan, M. Taxonomic evaluation of Euphorbiaceae sensu lato with special reference to Phyllanthaceae as a new family to the flora of Egypt. Biol. Forum 2019, 11, 47–64. [Google Scholar]
- Weiss, E.A. Oilseed Crops; Blackwell Science: London, UK, 2000; pp. 13–15. [Google Scholar]
- Kreissig, K. Identify Common Tropical and Subtropical Ornamental Plants by Flower Color; Springer: Berlin/Heidelberg, Germany, 2019; p. 40. [Google Scholar]
- Kim, H.; Lei, P.; Wang, A.; Liu, S.; Zhao, Y.; Huang, F.; Yu, Z.; Zhu, G.; He, Z.; Tan, D.; et al. Genetic diversity of castor bean (Ricinus communis L.) revealed by ISSR and RAPD markers. Agronomy 2021, 11, 457. [Google Scholar] [CrossRef]
- Foster, J.T.; Allan, G.J.; Chan, A.P.; Rabinowicz, P.D.; Ravel, J.; Jackson, P.J.; Keim, P. Single nucleotide polymorphisms for assessing genetic diversity in castor bean (Ricinus communis). BMC Plant Biol. 2010, 10, 13. [Google Scholar] [CrossRef]
- Máira Milani, M.; de Medeiros Nóbrega, M.B. Castor breeding. In Plant Breeding from Laboratories to Fields; Andersen, S.B., Ed.; IntechOpen: London, UK, 2013; pp. 239–254. [Google Scholar]
- Caupin, H.J. Products from castor oil: Past, present, and future. In Lipid Technologies and Applications; Gunstone, F.D., Padley, F.B., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 787–795. [Google Scholar]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Cândido, M.J.D.; Chen, G.; Crosby, W.; Tan, D.; He, X.; Lakshmamma, P.; Lavanya, C.; et al. A review on the challenges for increased production of castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor oil: Properties, uses, and optimization of processing parameters in commercial production. Lipid Insights 2016, 9, 1–12. [Google Scholar] [CrossRef]
- De Lima da Silva, N.; Maciel, M.; Batistella, C.; Filho, R. Optimization of biodiesel production from castor oil. Appl. Biochem. Biotechnol. 2006, 130, 405–414. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Global Castor Oil Market Research Report 2020. Available online: https://www.360researchreports.com/global-castor-oil-market-15041326 (accessed on 8 February 2021).
- Akande, T.; Odunsi, A.A.; Akinfala, E.O. A review of nutritional and toxicological implications of castor bean (Ricinus communis L.) meal in animal feeding systems. J. Anim. Physiol. Anim. Nutr. 2016, 100, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Worbs, S.; Köhler, K.; Pauly, D.; Avondet, M.A.; Schaer, M.; Dorner, M.B.; Dorner, B.G. Ricinus communis intoxications in human and veterinary medicine—A summary of real cases. Toxins 2011, 3, 1332–1372. [Google Scholar] [CrossRef] [PubMed]
- Lappi, D.A.; Kapmeyer, W.; Beglau, J.M.; Kaplan, N.O. The disulfide bond connecting the chains of ricin. Proc. Natl. Acad. Sci. USA 1978, 75, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Halling, K.C.; Halling, A.C.; Murray, E.E.; Ladin, B.F.; Houston, L.L.; Weaver, R.F. Genomic cloning and characterization of a ricin gene from Ricinus communis. Nucleic Acids Res. 1985, 13, 8019–8033. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S.; Fernandez-Puentes, C.; Carrasco, L.; Vazquez, D. Ribosome inactivation by the toxic lectins abrin and ricin. Eur. J. Biochem. 1975, 60, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tsurugi, K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987, 262, 8128–8130. [Google Scholar] [CrossRef]
- Fernandes, K.V.; Deus-de-Oliveira, N.; Godoy, M.G.; Guimarães, Z.A.; Nascimento, V.V.; Melo, E.J.; Freire, D.M.; Dansa-Petretski, M.; Machado, O.L. Simultaneous allergen inactivation and detoxification of castor bean cake by treatment with calcium compounds. Bras. J. Med. Biol. Res. 2012, 45, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Melo, W.C.; dos Santos, A.S.; Santa Anna, L.M.M.; Pereira, J.N. Acid and enzymatic hydrolysis of the residue from castor bean (Ricinus communis L.) oil extraction for ethanol production: Detoxification and biodiesel process integration. J. Braz. Chem. Soc. 2008, 19, 418–425. [Google Scholar] [CrossRef]
- Madeira, J.V.; Macedo, J.A.; Macedo, G.A. Detoxification of castor bean residues and the simultaneous production of tannase and phytase by solid-state fermentation using Paecilomyces variotii. Bioresour. Technol. 2011, 102, 7343–7348. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.L.; Cabral, G.B.; Vieira, P.M.; Baldoni, A.B.; Aragão, F.J.L. Bio-detoxification of ricin in castor bean (Ricinus communis L.) seeds. Sci. Rep. 2017, 7, 15385. [Google Scholar] [CrossRef]
- Alexandrov, O.S. Study of the upstream ricin gene sequences in different castor (Ricinus communis) varieties as a preliminary step in CRISPR/Cas9 editing. Res. Crops 2020, 21, 344–348. [Google Scholar]
- Bhatia, P.; Ashwath, N.; Senaranta, T.; Midmore, D.J. Tissue culture studies of tomato (Lycopersicon esculentum). Plant Cell Tissue Org. Cult. 2004, 78, 1–21. [Google Scholar] [CrossRef]
- Jabeen, N.; Chaudhry, Z.; Rashid, H.; Mirzaa, B. Effect of genotype and explants type on in vitro shoot regeneration of tomato (Lycopersicon esculantum Mill.). Pak. J. Bot. 2005, 37, 899–903. [Google Scholar]
- Rashid, R.; Bal, S.S. Effect of hormones on direct shoot regeneration in hypocotyl explants of tomato. Not. Sci. Biol. 2010, 2, 70–73. [Google Scholar] [CrossRef][Green Version]
- Tavallaie, F.Z.; Ghareyazie, B.; Bagheri, A.; Sharma, K. Lentil regeneration from cotyledon explant bearing a small part of the embryo axis. Plant Cell Tissue Org. Cult. 2011, 21, 169–180. [Google Scholar] [CrossRef][Green Version]
- Zimik, M.; Arumugam, N. Induction of shoot regeneration in cotyledon explants of the oilseed crop Sesamum indicum L. J. Genet. Eng. Biotechnol. 2017, 15, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Gubis, J.; Lajchova, Z.; Farago, J.; Jurekova, Z. Effect of genotype and explant type on shoot regeneration in tomato (Lycopersicon esculentum Mill.) in vitro. Czech J. Genet. Plant Breed. 2003, 39, 9–14. [Google Scholar] [CrossRef]
- Liu, G.; Huang, J.; Chen, L.; Bao, M. Plant regeneration from excised hypocotyl explants of Platanus acerifolia Willd. In Vitro Cell. Dev. Biol. Plant 2002, 38, 558–563. [Google Scholar] [CrossRef]
- Divya, K.; Anuradha, T.S.; Jami, S.K.; Kirti, P.B. Efficient regeneration from hypocotyl explants in three cotton cultivars. Biol. Plant. 2008, 52, 201–208. [Google Scholar] [CrossRef]
- Kaul, V.; Miller, R.M.; Hutchinson, J.F.; Richards, D. Shoot regeneration from stem and leaf explants of Dendranthema grandiflora Tzvelev (syn. Chrysanthemum morifolium Ramat.). Plant Cell Tissue Org. Cult. 1990, 21, 21–30. [Google Scholar] [CrossRef]
- Sheng, X.; Gu, H.; Yu, H.; Wang, J.; Zhao, Z.; Qi, Z. An efficient shoot regeneration system and Agrobacterium-mediated transformation with codA gene in a doubled haploid line of broccoli. Can. J. Plant Sci. 2016, 96, 1014–1020. [Google Scholar]
- Amano, R.; Momoi, R.; Omata, E.; Nakahara, T.; Kaminoyama, K.; Kojima, M.; Takebayashi, Y.; Ikematsu, S.; Okegawa, Y.; Sakamoto, T.; et al. Molecular and biochemical differences in leaf explants and the implication for regeneration ability in Rorippa aquatica (Brassicaceae). Plants 2020, 9, 1372. [Google Scholar] [CrossRef]
- Jung, W.-S.; Chung, I.-M.; Kim, S.-H.; Chi, H.-Y.; Yu, C.Y.; Ghimire, B.K. Direct shoot organogenesis from Lycium chinense Miller leaf explants and assessment of genetic stability using ISSR markers. Agronomy 2021, 11, 503. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Mendler-Drienyovszki, N.; Hanász, A.; Zsombik, L.; Dobránszki, J. Phytotoxicity and other adverse effects on the in vitro shoot cultures caused by virus elimination treatments: Reasons and solutions. Plants 2021, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived callus of Petunia hybrida cv. Mirage Rose. Biology 2020, 9, 228. [Google Scholar] [CrossRef]
- Young, R.; Kaul, V.; Williams, E.G. Clonal propagation in vitro from immature embryos and flower buds of Lycopersicon peruvianum and L. esculentum. Plant Sci. 1987, 52, 237–242. [Google Scholar] [CrossRef]
- Khaliluev, M.R.; Bogoutdinova, L.R.; Baranova, G.B.; Baranova, E.N.; Kharchenko, P.N.; Dolgov, S.V. Influence of genotype, explant type and component of culture medium on in vitro callus induction and shoot organogenesis of tomato (Solanum lycopersicum L.). Biol. Bull. 2014, 41, 512–521. [Google Scholar] [CrossRef]
- Khaliluev, M.R.; Kharchenko, P.N.; Dolgov, S.V. Development of regeneration system and study of transformation potential of a commercial tomato variety. Russ. Agric. Sci. 2010, 3, 175–179. [Google Scholar] [CrossRef]
- Osman, M.G.; Elhadi, E.A.; Khalafalla, M.M. Callus formation and organogenesis of tomato (Lycopersicon esculentum Mill., c.v. Omdurman) induced by thidiazuron. Afr. J. Biotechnol. 2010, 9, 4407–4413. [Google Scholar]
- Rahman, M.; Bari, M. Callus induction and cell culture of castor (Ricinus communis L. cv. Shabje). J. Bio-Sci. 2014, 20, 161–169. [Google Scholar] [CrossRef]
- Sujatha, M.; Reddy, T.P. Differential cytokinin effects on the stimulation of in vitro shoot proliferation from meristematic explants of castor (Ricinus communis L.). Plant Cell Rep. 1998, 17, 561–566. [Google Scholar] [CrossRef]
- Alam, I.; Sharmin, S.A.; Mondal, S.C.; Alam, M.J.; Khalekuzzaman, M.; Anisuzzaman, M.; Alam, M.F. In vitro micropropagation through cotyledonary node culture of castor bean (Ricinus communis L.). Aust. J. Crop. Sci. 2010, 4, 81–84. [Google Scholar]
- Danso, K.; Afful, N.T.; Annor, C.; Amoatey, H.M. In vitro regeneration of Ricinus communis L. and Jatropha curcas L. for biofuel production. Biotechnology 2011, 10, 400–407. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, X.Y.; Feng, Z.Z.; Geng, X.J.; Mu, S.M.; Huo, H.Y.; Tong, H.; Li, M.Z.; Li, Y.; Chi, Y.; et al. In vitro establishment of a highly effective method of castor bean (Ricinus communis L.) regeneration using shoot explants. J. Integr. Agric. 2016, 15, 1417–1422. [Google Scholar] [CrossRef]
- Gil-Correal, A.; Restrepo-Osorio, C.; Álvarez, J.; Villanueva-Mejía, D. Direct in vitro regeneration of castor bean plants (Ricinus communis) using epicotyls. Biosci. J. 2019, 35, 347–355. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Maramokhin, E.V.; Malakhova, K.V.; Zontikov, D.N. Features of anatomical and morphological structure of callus tissue of Schisandra chinensis (Turcz.) Bail. and Eleutherococcus senticosus Maxim. during cultured in vitro. Biomics 2018, 10, 274–280. [Google Scholar] [CrossRef]
- Börner, A.; Melz, G.; Lenton, J.R. Genetical and physiological studies of gibberellic acid insensitivity in semidwarf rye. Hereditas 1992, 116, 199–201. [Google Scholar] [CrossRef]
- Pinthus, M.J.; Gale, M.D.; Appleford, N.E.J.; Lenton, J.R. Effect of temperature on gibberellin (GA) responsiveness and on endogenous GA1 content of tall and dwarf wheat genotypes. Plant Physiol. 1989, 90, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Khafagi, I.K. Variation of callus induction and active metabolite accumulation in callus cultures of two varieties of Ricinus communis L. Biotechnology 2007, 6, 193–201. [Google Scholar]
- Liu, Y.; Yan, S.; Yang, F.; Li, D.; Tang, J.; Liu, G.; Lin, S.; Niu, S.; Yang, Y. High Concentration of benzyladenine solution stimulates anthers for inducing callus in Ricinus Communis L. IOP Conf. Ser. Mater. Sci. Eng. 2017, 275, 012011. [Google Scholar] [CrossRef]
| Plant Growth Regulators | Concentration of Plant Growth Regulators in MS Culture Media, mg/L | |||||||
|---|---|---|---|---|---|---|---|---|
| MS1 | MS2 | MS3 | MS4 | MS5 | MS6 | MS7 | MS8 | |
| Zeatin | – | – | – | – | 1 | 2 | – | – |
| 6-BAP | – | 2.5 | 5.0 | 5.0 | – | – | – | – |
| TDZ | – | – | – | – | – | – | 0.25 | 0.5 |
| GA | – | – | – | 1.0 | – | – | – | – |
| IAA | – | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Source of Variation | ss | df | ms | F05 | F |
|---|---|---|---|---|---|
| Total | 106,381.40 | 143 | – | – | – |
| Variants | 101,877.10 | 47 | 2167.60 | 1.63 | 46.20 * |
| A (genotype) | 14,693.75 | 1 | 14,693.76 | 3.94 | 313.16 * |
| B (explant) | 2029.57 | 2 | 1014.78 | 3.09 | 21.63 * |
| C (cultural medium) | 6237.06 | 7 | 891.01 | 2.10 | 18.99 * |
| AB | 3748.05 | 2 | 1874.03 | 3.09 | 39.94 * |
| AC | 5439.07 | 7 | 777.01 | 2.10 | 16.56 * |
| BC | 56,044.40 | 14 | 4003.10 | 1.79 | 85.32 * |
| ABC | 13,686.16 | 14 | 977.58 | 1.79 | 20.84 * |
| Error | 4504.34 | 96 | 46.92 | – | – |
| Culture Medium | Frequency of Shoot Organogenesis, % | |||||||
|---|---|---|---|---|---|---|---|---|
| Zanzibar Green | Impala Bronzovaya | |||||||
| Hypocotyl | Cotyledonous Leaf | Cotyledon Petiole | Mean (Culture Medium) 2 | Hypocotyl | Cotyledonous Leaf | Cotyledon Petiole | Mean (Culture Medium) 2 | |
| MS 1 | 0a 1 | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| MS 2 | 0a | 0a | 6.7efghij | 2.2bcde | 0a | 0a | 9.2fghijk | 3.6defgh |
| MS 3 | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| MS 4 | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| MS 5 | 10hijk | 0a | 11.6ijk | 7.2fghi | 10.1ghijk | 0a | 43.3n | 17.8j |
| MS 6 | 4.6bcdefghi | 0a | 19.3kl | 7.96h | 3.5cdefghi | 0a | 43.3mn | 15.6ij |
| MS 7 | 0a | 0a | 16.5jkl | 5.5efgh | 0a | 0a | 25.4l | 8.46gh |
| MS 8 | 0a | 0a | 0a | 0a | 0a | 0a | 6.7defghij | 2.2cde |
| mean (explant) 3 | 1.8c | 0a | 6.76d | — | 1.7bc | 0a | 16e | — |
| mean (genotype) 4 | 2.85a | 5.9a | ||||||
| Culture Medium | Values of √(X + 1) Expression, where X Is the Average Number of Shoots per Explant | |||||||
|---|---|---|---|---|---|---|---|---|
| Zanzibar Green | Impala Bronzovaya | |||||||
| Hypocotyl | Cotyledonous Leaf | Cotyledon Petiole | Mean (Culture Medium) 2 | Hypocotyl | Cotyledonous Leaf | Cotyledon Petiole | Mean (Culture Medium) 2 | |
| MS 1 | 1.00a 1 | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a |
| MS 2 | 1.00a | 1.00a | 1.61defghij | 1.20ab | 1.00a | 1.00a | 1.61fghij | 1.20ab |
| MS 3 | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a |
| MS 4 | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a |
| MS 5 | 2.00hijklm | 1.00a | 1.77ghijkl | 1.60ab | 2.00ijklm | 1.00a | 2.52mn | 1.84d |
| MS 6 | 1.61bcdefghij | 1.00a | 2.20lm | 1.60bcd | 1.61cdefghij | 1.00a | 2.58n | 1.73cd |
| MS 7 | 1.00a | 1.00a | 2.00jkl | 1.33ab | 1.00a | 1.00a | 2.16klm | 1.40ab |
| MS 8 | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.00a | 1.61efghij | 1.20a |
| mean (explant) 3 | 1.20a | 1.00a | 1.40ab | — | 1.20a | 1.00a | 1.70b | — |
| mean (genotype) 4 | 1.20a | 1.30a | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrov, O.S.; Petrov, N.R.; Varlamova, N.V.; Khaliluev, M.R. An Optimized Protocol for In Vitro Indirect Shoot Organogenesis of Impala Bronzovaya and Zanzibar Green Ricinus communis L. Varieties. Horticulturae 2021, 7, 105. https://doi.org/10.3390/horticulturae7050105
Alexandrov OS, Petrov NR, Varlamova NV, Khaliluev MR. An Optimized Protocol for In Vitro Indirect Shoot Organogenesis of Impala Bronzovaya and Zanzibar Green Ricinus communis L. Varieties. Horticulturae. 2021; 7(5):105. https://doi.org/10.3390/horticulturae7050105
Chicago/Turabian StyleAlexandrov, Oleg S., Nicolay R. Petrov, Natalia V. Varlamova, and Marat R. Khaliluev. 2021. "An Optimized Protocol for In Vitro Indirect Shoot Organogenesis of Impala Bronzovaya and Zanzibar Green Ricinus communis L. Varieties" Horticulturae 7, no. 5: 105. https://doi.org/10.3390/horticulturae7050105
APA StyleAlexandrov, O. S., Petrov, N. R., Varlamova, N. V., & Khaliluev, M. R. (2021). An Optimized Protocol for In Vitro Indirect Shoot Organogenesis of Impala Bronzovaya and Zanzibar Green Ricinus communis L. Varieties. Horticulturae, 7(5), 105. https://doi.org/10.3390/horticulturae7050105








