Estimation of Glucosinolates and Anthocyanins in Kale Leaves Grown in a Plant Factory Using Spectral Reflectance
Abstract
1. Introduction
2. Materials and Methods
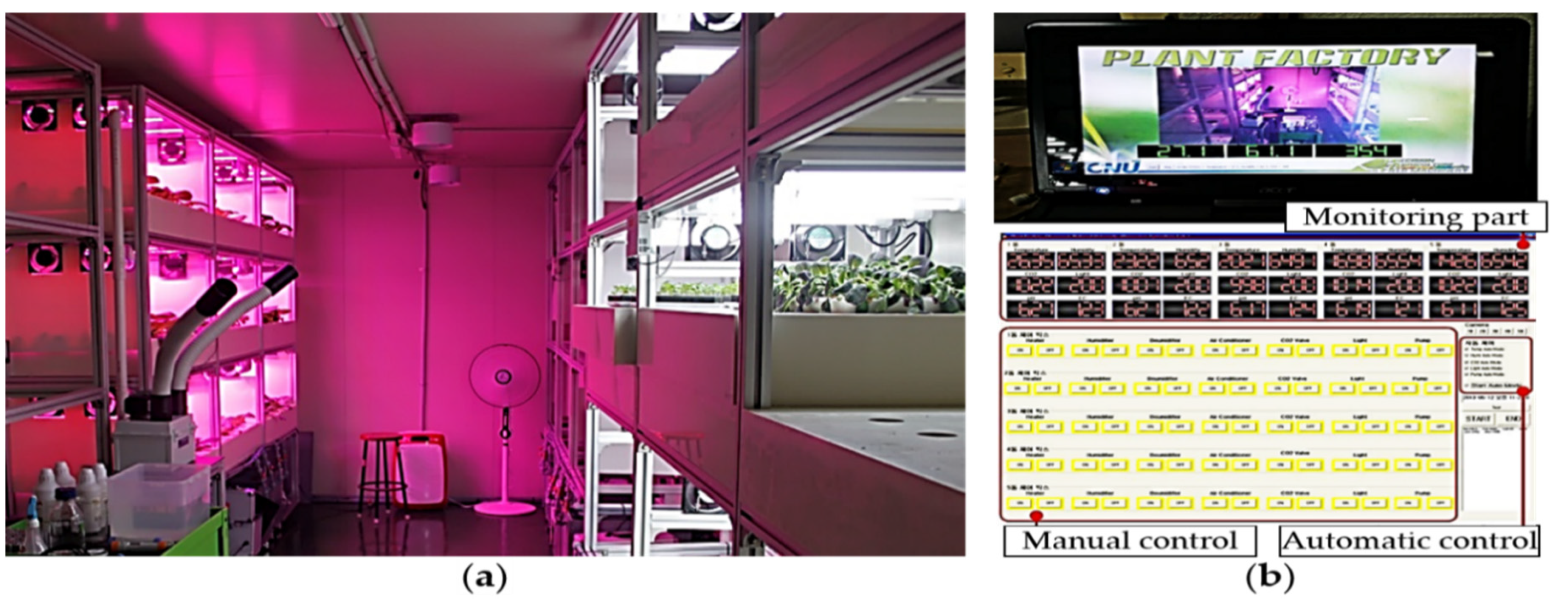
2.1. Kale Cultivation in a Plant Factory
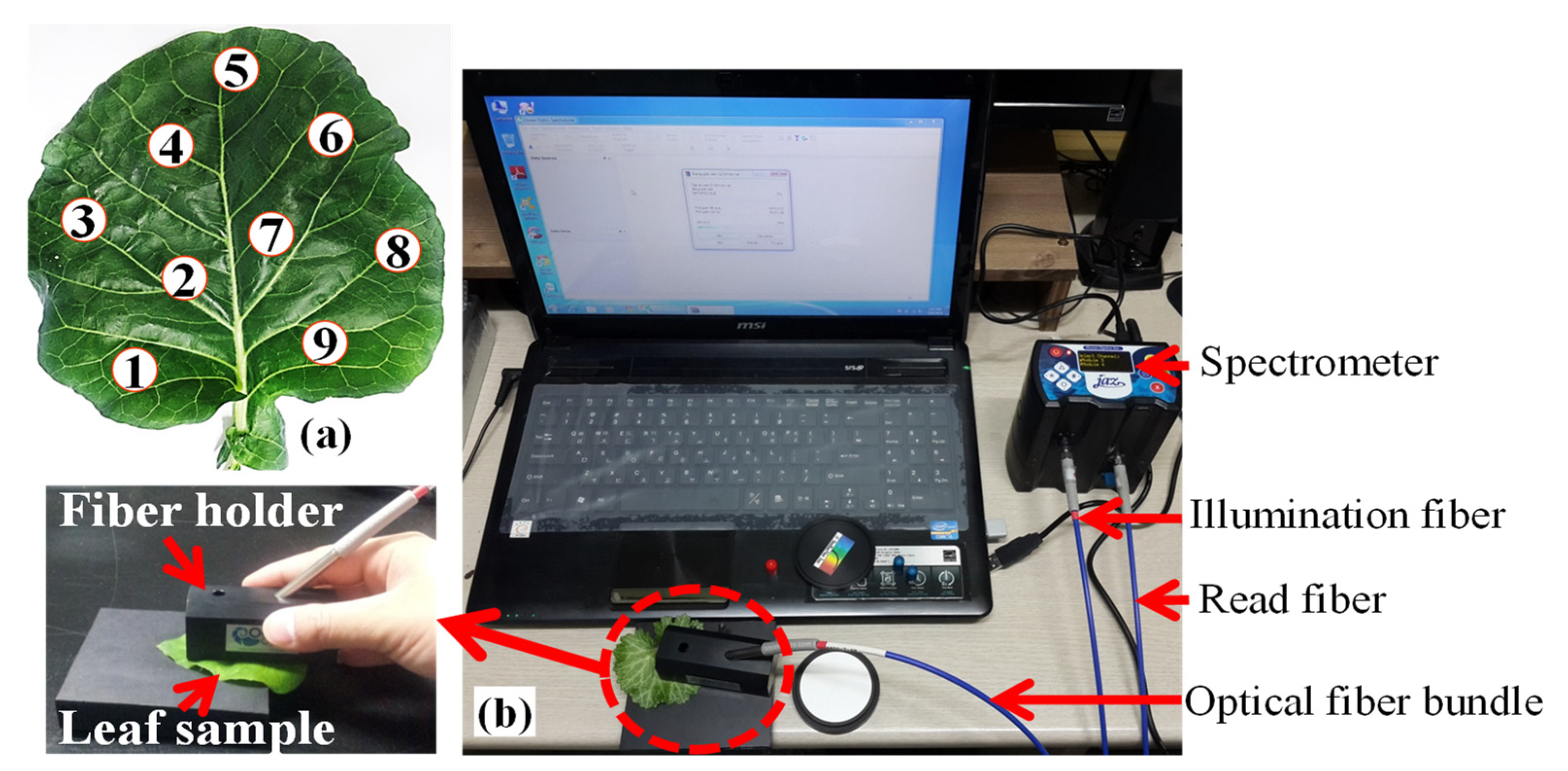
2.2. Leaf Sample Collection
2.3. Reflectance Spectra Acquisition
2.4. Extraction of Glucosinolates and Anthocyanins
2.5. Statistical Analyses Procedures
3. Results
3.1. Glucosinolate and Anthocyanin Contents
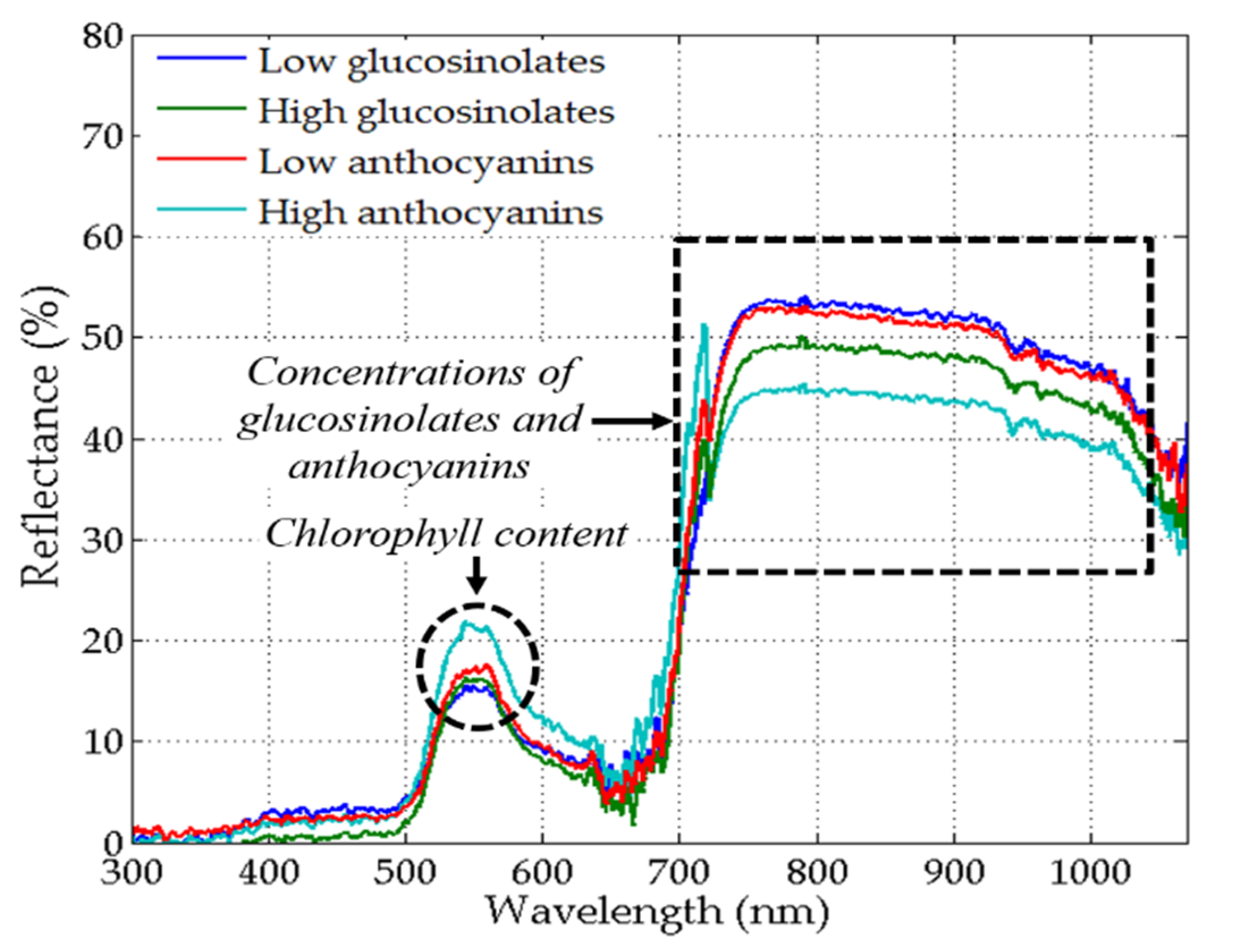
3.2. Characteristics of Spectral Data
3.3. Estimation Models of the Functional Components
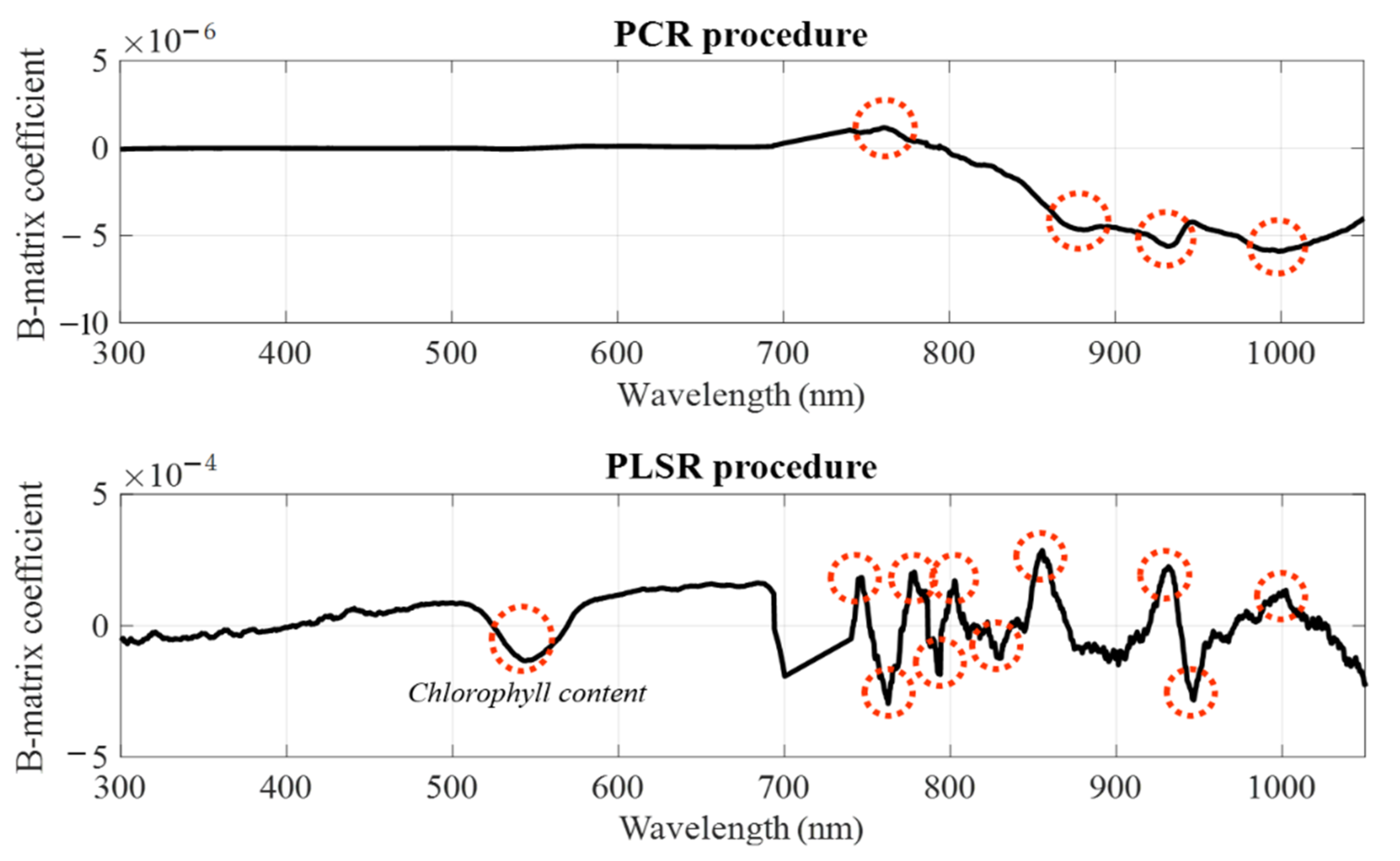
3.3.1. Performance of PCR, PLSR, and SMLR Using Raw Data
3.3.2. Performance of PCR, PLSR, and SMLR Using Transformed Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Sun, J.; Haytowitz, D.B.; Harnly, J.M.; Chen, P.; Pehrsson, P.R. Challenges of developing a valid dietary glucosin-olate database. J. Food Compos. Anal. 2017, 64, 78–84. [Google Scholar] [CrossRef]
- Esteve, M. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef]
- Kassie, F.; Uhl, M.; Rabot, S.; Grasl-Kraupp, B.; Verkerk, R.; Kundi, M.; Chabicovsky, M.; Schulte-Hermann, R.; Knasmüller, S. Chemoprevention of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced colonic and hepatic preneoplastic lesions in the F344 rat by cruciferous vegetables administered simultaneously with the carcinogen. Carcinogenesis 2003, 24, 255–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, B.; Liu, N.; Zhao, Y.; Yan, H.; Wang, Q. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- Vale, A.; Santos, J.; Brito, N.; Fernandes, D.; Rosa, E.; Oliveira, M.B.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef]
- Hahn, C.; Müller, A.; Kuhnert, N.; Albach, D. Diversity of Kale (Brassica oleraceavar.sabellica): Glucosinolate Content and Phylogenetic Relationships. J. Agric. Food Chem. 2016, 64, 3215–3225. [Google Scholar] [CrossRef]
- Yu, T.H.; Hsieh, S.P.; Su, C.M.; Huang, F.J.; Hung, C.C.; Yiin, L.M. Analysis of leafy vegetable nitrate using a modified spec-trometric method. Int. J. Anal. Chem. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Ngo, V.-D.; Jang, B.-E.; Park, S.-U.; Kim, S.-J.; Kim, Y.-J.; Chung, S.-O. Estimation of functional components of Chinese cabbage leaves grown in a plant factory using diffuse reflectance spectroscopy. J. Sci. Food Agric. 2019, 99, 711–718. [Google Scholar] [CrossRef]
- Dechant, B.; Cuntz, M.; Vohland, M.; Schulz, E.; Doktor, D. Estimation of photosynthesis traits from leaf reflectance spectra: Correlation to nitrogen content as the dominant mechanism. Remote Sens. Environ. 2017, 196, 279–292. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K.N. Alleviation of Adverse Effects of Ambient UV Stress on Growth and Some Potential Physiological Attributes in Soybean (Glycine max) by Seed Pre-treatment with Static Magnetic Field. J. Plant. Growth Regul. 2017, 36, 550–565. [Google Scholar] [CrossRef]
- Neto, A.J.S.; Lopes, D.C.; Toledo, J.V.; Zolnier, S.; Silva, T.G.F. Classification of sugarcane varieties using visible/near infrared spectral reflectance of stalks and multivariate methods. J. Agric. Sci. 2018, 156, 537–546. [Google Scholar] [CrossRef]
- Scotford, I.; Miller, P. Applications of Spectral Reflectance Techniques in Northern European Cereal Production: A Review. Biosyst. Eng. 2005, 90, 235–250. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Galvez-Sola, L.; Martínez-Nicolás, J.J.; Muelas-Domingo, R.; Nieves, M. Using Near-Infrared Spectroscopy in Agricultural Systems. In Developments in Near-Infrared Spectroscopy; Konstantinos, G., Kyprianidis, Eds.; Intech: Janeza Trdine 9, Croatia, 2017; pp. 98–127. [Google Scholar]
- Norris, K.H.; Barnes, R.F.; Moore, J.E.; Shenk, J.S. Predicting Forage Quality by Infrared Replectance Spectroscopy. J. Anim. Sci. 1976, 43, 889–897. [Google Scholar] [CrossRef]
- Mawlong, I.; Kumar, M.S.; Gurung, B.; Singh, K.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef]
- Bowie, B.T.; Chase, D.B.; Griffiths, P.R.; Pont, D. Factors Affecting the Performance of Bench—Top Raman Spectrometers. Part II: Effect of Sample. Appl. Spectrosc. 2000, 54, 200A–207A. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Xue, L.; Yang, L. Deriving leaf chlorophyll content of green-leafy vegetables from hyperspectral reflectance. ISPRS J. Photogramm. Remote Sens. 2009, 64, 97–106. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2007, 75, 272–281. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Zhou, S.; Yang, Y.; Ni, X.; Yang, J.; Zhu, Z.; Shi, C. Application of near-infrared reflectance spectroscopy to evaluate the lutein and β-carotene in Chinese kale. J. Food Compos. Anal. 2009, 22, 148–153. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Wang, S.; Tao, X.; Wang, Y.; Sun, A.; He, H. Assessment of Glucosinolates in Chinese Kale by Near-Infrared Spectroscopy. Int. J. Food Prop. 2014, 17, 1668–1679. [Google Scholar] [CrossRef]
- Kim, H.W.; Ko, H.C.; Baek, H.J.; Cho, S.M.; Jang, H.H.; Lee, Y.M.; Kim, J.B. Identification and quantification of glucosinolates in Korean leaf mustard germplasm (Brassica juncea var. integrifolia) by liquid chromatography–electrospray ionization/tandem mass spectrometry. Eur. Food Res. Technol. 2016, 242, 1479–1484. [Google Scholar] [CrossRef]
- Sahamishirazi, S.; Zikeli, S.; Fleck, M.; Claupein, W.; Graeff-Hoenninger, S. Development of a near-infrared spectroscopy method (NIRS) for fast analysis of total, indolic, aliphatic and individual glucosinolates in new bred open pollinating genotypes of broccoli (Brassica oleracea convar. botrytis var. italica). Food Chem. 2017, 232, 272–277. [Google Scholar] [CrossRef]
- Agati, G.; Tuccio, L.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Kowalski, A.; Grzegorzewska, M.; Kosson, R.; Kaniszewski, S. Nondestructive Optical Sensing of Flavonols and Chlorophyll in White Head Cabbage (Brassica oleraceaL. var.capitatasubvar.alba) Grown under Different Nitrogen Regimens. J. Agric. Food Chem. 2016, 64, 85–94. [Google Scholar] [CrossRef]
- Lin, K.-H.; Shih, F.-C.; Huang, M.-Y.; Weng, J.-H. Physiological Characteristics of Photosynthesis in Yellow-Green, Green and Dark-Green Chinese Kale (Brassica oleracea L. var. alboglabra Musil.) under Varying Light Intensities. Plants 2020, 9, 960. [Google Scholar] [CrossRef]
- Chung, S.O.; Chowdhury, M.; Ngo, V.D.; Jang, B.E.; Han, M.W.; Ko, H.J. Comparing Models and Wavelength Bands for Es-Timation of Functional Components of Kale and Chinese Cabbage. Available online: https://www.researchgate.net/profile/Milon_Chowdhury2/publication/343704587_Comparing_models_and_wavelength_bands_for_estimation_of_functional_components_of_Kale_and_Chinese_cabbage/links/5f3b24c5299bf13404cd4863/Comparing-models-and-wavelength-bands-for-estimation-of-functional-components-of-Kale-and-Chinese-cabbage.pdf (accessed on 25 January 2021).
- Noisopa, C.; Prapagdee, B.; Navanugraha, C.; Hutacharoen, R. Effects of bio-extracts on the growth of chinese kale. Agric. Nat. Resour. 2010, 44, 808–815. [Google Scholar]
- Filho, A.B.C.; Bianco, M.S.; Tardivo, C.F.; Pugina, G.C. Agronomic viability of New Zealand spinach and kale intercropping. An. Acad. Bras. Ciências 2017, 89, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Chung, S.O. Comparison of plant growth and glucosinolates of Chinese cabbage and kale crops under three cul-tivation conditions. J. Biosyst. Eng. 2018, 43, 30–36. [Google Scholar] [CrossRef]
- Kozai, T. Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Chowdhury, M.; Jang, B.; Kabir, M.; Kim, Y.; Na, K.; Park, S.; Chung, S. Factors affecting the accuracy and precision of ion-selective electrodes for hydroponic nutrient supply systems. Acta Hortic. 2020, 997–1004. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Yi, G.-E.; Robin, A.H.K.; Yang, K.; Park, J.-I.; Kang, J.-G.; Yang, T.-J.; Nou, I.-S. Identification and Expression Analysis of Glucosinolate Biosynthetic Genes and Estimation of Glucosinolate Contents in Edible Organs of Brassica oleracea Subspecies. Molecules 2015, 20, 13089–13111. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, J.-S.; Kim, D.; Kim, C.Y.; Son, J.E. Harvest strategies to maximize the annual production of bioactive compounds, glucosinolates, and total antioxidant activities of kale in plant factories. Hortic. Environ. Biotechnol. 2019, 60, 883–894. [Google Scholar] [CrossRef]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.B.; Haefele, S. The Impact of Biochar Application on Soil Properties and Plant Growth of Pot Grown Lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef]
- Ngo, V.-D.; Kang, S.-W.; Ryu, D.-K.; Chung, S.-O.; Park, S.-U.; Kim, S.-J.; Park, J.-T. Location of Sampling Points in Optical Reflectance Measurements of Chinese Cabbage and Kale Leaves. J. Biosyst. Eng. 2015, 40, 115–123. [Google Scholar] [CrossRef][Green Version]
- Lefsrud, M.; Kopsell, D.; Wenzel, A.; Sheehan, J. Changes in kale (Brassica oleracea L. var. acephala) carotenoid and chlorophyll pigment concentrations during leaf ontogeny. Sci. Hortic. 2007, 112, 136–141. [Google Scholar] [CrossRef]
- Chung, S.-O.; Kang, S.-W.; Bae, K.-S.; Ryu, M.-J.; Kim, Y.-J. The potential of remote monitoring and control of protected crop production environment using mobile phone under 3G and Wi-Fi communication conditions. Eng. Agric. Environ. Food 2015, 8, 251–256. [Google Scholar] [CrossRef]
- Chung, S.-O.; Ngo, V.-D.; Kabir, S.N.; Hong, S.-J.; Park, S.-U.; Kim, S.-J.; Park, J.-T. Number of sampling leaves for reflectance measurement of Chinese cabbage and kale. Korean J. Agric. Sci. 2014, 41, 169–175. [Google Scholar] [CrossRef][Green Version]
- Kim, S.-J.; Ishii, G. Glucosinolate profiles in the seeds, leaves and roots of rocket salad (Eruca sativaMill.) and anti-oxidative activities of intact plant powder and purified 4-methoxyglucobrassicin. Soil Sci. Plant. Nutr. 2006, 52, 394–400. [Google Scholar] [CrossRef]
- Park, N.I.; Li, X.; Suzuki, T.; Kim, S.-J.; Woo, S.-H.; Park, C.H.; Park, S.U. Differential Expression of Anthocyanin Biosynthetic Genes and Anthocyanin Accumulation in Tartary Buckwheat Cultivars ‘Hokkai T8’ and ‘Hokkai T10’. J. Agric. Food Chem. 2011, 59, 2356–2361. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, J.K.; Park, S.; Lee, S.-W.; Li, X.; Kim, Y.B.; Uddin, R.; Park, N.I.; Kim, S.-J.; Park, S.U. Metabolic Profiling of Glucosinolates, Anthocyanins, Carotenoids, and Other Secondary Metabolites in Kohlrabi (Brassica oleraceavar.gongylodes). J. Agric. Food Chem. 2012, 60, 8111–8116. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef]
- Brooks, M.S.L.; Celli, G.B. Anthocyanins From Natural Sources: Exploiting Targeted Delivery for Improved Health; Royal Society of Chemistry: London, UK, 2019; Volume 12. [Google Scholar]
- Luthria, D.L.; Mukhopadhyay, S.; Lin, L.-Z.; Harnly, J.M. A Comparison of Analytical and Data Preprocessing Methods for Spectral Fingerprinting. Appl. Spectrosc. 2011, 65, 250–259. [Google Scholar] [CrossRef]
- Osborne, J.W. Improving your data transformations: Applying the Box-Cox transformation. Pract. Assess. Res. Eval. 2010, 15, 1–9. [Google Scholar]
- Kawamura, K.; Tsujimoto, Y.; Rabenarivo, M.; Asai, H.; Andriamananjara, A.; Rakotoson, T. Vis-NIR Spectroscopy and PLS Regression with Waveband Selection for Estimating the Total C and N of Paddy Soils in Madagascar. Remote Sens. 2017, 9, 1081. [Google Scholar] [CrossRef]
- Mahesh, S.; Jayas, D.S.; Paliwal, J.; White, N.D.G. Comparison of Partial Least Squares Regression (PLSR) and Principal Components Regression (PCR) Methods for Protein and Hardness Predictions using the Near-Infrared (NIR) Hyperspectral Images of Bulk Samples of Canadian Wheat. Food Bioprocess. Technol. 2015, 8, 31–40. [Google Scholar] [CrossRef]
- Yaroshchyk, P.; Death, D.L.; Spencer, S.J. Comparison of principal components regression, partial least squares regression, multi-block partial least squares regression, and serial partial least squares regression algorithms for the analysis of Fe in iron ore using LIBS. J. Anal. At. Spectrom. 2012, 27, 92–98. [Google Scholar] [CrossRef]
- Ghani, I.M.M.; Ahmad, S. Stepwise Multiple Regression Method to Forecast Fish Landing. Procedia-Soc. Behav. Sci. 2010, 8, 549–554. [Google Scholar] [CrossRef]
- Zhan, X.; Liang, X.; Xu, G.; Zhou, L. Influence of plant root morphology and tissue composition on phenanthrene uptake: Stepwise multiple linear regression analysis. Environ. Pollut. 2013, 179, 294–300. [Google Scholar] [CrossRef]
- Neto, A.J.S.; Moura, L.D.O.; Lopes, D.D.C.; Carlos, L.D.A.; Martins, L.M.; Ferraz, L.D.C.L. Non-destructive prediction of pigment content in lettuce based on visible-NIR spectroscopy. J. Sci. Food Agric. 2016, 97, 2015–2022. [Google Scholar] [CrossRef]
- Kira, O.; Linker, R.; Gitelson, A. Non-destructive estimation of foliar chlorophyll and carotenoid contents: Focus on informative spectral bands. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 251–260. [Google Scholar] [CrossRef]
- Velasco, P.; Cartea, M.E.; González, M.E.C.; Vilar, M.; Ordás, A. Factors Affecting the Glucosinolate Content of Kale (Brassica oleraceaacephala Group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef]
- Toledo-Martín, E.M.; Font, R.; Obregón-Cano, S.; De Haro-Bailón, A.; Villatoro-Pulido, M.; Del Río-Celestino, M. Rapid and Cost-Effective Quantification of Glucosinolates and Total Phenolic Content in Rocket Leaves by Visible/Near-Infrared Spectroscopy. Molecules 2017, 22, 851. [Google Scholar] [CrossRef]
- Steindal, A.L.H.; Rødven, R.; Hansen, E.; Mølmann, J. Effects of photoperiod, growth temperature and cold acclimatisation on glucosinolates, sugars and fatty acids in kale. Food Chem. 2015, 174, 44–51. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Morphological and Physiological Responses in Basil and Brassica Species to Different Proportions of Red, Blue, and Green Wavelengths in Indoor Vertical Farming. J. Am. Soc. Hortic. Sci. 2020, 145, 267–278. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Muñoz-Rueda, A.; Mena-Petite, A. Interacting effects of high light and elevated CO2 on the nutraceutical quality of two differently pigmented Lactuca sativa cultivars (Blonde of Paris Batavia and Oak Leaf). Sci. Hortic. 2015, 191, 38–48. [Google Scholar] [CrossRef]
- Marin, A.; Ferreres, F.; Barberá, G.G.; Gil, M.I. Weather Variability Influences Color and Phenolic Content of Pigmented Baby Leaf Lettuces throughout the Season. J. Agric. Food Chem. 2015, 63, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current Review of the Modulatory Effects of LED Lights on Photosynthesis of Secondary Metabolites and Future Perspectives of Microgreen Vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Nosenko, T.; Hutsalo, I.; Nosenko, V.; Levchuk, I.; Litvynchuk, S. Analysis of near infrared reflectance spectrum of rape seed with different content of erucic acid. Ukr. J. Food Sci. 2013, 94–99. [Google Scholar]
- Challinor, A.J.; Parkes, B.; Ramírez-Villegas, J. Crop yield response to climate change varies with cropping intensity. Glob. Chang. Biol. 2015, 21, 1679–1688. [Google Scholar] [CrossRef] [PubMed]


| Environmental Factors | Experimental Treatments | Specification of the Used Sensors | |
|---|---|---|---|
| Target Levels | Obtained Levels | ||
| Temperature (°C) | 14 ± 1 17 ± 1 20 ± 1 23 ± 1 25 ± 1 | 14.58 ± 0.74 17.34 ± 1.8 20.25 ± 0.69 23.26 ± 0.52 25.97 ± 1.64 | Model: ETH-01DV Range: −40~125 ℃ Resolution: 14 bit Accuracy: ± 1.3 ℃ |
| Humidity (%) | 45 ± 5 55 ± 5 65 ± 5 75 ± 5 85 ± 5 | 45.78 ± 6.23 58.06 ± 4.35 67.66 ± 4.67 72.66 ± 4.49 83.85 ± 4.65 | Model: ETH-01DV Range: 0~100% Resolution: 14bit Accuracy: ± 4.5% |
| CO2 (ppm) | 400 ± 100 700 ± 100 1000 ± 100 1300 ± 100 1600 ± 100 | 475.62 ± 106.3 723.9 ± 140.6 1008.75 ± 175.36 1375.5 ± 125.11 1693.21 ± 137.2 | Model: SH-300-DX Range: 0 ~ 5000 ppm Response time: < 30 s Accuracy: ± 2% |
| Light source (light emitting diode color ratio) | 1 R:B:W, R:B, R:W, Fluorescent | - | - |
| Photosynthetic photon flux density (µmol m−2s−1) | 100, 130, 160, 190, 220 | - | Model: GY-30 Range: 1–65,535 lux Resolution: 16-bit Accuracy: ± 3% |
| Photoperiod (day/night hours) | 12/12, 14/10, 16/8, 18/6, 20/4 | - | Time switch: MaxiRex 5QT Prds rating: AC 230 V, 60 Hz Load capacity: 16 A |
| Electrical conduction (µS cm−1) | 0.80 ± 0.2 1.00 ± 0.2 1.20 ± 0.2 1.40 ± 0.2 1.60 ± 0.2 | 0.86 ± 0.3 1.02 ± 0.34 1.28 ± 0.22 1.39 ± 0.24 1.63 ± 0.25 | Model: conductivity probe Range: 2~20,000 μScm−1 Resolution: 10 μScm−1 Accuracy: ± 4% |
| Factors | Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2nd Week after Transplantation | 4th Week after Transplantation | |||||||||
| Temperature (°C) | 14 | 17 | 20 | 23 | 26 | 14 | 17 | 20 | 23 | 26 |
| Yield (mg) | 11.91 ± 1.2 | 12.59 ± 0.3 | 13.31 ± 0.2 | 13.02 ± 0.3 | 13.24 ± 0.3 | 21.30 ± 4.1 | 23.64 ± 6.2 | 28.29 ± 4.3 | 25.38 ± 3.2 | 22.15 ± 4.8 |
| Total GLSs 1 (μmol g−1 DW) 1 | 86.21 ± 5.9 | 65.24 ± 13.4 | 46.32 ± 19.2 | 29.75 ± 14.1 | 13.81 ± 7.7 | 37.12 ± 17.4 | 33.80 ± 5.1 | 23.94 ± 5.5 | 22.42 ± 20.1 | 12.42 ± 0.5 |
| ATCs2 (µg g−1) | 8.9 ± 6.2 | 47.9 ± 4.3 | 65.4 ± 2.5 | 100.5 ± 8.4 | 90.8 ± 18.2 | 29.2 ± 23.4 | 51.1 ± 48.1 | 126.4 ± 110.9 | 180.5 ± 57.4 | 133.6 ± 56.7 |
| Humidity (%) | 45 | 55 | 65 | 75 | 85 | 45 | 55 | 65 | 75 | 85 |
| Yield (mg) | 5.5 ± 0.1 | 8.22 ± 1.2 | 6.33 ± 1.4 | 7.76 ± 1.1 | 8.89 ± 1.5 | 23.42 ± 9.2 | 29.39 ± 3.5 | 33.01 ± 4.9 | 25.67 ± 0.9 | 34.76 ± 6.8 |
| Total GLSs (μmol g−1 DW) 1 | 1.54 ± 1.54 | 1.62 ± 1.6 | 1.17 ± 1.2 | 1.18 ± 1.2 | 1.17 ± 1.2 | 0.86 ± 0.3 | 0.99 ± 0.2 | 1.12 ± 0.3 | 0.88 ± 0.1 | 0.87 ± 0.2 |
| ATCs (µg g−1) | 97.1 ± 37.4 | 116.9 ± 47.2 | 164.8 ± 40.7 | 107.1 ± 89.9 | 146.1 ± 29.6 | 75.9 ± 67.0 | 85.7 ± 23.9 | 174 ± 17.5 | 133.6 ± 115.7 | 117 ± 105.9 |
| CO2 (ppm) | 400 | 700 | 1000 | 1300 | 1600 | 400 | 700 | 1000 | 1300 | 1600 |
| Yield (mg) | 10.01 ± 1.8 | 11.33 ± 1.7 | 10.38 ± 1.4 | 8.91 ± 0.4 | 7.49 ± 0.6 | 22.15 ± 4.8 | 28.38 ± 3.2 | 18.29 ± 4.3 | 23.64 ± 6.2 | 25.30 ± 4.1 |
| Total GLSs (μmol g−1 DW) 1 | 3.06 ± 0.3 | 2.37 ± 0.4 | 3.71 ± 0.8 | 8.08 ± 4.1 | 3.87 ± 0.8 | 3.64 ± 1.5 | 2.42 ± 0.9 | 2.20 ± 0.3 | 5.08 ± 0.8 | 4.48 ± 2.5 |
| ATCs (µg g−1) | 258.5 ± 96.9 | 285.7 ± 74.7 | 249.4 ± 24.4 | 279.4 ± 124.2 | 220.1 ± 19.9 | 224.2 ± 79.8 | 287.8 ± 18.0 | 313.16 ± 112.1 | 322.70 ± 45.1 | 171.5 ± 47.70 |
| Light source 3 | R:B:W | R:B | R:W | F - | R:B:W | R:B | R:W | F - | ||
| Yield (mg) | 12.7 ± 0.39 | 14.1 ± 0.77 | 11.9 ± 0.16 | 10.3 ± 0.27 - | 23 ± 1.7 | 21.9 ± 1.7 | 20.7± 2.4 | 17.3 ± 2.3 - | ||
| Total GLSs (μmol g−1 DW) 1 | 49.33 ± 66.80 | 14.27 ± 5.31 | 15.42 ± 13.30 | 63.38 ± 68.02 - | 79.05 ± 25.37 | 118.51 ± 16.33 | 99.04 ± 63.11 | 103.14 ± 13.42 - | ||
| Intensity (µmol m−2s−1) | 100 | 130 | 160 | 190 | 220 | 100 | 130 | 160 | 190 | 220 |
| Yield (mg) | 9.45 ± 0.82 | 8.37 ± 0.2 | 16.23 ± 0.36 | 12.72 ± 0.27 | 8.3 ± 0.25 | 17.24 ± 2.3 | 18.5 ± 1.74 | 24.48 ± 1.6 | 27.57 ± 0.74 | 18.07 ± 0.66 |
| Total GLSs (μmol g−1 DW) 1 | 9.08 ± 6.8 | 15.18 ± 0.6 | 11.16 ± 5.8 | 10.81 ± 5.1 | 10.93 ± 7.7 | 33.39 ± 2.22 | 93.17 ± 0.9 | 11.21 ± 3.7 | 20.54 ± 12.1 | 24.75 ± 14.9 |
| Photoperiod (h) | 12/12 | 14/10 | 16/8 | 18/6 | 20/4 | 12/12 | 14/10 | 16/8 | 18/6 | 20/4 |
| Yield (mg) | 9.5 ± 0.91 | 8.6 ±0.40 | 12.23 ± 0.36 | 10.21 ± 0.47 | 7.6 ± 0.5 | 28.45 ± 2.18 | 18.71 ± 0.48 | 24.48 ± 1.58 | 19.21 ± 0.49 | 17.75 ± 0.53 |
| Total GLSs (μmol g−1 DW) 1 | 9.22 ± 4.8 | 9.01 ± 4.0 | 11.16 ± 5.8 | 21.21 ± 2.7 | 6.50 ± 0.9 | 19.35 ± 9.9 | 28.23 ± 16.9 | 11.21 ± 3.7 | 13.78 ± 12.1 | 5.46 ± 2.1 |
| EC (µS cm−1) | 0.80 | 1.00 | 1.20 | 1.40 | 1.60 | 0.80 | 1.00 | 1.20 | 1.40 | 1.60 |
| Yield (mg) | 14.0 ±0.9 | 16.8 ±1.5 | 17.4 ± 0.3 | 15.4 ± 1.6 | 14.4 ± 1.1 | 33.0 ± 2.2 | 45.6 ± 1.5 | 34.9 ± 0.4 | 24.0 ± 1.2 | 30.3 ± 1.8 |
| Total GLSs (μmol g−1 DW) 1 | 91.50 ± 30.77 | 82.89 ± 52.70 | 166.93 ± 23.98 | 92.75 ± 11.08 | 169.99 ± 14.59 | 88.01 ± 33.40 | 42.59 ± 23.70 | 76.03 ± 12.98 | 96.15 ± 27.35 | 92.41 ± 33.37 |
| Component | No. of Samples | Min | Max | Mean ± STD | |
|---|---|---|---|---|---|
| Glucosinolates (μmol g−1 DW) 1 | Progoitrin | 55 | 1.43 | 96.32 | 64.57 ± 44.06 |
| Sinigrin | 157 | 0.05 | 14.52 | 6.62 ± 9.19 | |
| Glucoalyssin | 22 | 0.74 | 6.03 | 2.77 ± 4.49 | |
| Glucobrassicanapin | 7 | 1.85 | 4.23 | 2.52 ± 1.20 | |
| Glucobrassicin | 187 | 0.05 | 16.77 | 8.29 ± 12.63 | |
| 4-methoxyglucobrassicin | 149 | 0.02 | 2.58 | 1.06 ± 1.72 | |
| Gluconasturtiin | 23 | 1.26 | 3.35 | 0.75 ± 1.05 | |
| Neoglucobrassicin | 146 | 0.03 | 1.56 | 0.81 ± 1.06 | |
| Glucoraphanin | 39 | 0.29 | 5.2 | 3.25 ± 5.51 | |
| Gluconapin | 86 | 0.13 | 1.69 | 0.78 ± 0.90 | |
| Anthocyanins (µg g−1) | Cyanidin | 72 | 0.02 | 217.56 | 135.98 ± 93.91 |
| Pelargonidin | 71 | 0.02 | 0.25 | 0.13 ± 0.11 | |
| Delphinidin | 33 | 0.03 | 0.22 | 0.07 ± 0.10 | |
| Malvidin | 68 | 0.07 | 11.82 | 7.39 ± 4.83 | |
| Components | PCR | PLSR | SMLR | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | ||
| Glucosinolates (μmol g−1 DW) | Sinigrin | 0.38 | 3.02 | 0.63 | 2.33 | 0.84 | 1.44 |
| Glucobrassicin | 0.23 | 3.69 | 0.90 | 1.30 | 0.92 | 0.90 | |
| 4-methoxyglucobrassicin | 0.44 | 0.49 | 0.62 | 0.41 | 0.84 | 0.24 | |
| Neoglucobrassicin | 0.46 | 0.25 | 0.61 | 0.21 | 0.85 | 0.12 | |
| Gluconapin | 0.35 | 0.35 | 0.53 | 0.29 | 0.89 | 0.12 | |
| Anthocyanins (µg g−1) | Cyanidin | 0.28 | 54.63 | 0.56 | 42.54 | 0.14 | 60.37 |
| Malvidin | 0.44 | 2.55 | 0.91 | 1.04 | 0.67 | 2.09 | |
| Pelargonidin | 0.42 | 0.04 | 0.74 | 0.03 | 0.46 | 0.04 | |
| Components | Transformation | PCR | PLSR | SMLR | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | |||
| Glucosinolate (μmol g−1 DW) | Sinigrin | Ref2 | 0.35 | 3.11 | 0.64 | 2.30 | 0.84 | 1.41 |
| √Ref | 0.41 | 2.95 | 0.65 | 2.27 | 0.85 | 1.56 | ||
| eRef | 0.40 | 2.97 | 0.50 | 2.72 | 0.84 | 1.43 | ||
| 1/Ref | 0.01 | 3.82 | 0.09 | 3.66 | 0.85 | 1.36 | ||
| Ln(Ref) | 0.41 | 2.96 | 0.59 | 2.45 | 0.86 | 1.32 | ||
| Glucobrassicin | Ref2 | 0.29 | 3.55 | 0.35 | 3.38 | 0.91 | 1.04 | |
| √Ref | 0.23 | 3.69 | 0.96 | 0.78 | 0.91 | 1.06 | ||
| eRef | 0.28 | 3.56 | 0.60 | 2.67 | 0.88 | 1.30 | ||
| 1/Ref | 0.02 | 4.18 | 0.002 | 4.21 | 0.92 | 0.88 | ||
| Ln(Ref) | 0.24 | 3.67 | 0.86 | 1.57 | 0.92 | 0.88 | ||
| 4-ethoxyglucobrassicin | Ref2 | 0.40 | 0.51 | 0.60 | 0.41 | 0.83 | 0.25 | |
| √Ref | 0.46 | 0.48 | 0.65 | 0.39 | 0.86 | 0.22 | ||
| eRef | 0.42 | 0.50 | 0.62 | 0.40 | 0.82 | 0.26 | ||
| 1/Ref | 0.04 | 0.65 | 0.04 | 0.65 | 0.87 | 0.21 | ||
| Ln(Ref) | 0.44 | 0.49 | 0.80 | 0.29 | 0.86 | 0.23 | ||
| Neoglucobrassicin | Ref2 | 0.38 | 0.27 | 0.62 | 0.21 | 0.86 | 0.12 | |
| √Ref | 0.42 | 0.26 | 0.63 | 0.20 | 0.85 | 0.12 | ||
| eRef | 0.43 | 0.25 | 0.62 | 0.21 | 0.82 | 0.14 | ||
| 1/Ref | 0.06 | 0.33 | 0.10 | 0.32 | 0.86 | 0.12 | ||
| Ln(Ref) | 0.42 | 0.26 | 0.77 | 0.16 | 0.87 | 0.11 | ||
| Gluconapin | Ref2 | 0.20 | 0.38 | 0.68 | 0.24 | 0.88 | 0.12 | |
| √Ref | 0.50 | 0.30 | 0.86 | 0.16 | 0.89 | 0.12 | ||
| eRef | 0.37 | 0.34 | 0.61 | 0.27 | 0.89 | 0.12 | ||
| 1/Ref | 0.14 | 0.40 | 0.20 | 0.39 | 0.88 | 0.13 | ||
| Ln(Ref) | 0.51 | 0.30 | 0.88 | 0.15 | 0.89 | 0.12 | ||
| Anthocyanins (µg g−1) | Cyanidin | Ref2 | 0.02 | 64.38 | 0.004 | 64.26 | 0.16 | 60 |
| √Ref | 0.24 | 56.18 | 0.50 | 45.54 | 0.14 | 60.58 | ||
| eRef | 0.27 | 54.86 | 0.46 | 47.29 | 0.14 | 60.78 | ||
| 1/Ref | 0.01 | 64.15 | 0.01 | 64.09 | 0.12 | 61.29 | ||
| Ln(Ref) | 0.20 | 57.39 | 0.68 | 36.59 | 0.13 | 60.81 | ||
| Malvidin | Ref2 | 0.03 | 3.41 | 0.36 | 2.73 | 0.22 | 3.08 | |
| √Ref | 0.42 | 2.60 | 0.96 | 0.63 | 0.71 | 1.97 | ||
| eRef | 0.42 | 2.60 | 0.86 | 1.30 | 0.12 | 3.28 | ||
| 1/Ref | 0.02 | 3.41 | 0.02 | 3.38 | 0.48 | 2.55 | ||
| Ln(Ref) | 0.41 | 2.62 | 0.97 | 0.61 | 0.74 | 1.90 | ||
| Pelargonidin | Ref2 | 0.01 | 0.06 | 0.002 | 0.06 | 0.40 | 0.04 | |
| √Ref | 0.50 | 0.04 | 0.69 | 0.03 | 0.45 | 0.04 | ||
| eRef | 0.36 | 0.04 | 0.66 | 0.03 | 0.08 | 0.05 | ||
| 1/Ref | 0.02 | 0.06 | 0.03 | 0.06 | 0.09 | 0.05 | ||
| Ln(Ref) | 0.36 | 0.04 | 0.91 | 0.02 | 0.38 | 0.04 | ||
| Plant | Cultivation Method | Dominating Component (Analyzed by HPLC) | Dominating Component (Analyzed by Spectroscopy) | Wavelengths (nm) |
|---|---|---|---|---|
| Kale | Aeroponic (Plant factory) | Progoitrin, Sinigrin | Sinigrin, Glucobrassicin | 742, 761, 787, 796, 805, 833, 855, 932, 947, 1000 |
| Kale | Soil-based (Closed-chamber) | Glucoiberin, Glucoraphanin, Sinigrin | - | - |
| Kale | Open field | Glucobrassicin, Sinigrin | - | - |
| Chinese cabbage | Aeroponic (Plant factory) | Neoglucobrassicin, 4-methoxyglucobrassicin | Glucobrassicin, 4-methoxyglucobrassicin | 365, 388, 440, 545, 607, 651, 798, 838, 860, 870, 932, 950 |
| Mustard leaf | Open field | Sinigrin, Glucoiberverin, Gluconasturtiin | - | - |
| Rocket Leaf | Soil-based (Greenhouse) | - | Glucoerucin, Gluconasturtiin,4-hydroxyglucobrassicin | 548, 610, 680, 1432, 1696, 1730, 1920, 2054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, M.; Ngo, V.-D.; Islam, M.N.; Ali, M.; Islam, S.; Rasool, K.; Park, S.-U.; Chung, S.-O. Estimation of Glucosinolates and Anthocyanins in Kale Leaves Grown in a Plant Factory Using Spectral Reflectance. Horticulturae 2021, 7, 56. https://doi.org/10.3390/horticulturae7030056
Chowdhury M, Ngo V-D, Islam MN, Ali M, Islam S, Rasool K, Park S-U, Chung S-O. Estimation of Glucosinolates and Anthocyanins in Kale Leaves Grown in a Plant Factory Using Spectral Reflectance. Horticulturae. 2021; 7(3):56. https://doi.org/10.3390/horticulturae7030056
Chicago/Turabian StyleChowdhury, Milon, Viet-Duc Ngo, Md Nafiul Islam, Mohammod Ali, Sumaiya Islam, Kamal Rasool, Sang-Un Park, and Sun-Ok Chung. 2021. "Estimation of Glucosinolates and Anthocyanins in Kale Leaves Grown in a Plant Factory Using Spectral Reflectance" Horticulturae 7, no. 3: 56. https://doi.org/10.3390/horticulturae7030056
APA StyleChowdhury, M., Ngo, V.-D., Islam, M. N., Ali, M., Islam, S., Rasool, K., Park, S.-U., & Chung, S.-O. (2021). Estimation of Glucosinolates and Anthocyanins in Kale Leaves Grown in a Plant Factory Using Spectral Reflectance. Horticulturae, 7(3), 56. https://doi.org/10.3390/horticulturae7030056











