Supplementary Light with Increased Blue Fraction Accelerates Emergence and Improves Development of the Inflorescence in Aechmea, Guzmania and Vriesea
Abstract
:1. Introduction
2. Materials and Methods
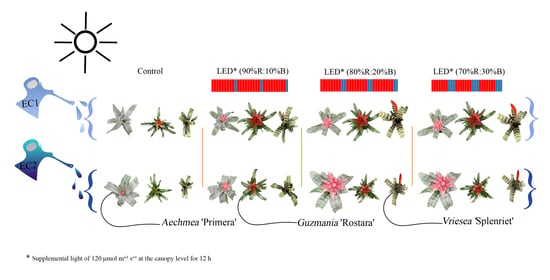
2.1. Plant Material and Growth Conditions
2.2. Flower Induction
2.3. Plant Growth, Morphology, and Biomass Allocation
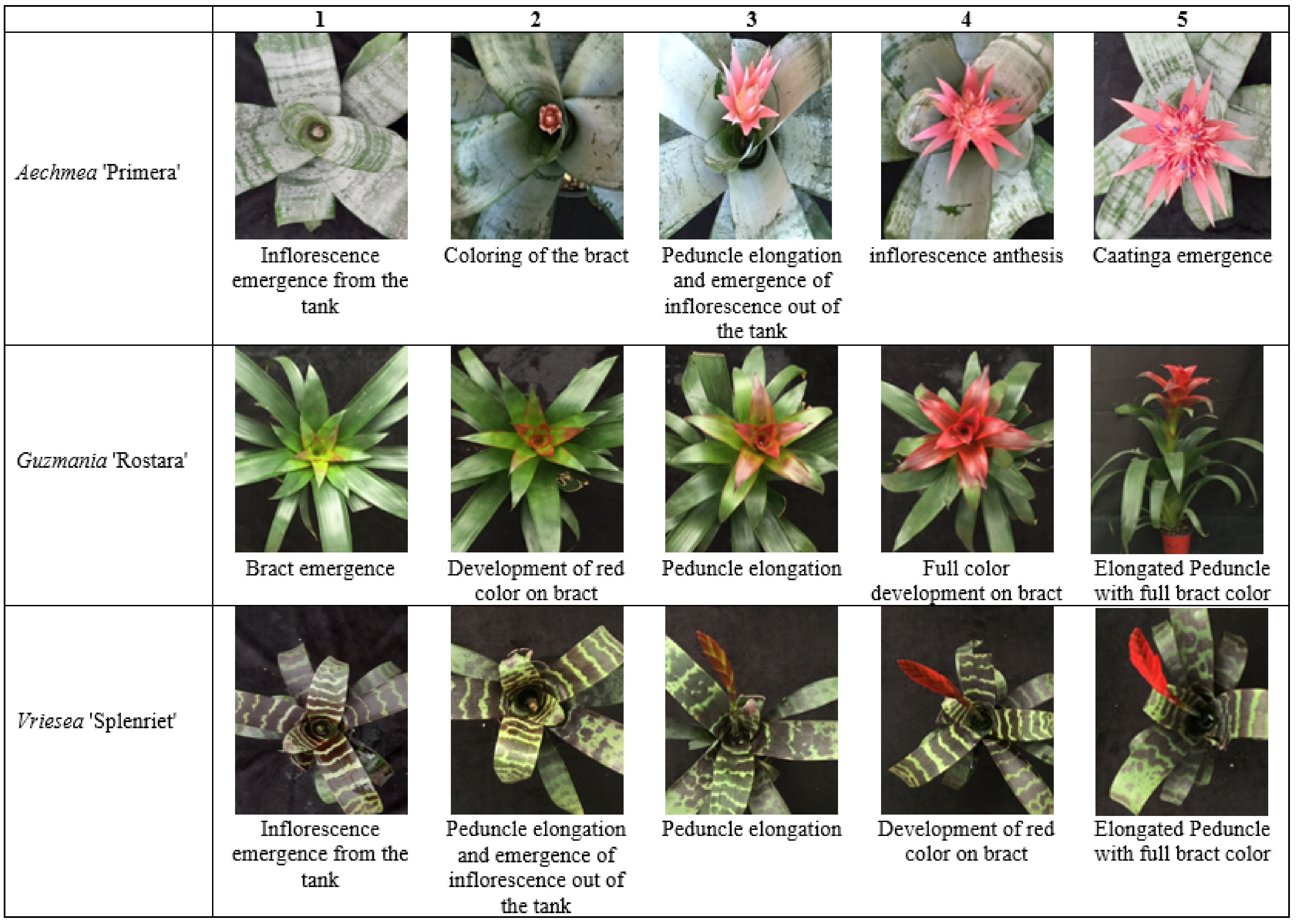
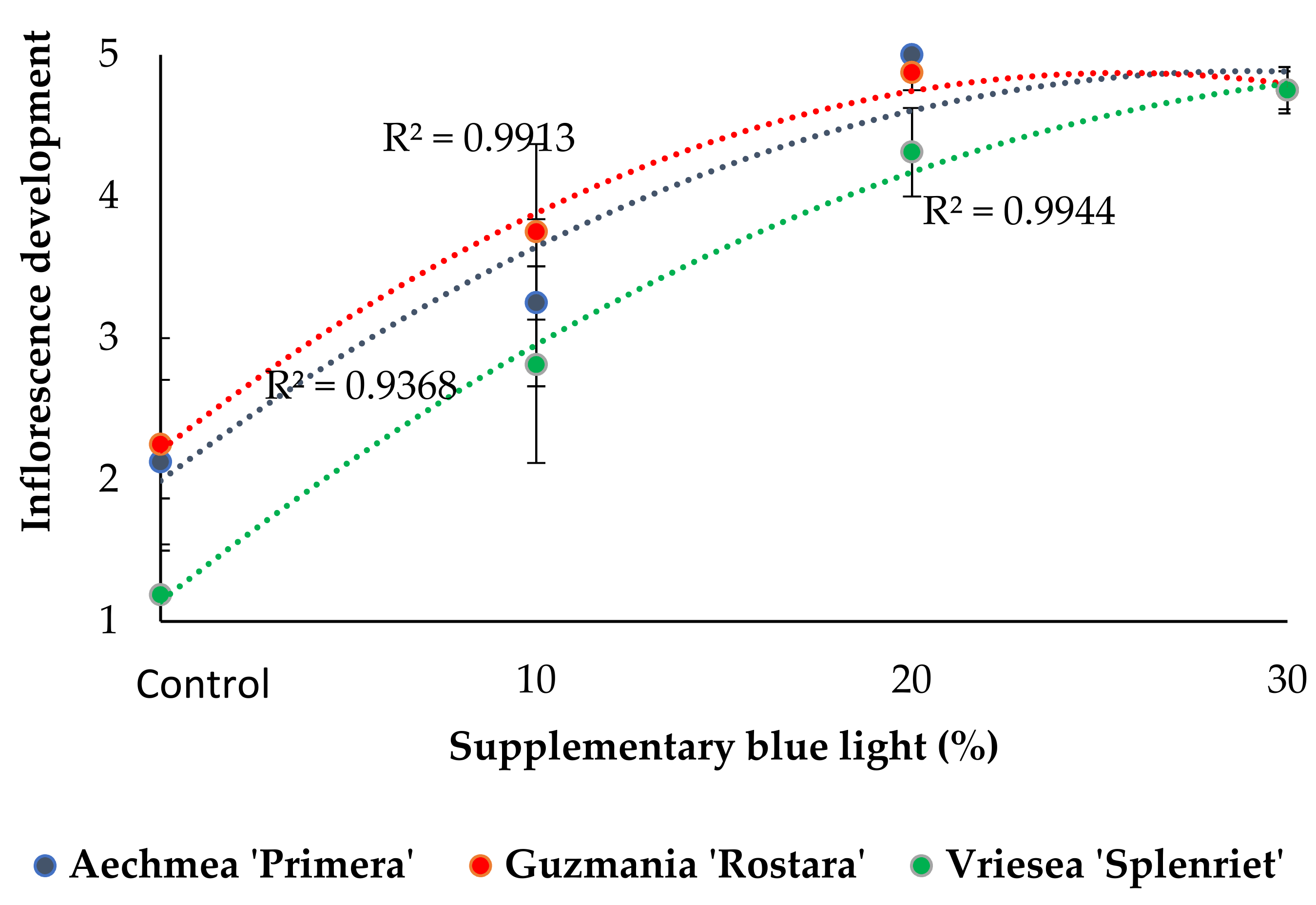
2.4. Inflorescence Emergence and Development
2.5. Leaf Osmotic Potential
2.6. SPAD Value and Leaf Photosynthetic Pigment Content
2.7. Chlorophyll Fluorescence Imaging
2.8. Polyphasic Chlorophyll Fluorescence Transient (OJIP) Evaluation
2.9. Experimental Design and Statistical Analysis
3. Results
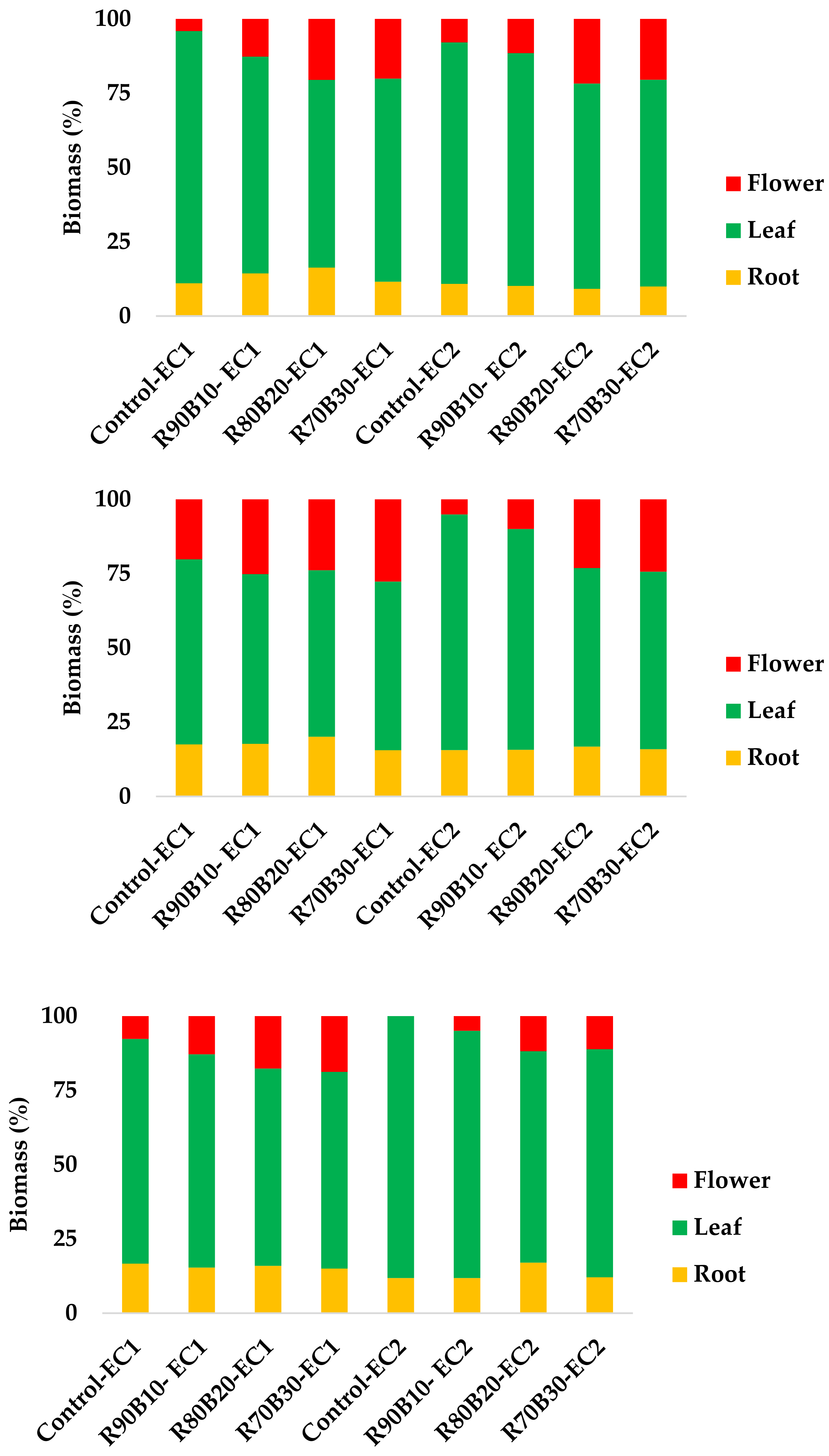
3.1. Plant Growth, Morphology, and Biomass Allocation
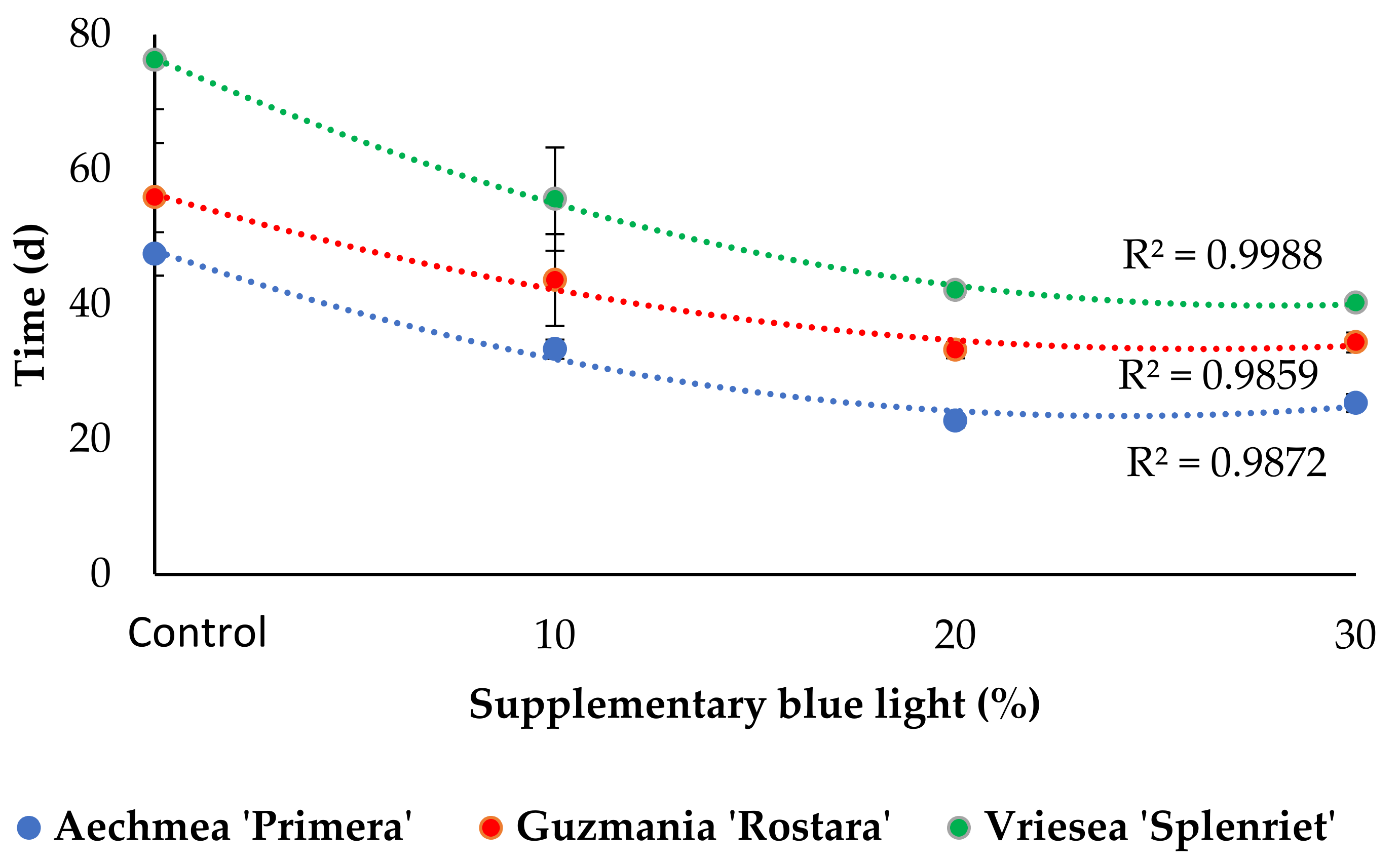
3.2. Inflorescence Emergence and Development
3.3. Leaf Osmotic Potential
3.4. SPAD Value and Leaf Photosynthetic Pigment Content
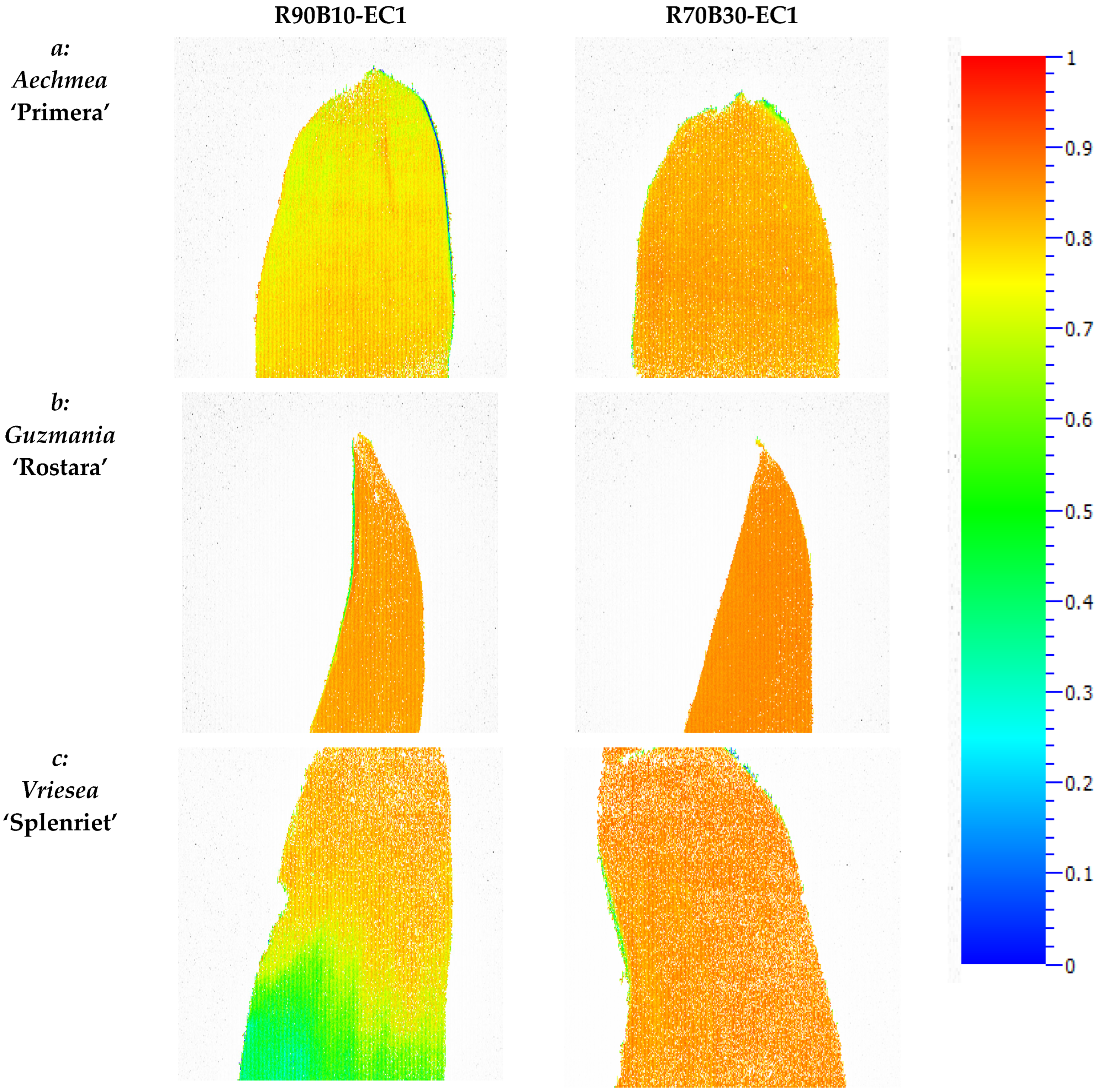
3.5. Chlorophyll Fluorescence Imaging
3.6. Photosynthetic Efficiency in Response to Light Intensity
4. Discussion
4.1. The Optimum EC for Growth Mostly Depends on the Species Rather than on Light Level
4.2. Increased Supplementary Light B Fraction Shortens the Time to Commercial Development Stage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acebey, A.; Krömer, T.; Maass, B.L.; Kessler, M. Ecoregional distribution of potentially useful species of Araceae and Bromeliaceae as non-timber forest products in Bolivia. Biodivers. Conserv. 2010, 19, 2553–2564. [Google Scholar] [CrossRef] [Green Version]
- Hornung-Leoni, C.T. Avances sobre usos etnobotánicos de las Bromeliaceae en Latinoamérica. Bol. Latinoam. Del Caribe De Plantas Med. Aromat. 2011, 10, 297–314. [Google Scholar]
- Ladino, G.; Ospina-Bautista, F.; Estévez Varón, J.; Jerabkova, L.; Kratina, P. Ecosystem services provided by bromeliad plants: A systematic review. Ecol. Evol. 2019, 9, 7360–7372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, B.; Schenkels, L.; Ceusters, J.; De Proft, M. Water and nutrient uptake in Vriesea cultivars: Trichomes vs. roots. Environ. Exp. Bot. 2017, 136, 21–30. [Google Scholar] [CrossRef]
- Vanhoutte, B.; Ceusters, J.; De Proft, M. The ‘tubing’ phenomenon in commercial cultivation of Guzmania: Morphology, physiology and anatomy. Sci. Hortic. 2016, 205, 112–118. [Google Scholar] [CrossRef]
- Dukovski, D.; Bernatzky, R.; Han, S. Flowering induction of Guzmania by ethylene. Sci. Hortic. 2006, 110, 104–108. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Lei, M.; Li, Z.; Jing, Y.; Hu, H.; Zhu, S.; Xu, L. Small RNA sequencing revealed various microRNAs involved in ethylene-triggered flowering process in Aechmea fasciata. Sci. Rep. 2020, 10, 7348. [Google Scholar] [CrossRef]
- Bermudez Müller, D.; Kanashiro, S.; Jocys, T.; Reis Tavares, A. Captación de nitrógeno por las bromelias ornamentales durante las etapas de desarrollo atmosférico y de tanque. Idesia Arica 2020, 38, 105–111. [Google Scholar] [CrossRef]
- Londers, E. Leaf Damage in CAM Bromeliads: A Hydrophysiological Approach. Ph.D. Thesis, Catholic University of Leuven, Leuven, Belgium, 2006. [Google Scholar]
- Fanourakis, D.; Hyldgaard, B.; Giday, H.; Aulik, I.; Bouranis, D.; Körner, O.; Ottosen, C.O. Stomatal anatomy and closing ability is affected by supplementary light intensity in rose (Rosa hybrida L.). Hortic. Sci. 2019, 46, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, H.K.; Fanourakis, D.; Tsaniklidis, G.; Bouranis, D.; Nejad, A.R.; Ottosen, C.O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hortic. 2020, 267, 109354. [Google Scholar] [CrossRef]
- van Telgen, H. Bloei-Inductie Bij Bromeliaceae: Literatuurstudie Naar Effectiviteit van Bloei-Inductie en Bloeiresultaat in Relatie Tot Inductierijpheid; Praktijkonderzoek Plant & Omgeving B.V.: Wageningen, The Netherlands, 2003. [Google Scholar]
- Zimmer, K. Bromelien: Botanik und Anzucht ausgewählter Arten; Paul Parey: Berlin, Germany; Hamburg, Germany, I-1: 1986; ISBN 3-489-62-24-0. [Google Scholar]
- van Noort, F.; Kromwijk, J.; Snel, J.; Warmenhoven, M.; Meinen, E.; Li, T.; Kempkes, F.; Marcelis, L. ‘Grip op Licht’bij Potanthurium en Bromelia: Meer Energie Besparing bij Het Nieuwe Telen Potplanten met Diffuus Licht en Verbeterde Monitoring Meer Natuurlijk; Wageningen UR Glastuinbouw: Bleiswijk, The Netherlands, 2013. [Google Scholar]
- Moosavi-Nezhad, M.; Salehi, R.; Aliniaeifard, S.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D.; Żuk-Gołaszewska, K.; Kalaji, H.M. Blue light improves photosynthetic performance during healing and acclimatization of grafted watermelon seedlings. Int. J. Mol. Sci. 2021, 22, 8043. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Hajilou, J.; Tabatabaei, S.J. Photosynthetic and growth responses of olive to proline and salicylic acid under salinity condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.; Yang, Q.; Li, T. Lettuce growth, morphology and critical leaf trait responses to far-red light during cultivation are low fluence and obey the reciprocity law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, U.; Zotz, G. ‘And then there were three’: Highly efficient uptake of potassium by foliar trichomes of epiphytic bromeliads. Ann. Bot. 2010, 106, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Males, J. Think tank: Water relations of Bromeliaceae in their evolutionary context. Bot. J. Linn. Soc. 2016, 181, 415–440. [Google Scholar] [CrossRef] [Green Version]
- Matiz, A.; Mioto, P.T.; Mayorga, A.Y.; Freschi, L.; Mercier, H. CAM photosynthesis in bromeliads and agaves: What can we learn from these plants. Photosynthesis 2013, 1, 91–134. [Google Scholar]
- Males, J. Concerted anatomical change associated with crassulacean acid metabolism in the Bromeliaceae. Funct. Plant Biol. 2018, 45, 681–695. [Google Scholar] [CrossRef]
- Beltrán, J.; Lasso, E.; Madriñán, S.; Virgo, A.; Winter, K. Juvenile tank-bromeliads lacking tanks: Do they engage in CAM photosynthesis? Photosynthesis 2013, 51, 55–62. [Google Scholar] [CrossRef]
- García Victoria, N.; Warmenhoven, M. Supplementary light and higher Fertigation EC in the cultivation of Bromelia improve quality and accelerate growth. In Proceedings of the VII International Symposium on Light in Horticultural Systems, Wageningen, The Netherlands, 15–18 October 2012; Volume 956, pp. 299–306. [Google Scholar]
- Warmenhoven, M.; Victoria, N.G. Toepassing Assimilatiebelichting bij Bromelia: Belichtingsniveau en Belichtingsduur in Relatie tot Voeding; Wageningen UR Glastuinbouw: Bleiswijk, The Netherlands, 2008. [Google Scholar]
- Hassanvand, F.; Nejad, A.R.; Fanourakis, D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind. Crop. Prod. 2019, 134, 19–25. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Van Labeke, M.C. Long-term effects of red-and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano, S.; Trivellini, A.; Ferrante, A.; Romano, D. Physiological mechanisms for delaying the leaf yellowing of potted geranium plants. Sci. Hortic. 2018, 242, 146–154. [Google Scholar] [CrossRef]
- Hoel, B.O.; Solhaug, K.A. Effect of irradiance on chlorophyll estimation with the Minolta SPAD-502 leaf chlorophyll meter. Ann. Bot. 1998, 82, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Bergsträsser, S.; Fanourakis, D.; Schmittgen, S.; Cendrero-Mateo, M.P.; Jansen, M.; Scharr, H.; Rascher, U. HyperART: Non-invasive quantification of leaf traits using hyperspectral absorption-reflectance-transmittance imaging. Plant Methods 2015, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents; Portland Press Limited: London, UK, 1983. [Google Scholar]
- Kalhor, M.S.; Aliniaeifard, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef]
- Giampaoli, P.; Santos, D.S.; Mollo, L.; Kanashiro, S.; Tavares, A.R. Effect of fertilisation with urea on development in the ornamental bromeliad Aechmea fasciata. Rev. Ciência Agronômica 2017, 48, 657–662. [Google Scholar] [CrossRef]
- Mercier, H.; Kerbauy, G.; Sotta, B.; Miginiac, E. Effects of NO3−, NH4+ and urea nutrition on endogenous levels of IAA and four cytokinins in two epiphytic bromeliads. Plant Cell Environ. 1997, 20, 387–392. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar]
| Element | Concentration (mmol L−1) | |
|---|---|---|
| EC1 | EC2 | |
| K | 6.78 | 13.56 |
| Mg | 0.83 | 1.66 |
| NO3− | 7.48 | 14.96 |
| NH4 | 1.25 | 2.5 |
| PO₄3− | 0.55 | 1.1 |
| SO₄2− | 0.837 | 1.674 |
| Fe | 0.013 | |
| Mn | 0.0026 | |
| Zn | 0.0024 | |
| Cu | 0.0025 | |
| Na | 0.00004 | |
| Mo | 0.00004 | |
| EC (dS m−l) | Light Regime | Plant Height (cm) | Number of Offshoots | Crown Thickness (mm) | Tank Volume (mL) | SLA (cm2 g−1) | Leaf | Root | Flower | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Area (cm2) | FW (g) | DW (g) | FW (g) | DW (g) | Volume (cm3) | FW (g) | DW (g) | |||||||
| 1 | Control | 42.1 | 0.50 | 32.8 | 25.9 | 112.5 | 16.0 | 3680.5 | 344.2 c | 35.7 | 40.3 | 4.59 | 47.0 | 27.6 | 1.77 |
| R90B10 | 43.3 | 0.75 | 32.7 | 18.8 | 108.6 | 14.5 | 3838.5 | 366.7 bc | 40.2 | 63.3 | 7.90 | 62.8 | 80.6 | 7.11 | |
| R80B20 | 48.3 | 3.50 | 35.9 | 38.8 | 125.9 | 12.3 | 3508.5 | 348.8 bc | 32.2 | 60.5 | 8.36 | 67.0 | 110.1 | 10.68 | |
| R70B30 | 46.5 | 1.50 | 32.3 | 29.0 | 110.9 | 14.3 | 3953.2 | 351.6 bc | 40.8 | 56.8 | 6.90 | 57.5 | 110.6 | 11.92 | |
| 2 | Control | 39.8 | 0.50 | 32.5 | 25.3 | 106.2 | 16.8 | 3123.5 | 334.6 c | 33.5 | 35.3 | 4.39 | 39.0 | 44.5 | 3.29 |
| R90B10 | 45.0 | 2.50 | 32.1 | 19.3 | 100.2 | 16.0 | 4244.3 | 419.2 a | 49.1 | 49.5 | 6.46 | 52.0 | 70.8 | 7.35 | |
| R80B20 | 50.8 | 1.75 | 34.5 | 22.0 | 90.50 | 14.5 | 3816.5 | 412.4 a | 48.2 | 54.3 | 6.41 | 59.3 | 127.6 | 15.23 | |
| R70B30 | 45.5 | 2.75 | 35.5 | 23.5 | 107.8 | 16.3 | 4103.7 | 395.1 ab | 44.7 | 57.9 | 6.23 | 70.0 | 110.2 | 12.82 | |
| p value | Light regime | 0.014 * | 0.028 * | 0.247 ns | 0.113 ns | 0.905 ns | 0.2 ns | 0.165 ns | 0.109 ns | 0.124 ns | 0.039 * | 0.003 ** | 0.047 * | 0.0002 ** | 0.0001 ** |
| EC | 0.88 ns | 0.527 ns | 0.877 ns | 0.038 * | 0.034 * | 0.046 * | 0.703 ns | 0.0004 ** | 0.013 * | 0.252 ns | 0.053 ns | 0.501 ns | 0.321 ns | 0.035 * | |
| Light regime × EC | 0.61 ns | 0.097 ns | 0.636 ns | 0.093 ns | 0.203 ns | 0.89 ns | 0.399 ns | 0.024 * | 0.749 ns | 0.768 ns | 0.423 ns | 0.375 ns | 0.257 ns | 0.246 ns | |
| EC (dS m−l) | Light Regime | Plant Height (cm) | Number of Offshoots | Crown Thickness (mm) | Tank Volume (mL) | SLA (cm2 g−1) | Leaf | Root | Flower | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Area (cm2) | FW (g) | DW (g) | FW (g) | DW (g) | Volume (cm3) | FW (g) | DW (g) | |||||||
| 1 | Control | 31.9 | 0.00 | 14.1 | 3.55 | 180.7 | 23.5 b | 1490.7 c | 58.8 c | 8.85 | 12.4 | 2.49 | 13.3 | 25.7 | 2.97 |
| R90B10 | 37.5 | 0.25 | 18.4 | 5.45 | 155.0 | 26.0 ab | 1939.9 ac | 79.7 ab | 13.97 | 19.7 | 4.27 | 22.0 | 49.0 | 6.15 | |
| R80B20 | 38.3 | 0.00 | 17.8 | 5.10 | 167.7 | 27.3 a | 2217.4 ab | 80.6 ab | 14.07 | 22.9 | 5.05 | 26.5 | 46.2 | 6.00 | |
| R70B30 | 41.3 | 0.25 | 18.5 | 15.75 | 152.7 | 28.0 a | 2310.8 a | 98.5 a | 16.47 | 22.1 | 4.50 | 23.5 | 59.7 | 8.02 | |
| 2 | Control | 28.8 | 0.00 | 13.5 | 3.05 | 168.0 | 26.5 ab | 1498.4 c | 66.1 bc | 9.53 | 10.4 | 1.91 | 11.0 | 5.88 | 0.62 |
| R90B10 | 36.9 | 0.50 | 17.5 | 3.40 | 147.6 | 28.8 a | 2043.9 ab | 99.2 a | 14.77 | 17.9 | 3.17 | 16.8 | 19.1 | 2.05 | |
| R80B20 | 37.6 | 0.50 | 16.1 | 4.87 | 150.8 | 27.0 ab | 1875.0 abc | 83.8 ab | 13.77 | 19.3 | 3.77 | 19.8 | 44.5 | 5.26 | |
| R70B30 | 42.6 | 0.25 | 19.3 | 9.37 | 138.3 | 26.3 ab | 1762.1 bc | 82.0 ab | 13.44 | 21.5 | 3.59 | 20.3 | 47.6 | 5.48 | |
| p value | Light regime | 0.002 ** | 0.573 ns | 0.02 * | 0.0001 ** | 0.008 ** | 0.377 ns | 0.048 * | 0.028 * | 0.006 ** | 0.002 ** | 0.004 ** | 0.004 ** | 0.0001 ** | 0.0001 ** |
| EC | 0.58 ns | 0.299 ns | 0.666 ns | 0.012 * | 0.095 ns | 0.136 ns | 0.032 * | 0.394 ns | 0.503 ns | 0.119 ns | 0.0007 ** | 0.003* * | 0.005 ** | 0.001 ** | |
| Light regime × EC | 0.698 ns | 0.703 ns | 0.939 ns | 0.054 ns | 0.97 ns | 0.037 * | 0.046 * | 0.039 * | 0.208 ns | 0.846 ns | 0.701 ns | 0.573 ns | 0.266 ns | 0.303 ns | |
| EC (dS m−l) | Light Regime | Plant Height (cm) | Crown Thickness (mm) | Tank Volume (mL) | SLA (cm2 g−1) | Leaf | Root | Flower | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Area (cm2) | FW (g) | DW (g) | FW (g) | DW (g) | Volume (cm3) | FW (g) | DW (g) | ||||||
| 1 | Control | 16.8 | 18.3 a | 9.5 | 135.0 | 14.3 | 748.9 | 51.0 | 5.86 | 3.25 | 1.31 | 4.5 | 7.13 | 0.69 |
| R90B10 | 16.5 | 19.0 a | 13.0 | 148.5 | 15.8 | 989.0 | 55.7 | 7.30 | 3.13 | 1.67 | 4.5 | 13.8 | 1.61 | |
| R80B20 | 18.5 | 18.9 a | 21.3 | 130.5 | 16.3 | 1158 | 69.3 | 9.53 | 3.27 | 2.23 | 6.1 | 23.6 | 2.57 | |
| R70B30 | 17.0 | 18.3 a | 23.8 | 133.6 | 16.0 | 935.4 | 56.9 | 7.52 | 3.25 | 1.75 | 5.8 | 22.6 | 2.15 | |
| 2 | Control | 14.8 | 14.3 b | 4.25 | 132.2 | 10.5 | 488.3 | 31.3 | 3.88 | 1.27 | 0.52 | 2.6 | 0 | 0 |
| R90B10 | 15.5 | 20.5 a | 21.3 | 124.7 | 13.0 | 675.9 | 45.0 | 5.73 | 3.25 | 0.79 | 3.1 | 4.97 | 0.39 | |
| R80B20 | 16.8 | 17.7 a | 7.75 | 138.7 | 14.0 | 744.8 | 47.1 | 5.91 | 3.76 | 1.36 | 4.0 | 11.7 | 1.01 | |
| R70B30 | 17.3 | 18.5 a | 19.5 | 142.9 | 14.3 | 935.3 | 55.0 | 7.09 | 3.52 | 1.16 | 3.5 | 14.3 | 1.20 | |
| p value | Light regime | 0.408 ns | 0.009 ** | 0.093 ns | 0.969 ns | 0.045 * | 0.004 ** | 0.017 * | 0.003 ** | 0.083 ns | 0.033 * | 0.182 ns | 0.005 ** | 0.003 ** |
| EC | 0.105 ns | 0.181 ns | 0.403 ns | 0.694 ns | 0.0002 ** | 0.001 ** | 0.003 ** | 0.003 ** | 0.412 ns | 0.0005 ** | 0.0005 ** | 0.001 ** | 0.0003 ** | |
| Light regime × EC | 0.615 ns | 0.042 * | 0.381 ns | 0.192 ns | 0.562 ns | 0.133 ns | 0.252 ns | 0.217 ns | 0.064 ns | 0.92 ns | 0.878 ns | 0.867 ns | 0.529 ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javadi Asayesh, E.; Aliniaeifard, S.; Askari, N.; Roozban, M.R.; Sobhani, M.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D. Supplementary Light with Increased Blue Fraction Accelerates Emergence and Improves Development of the Inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae 2021, 7, 485. https://doi.org/10.3390/horticulturae7110485
Javadi Asayesh E, Aliniaeifard S, Askari N, Roozban MR, Sobhani M, Tsaniklidis G, Woltering EJ, Fanourakis D. Supplementary Light with Increased Blue Fraction Accelerates Emergence and Improves Development of the Inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae. 2021; 7(11):485. https://doi.org/10.3390/horticulturae7110485
Chicago/Turabian StyleJavadi Asayesh, Elahe, Sasan Aliniaeifard, Naser Askari, Mahmood Reza Roozban, Mohammadhadi Sobhani, Georgios Tsaniklidis, Ernst J. Woltering, and Dimitrios Fanourakis. 2021. "Supplementary Light with Increased Blue Fraction Accelerates Emergence and Improves Development of the Inflorescence in Aechmea, Guzmania and Vriesea" Horticulturae 7, no. 11: 485. https://doi.org/10.3390/horticulturae7110485
APA StyleJavadi Asayesh, E., Aliniaeifard, S., Askari, N., Roozban, M. R., Sobhani, M., Tsaniklidis, G., Woltering, E. J., & Fanourakis, D. (2021). Supplementary Light with Increased Blue Fraction Accelerates Emergence and Improves Development of the Inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae, 7(11), 485. https://doi.org/10.3390/horticulturae7110485