Nanobiotechnological Approaches to Enhance Potato Resistance against Potato Leafroll Virus (PLRV) Using Glycyrrhizic Acid Ammonium Salt and Salicylic Acid Nanoparticles
Abstract
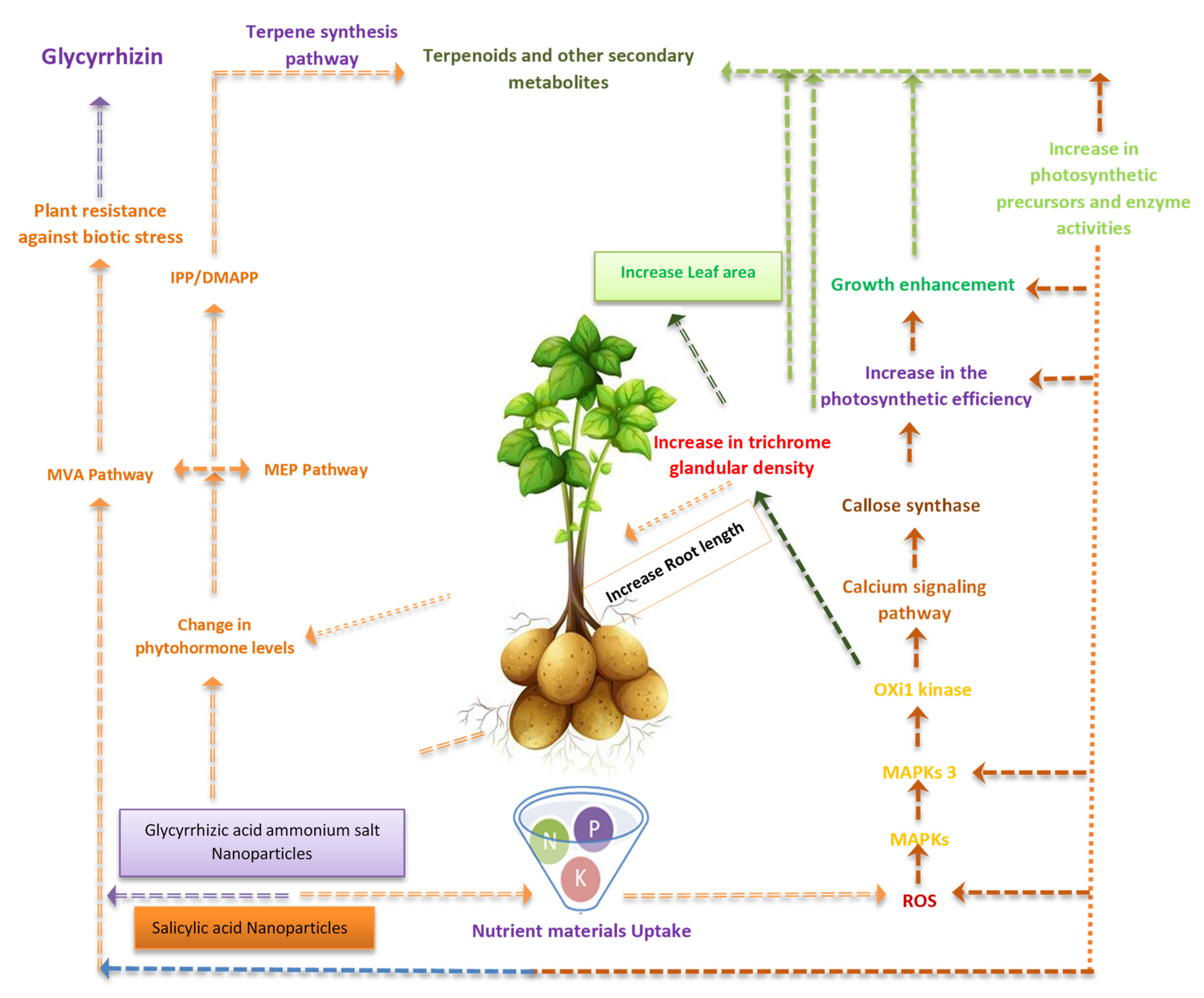
:1. Introduction
1.1. Glycyrrhizic Acid
1.2. Salicylic Acid (SA)
1.3. Plant Defense-Related Genes
1.4. Docking Studies
2. Materials and Methods
2.1. Docking Studies
2.2. Tested Compounds Optimization
2.3. Target Potato Leaf Roll Viral Protein Coat Active Site Optimization
2.4. Docking of the Tested Molecules to Viral Main Protein Coat Binding Site
2.5. Sample Collection
2.6. Media Preparation
2.7. Glycyrrhizic Acid Ammonium Salt Nanoparticles Preparation
2.8. Preparation and Characterization of SA Nanoparticles
2.9. Characterization of Nanomaterials by Using Dynamic Light Scattering (DLS)
2.10. RNA Extraction
2.11. Primer Design and Gene Expression
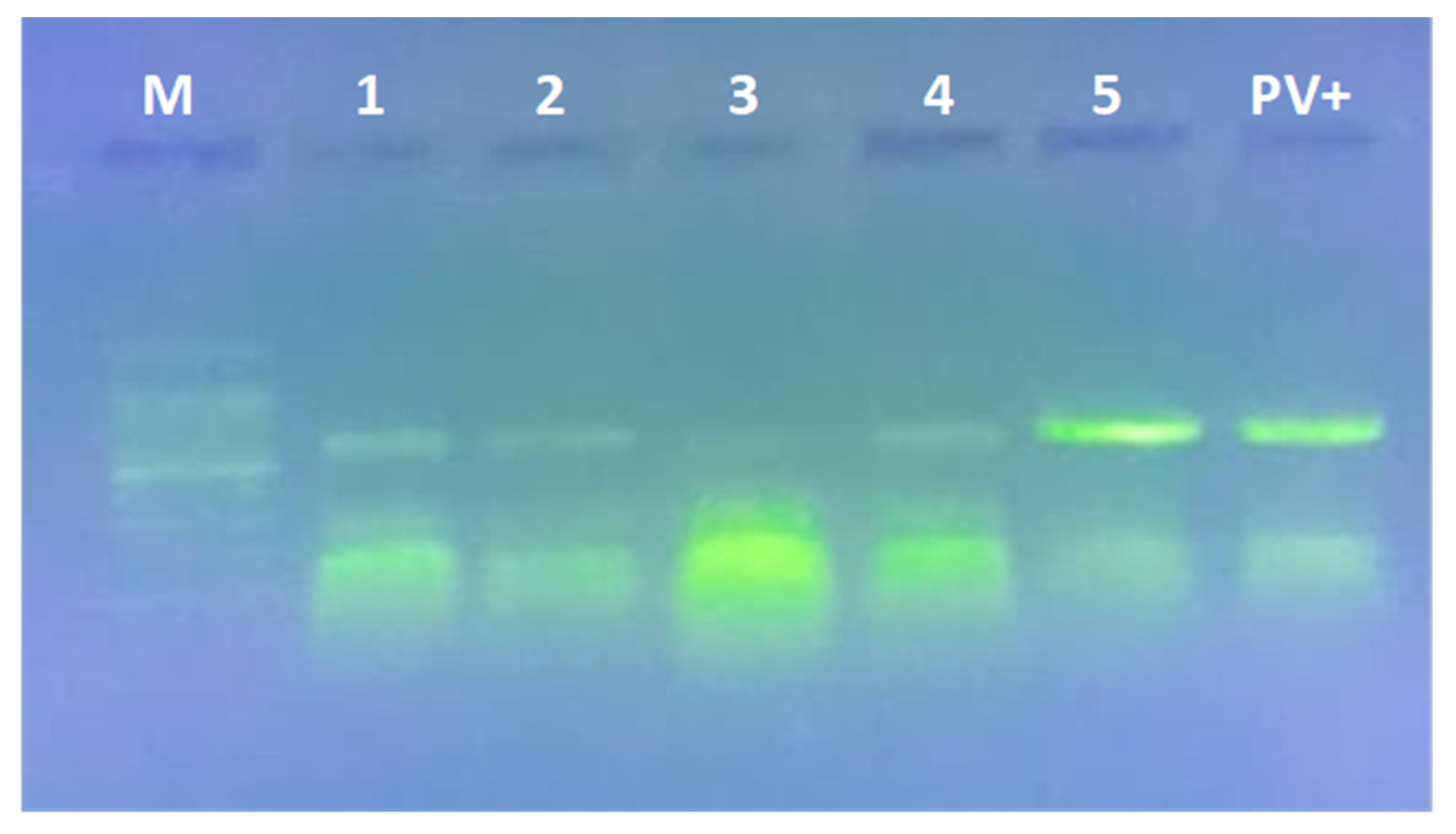
2.12. RT-PCR Detection
2.13. Gel Electrophoresis Preparation
2.14. Statistical Analysis
3. Results
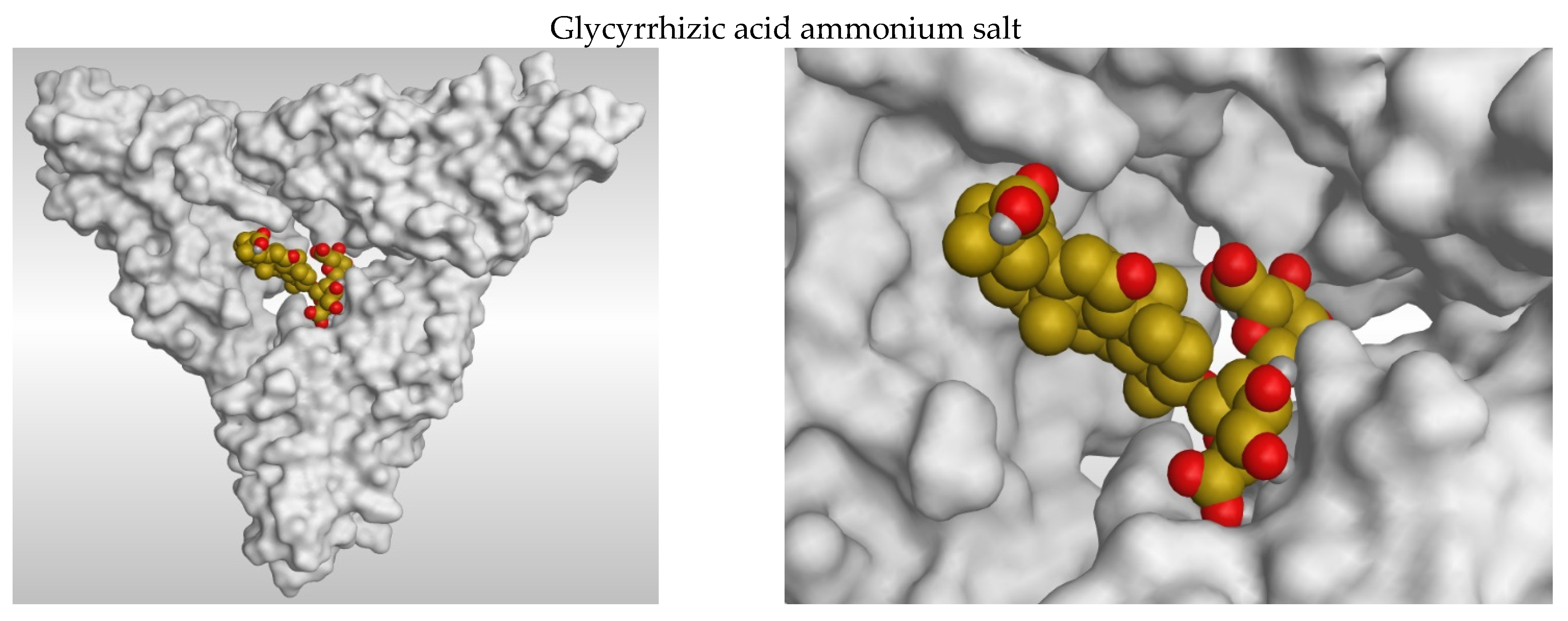
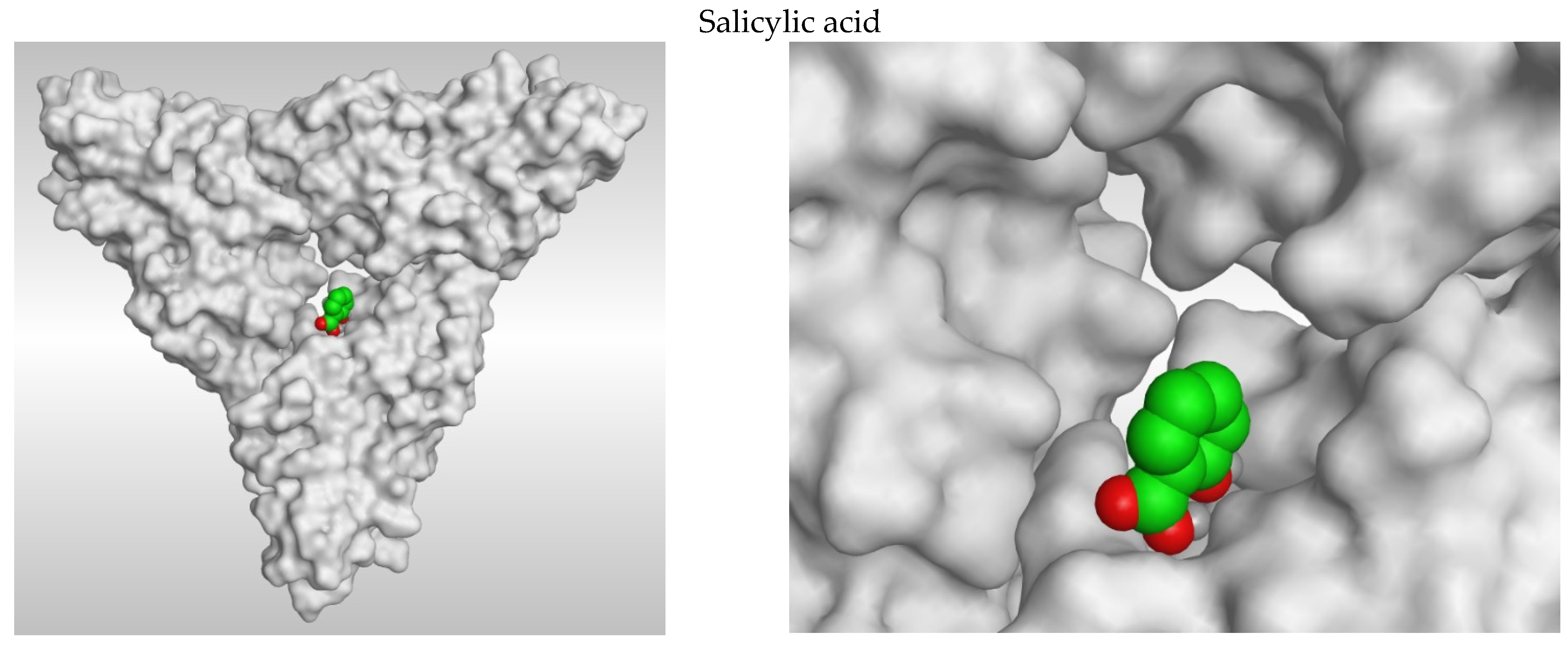
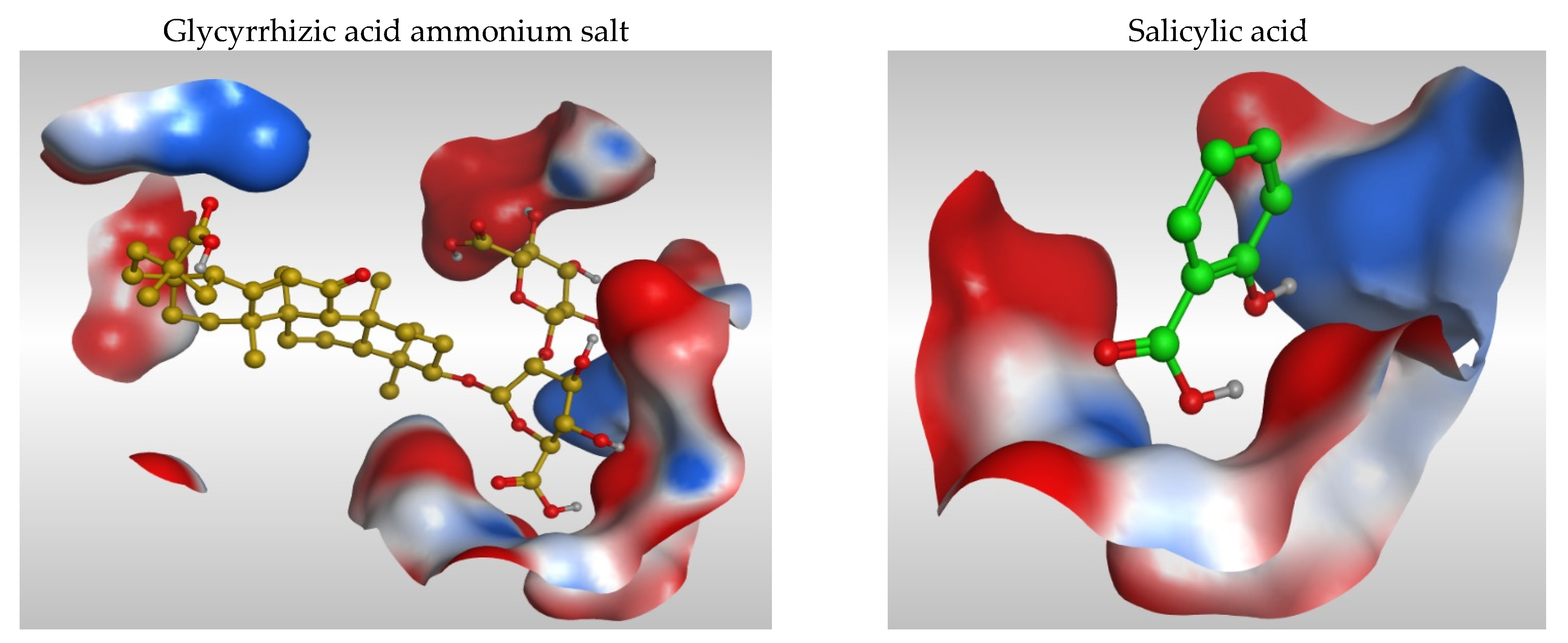
3.1. Docking Studies
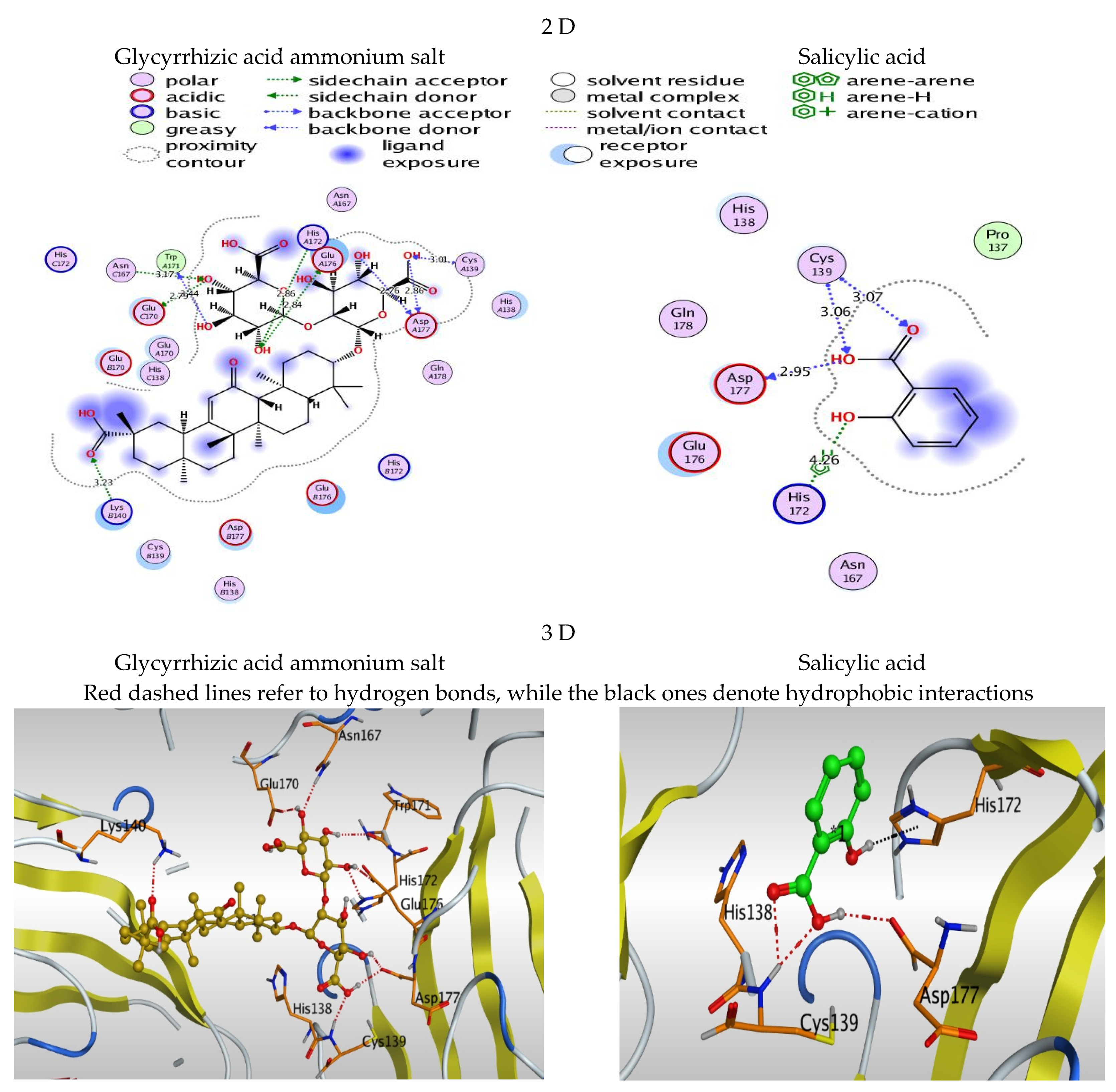
3.2. 2D and 3D Images of Docked Molecules Showing That
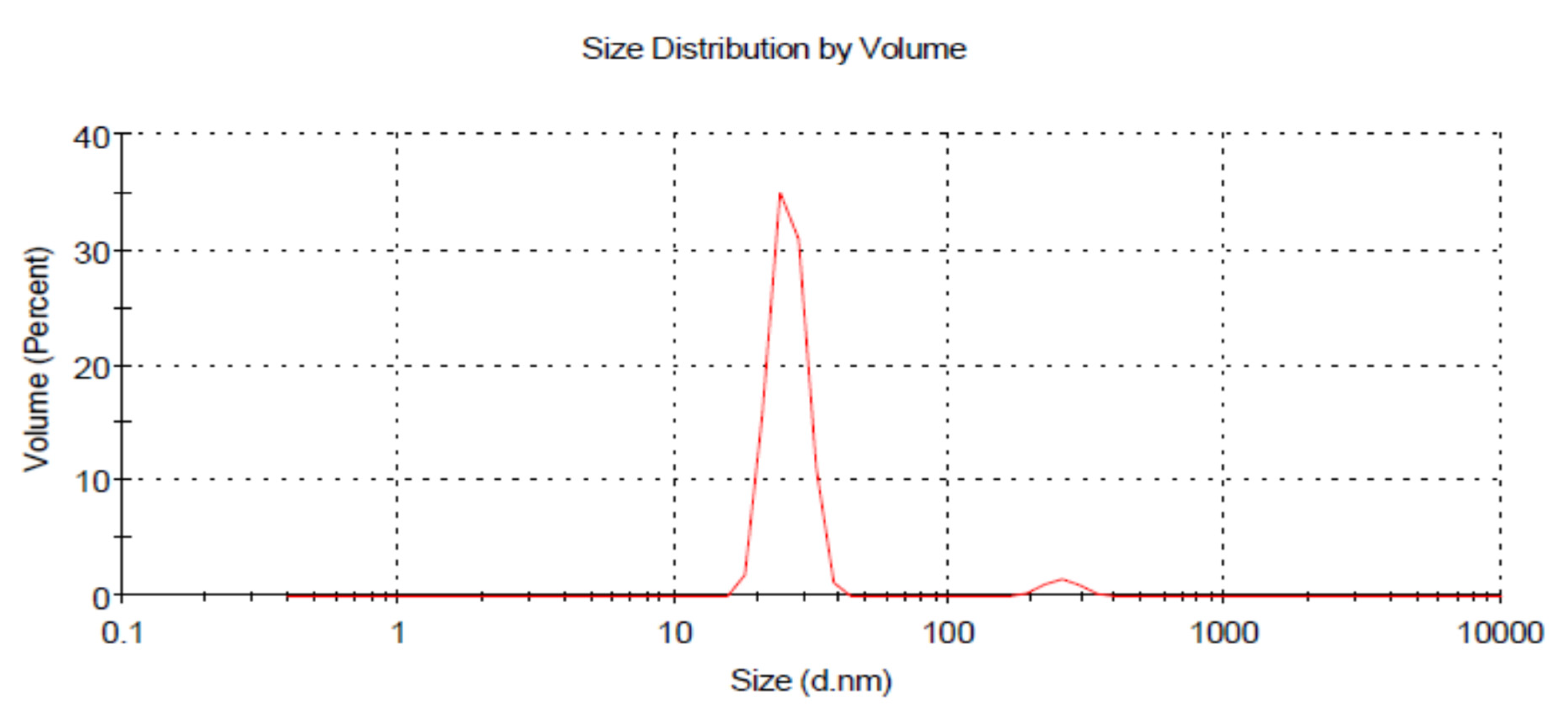
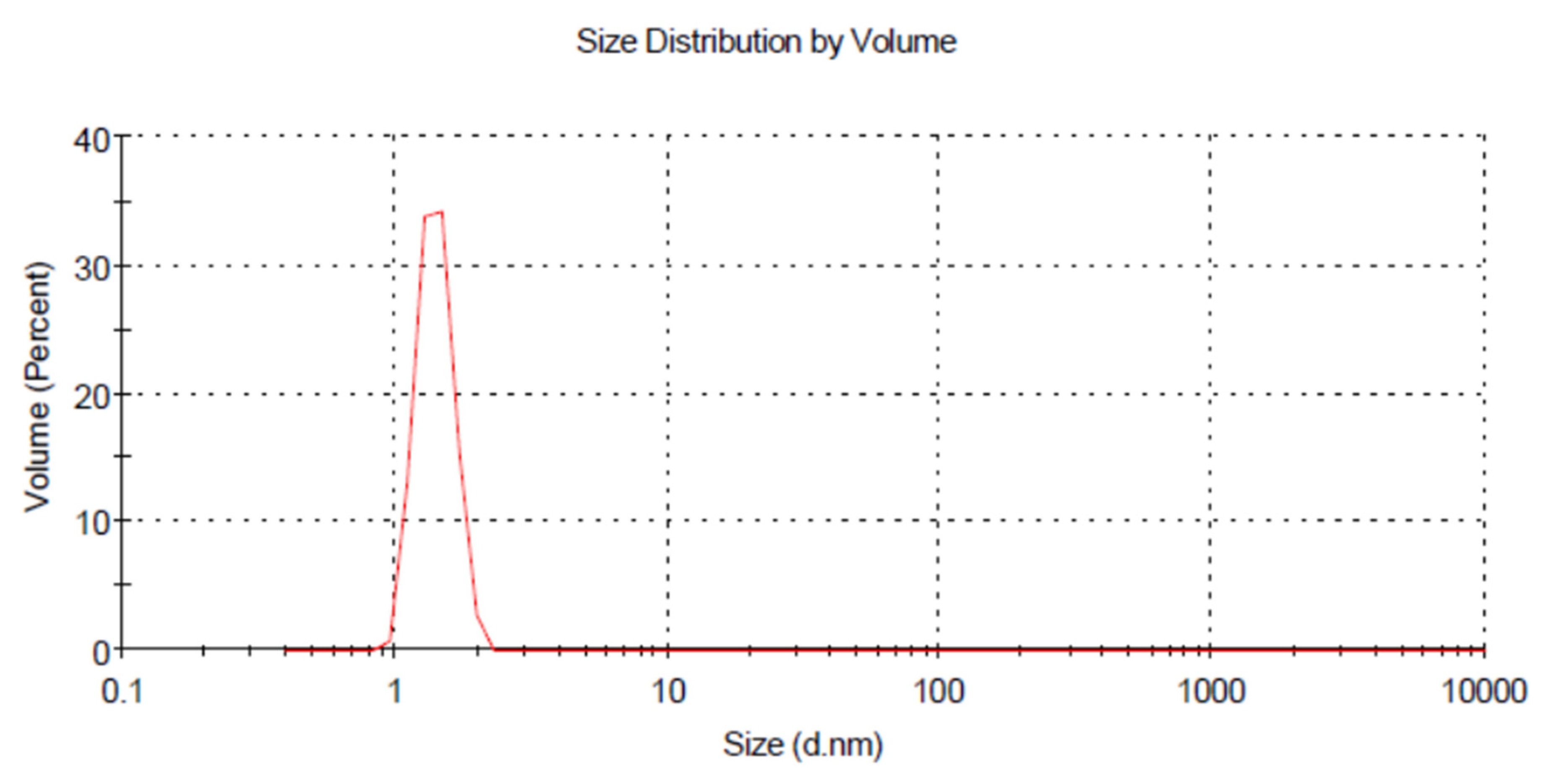
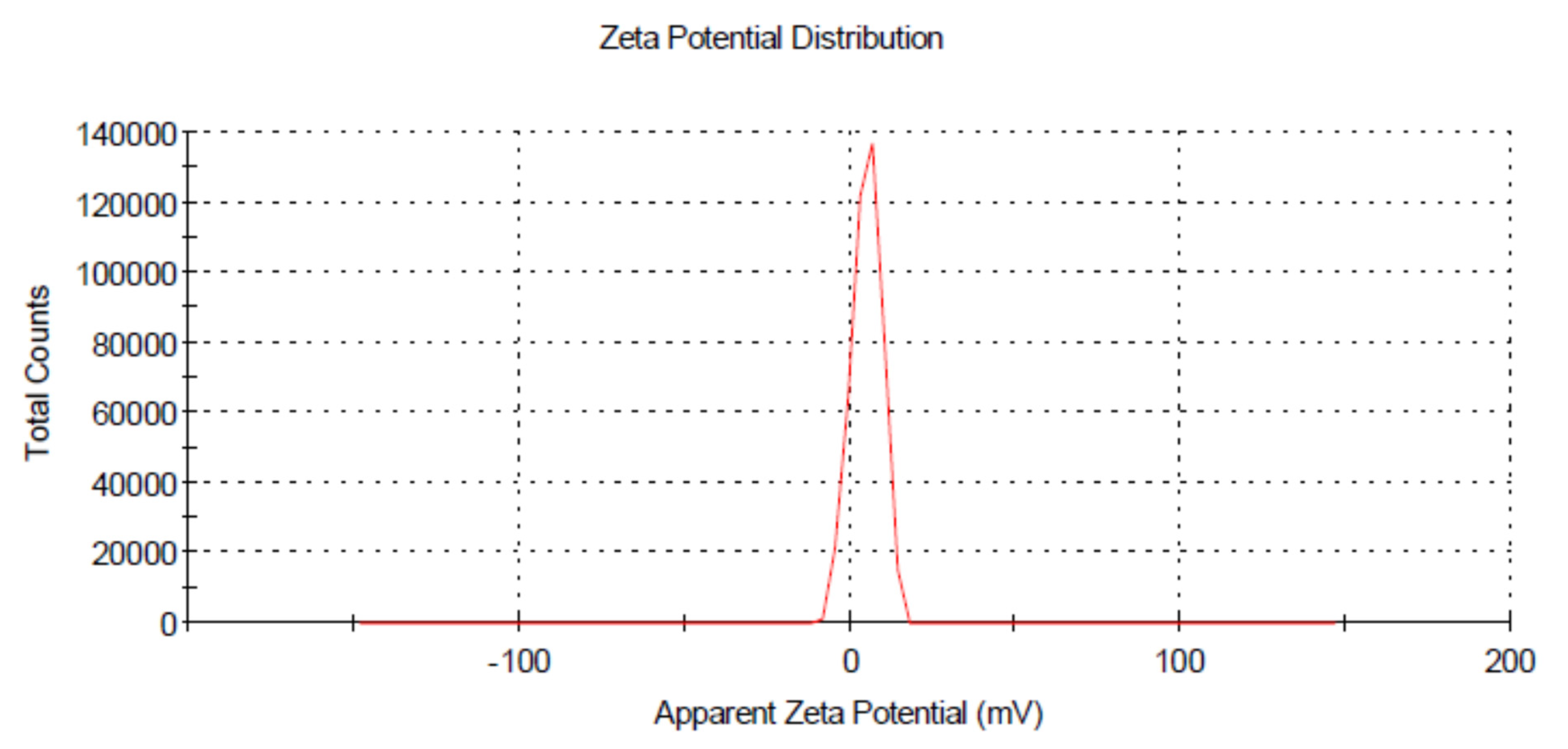
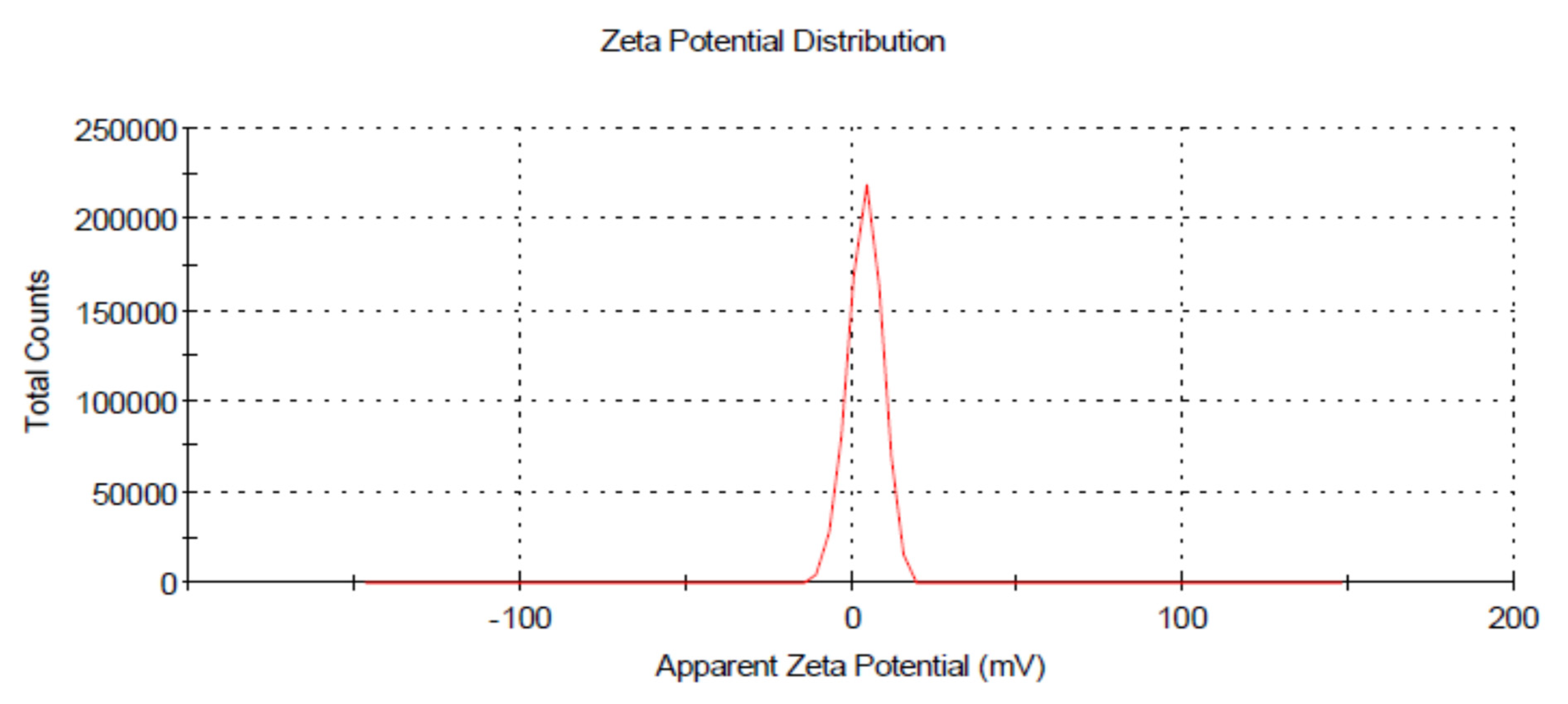
3.3. Dynamic Light Scattering (DLS)
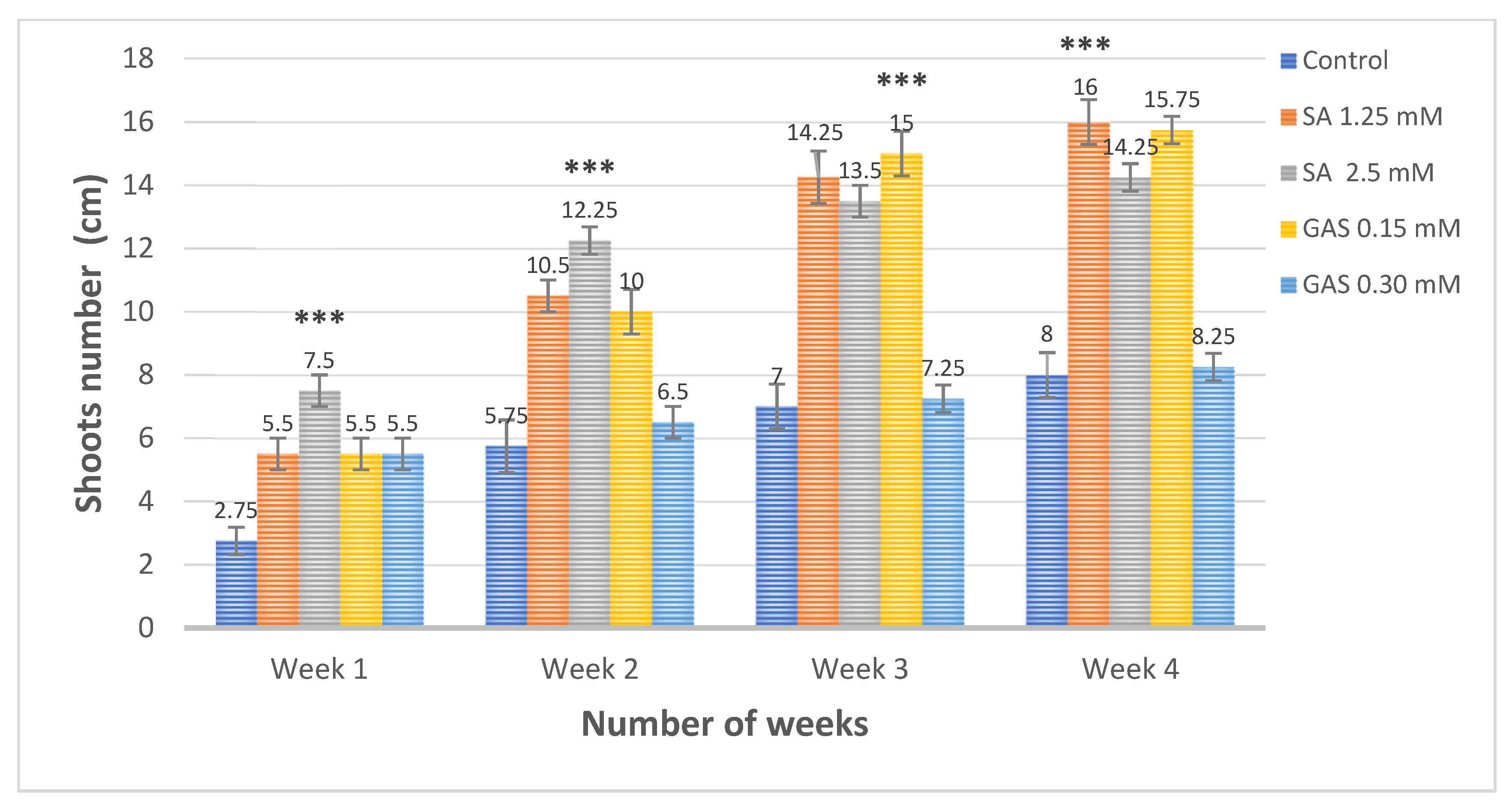
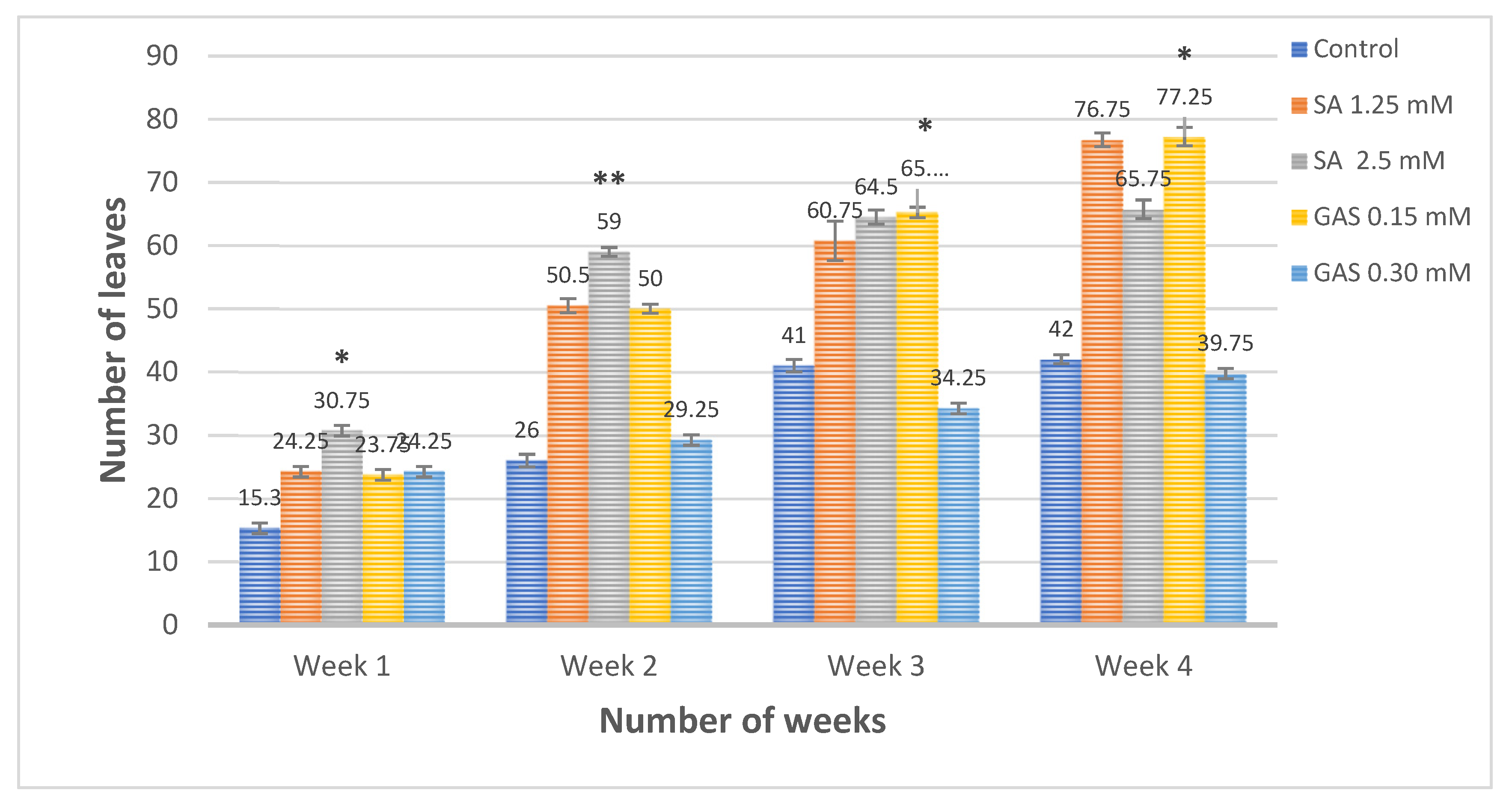
3.4. Effect of Salicylic Acid and Glycyrrhizic Acid Ammonium Salt Nanoparticles on the Shoots and Leaves Number Compared to Positive Control
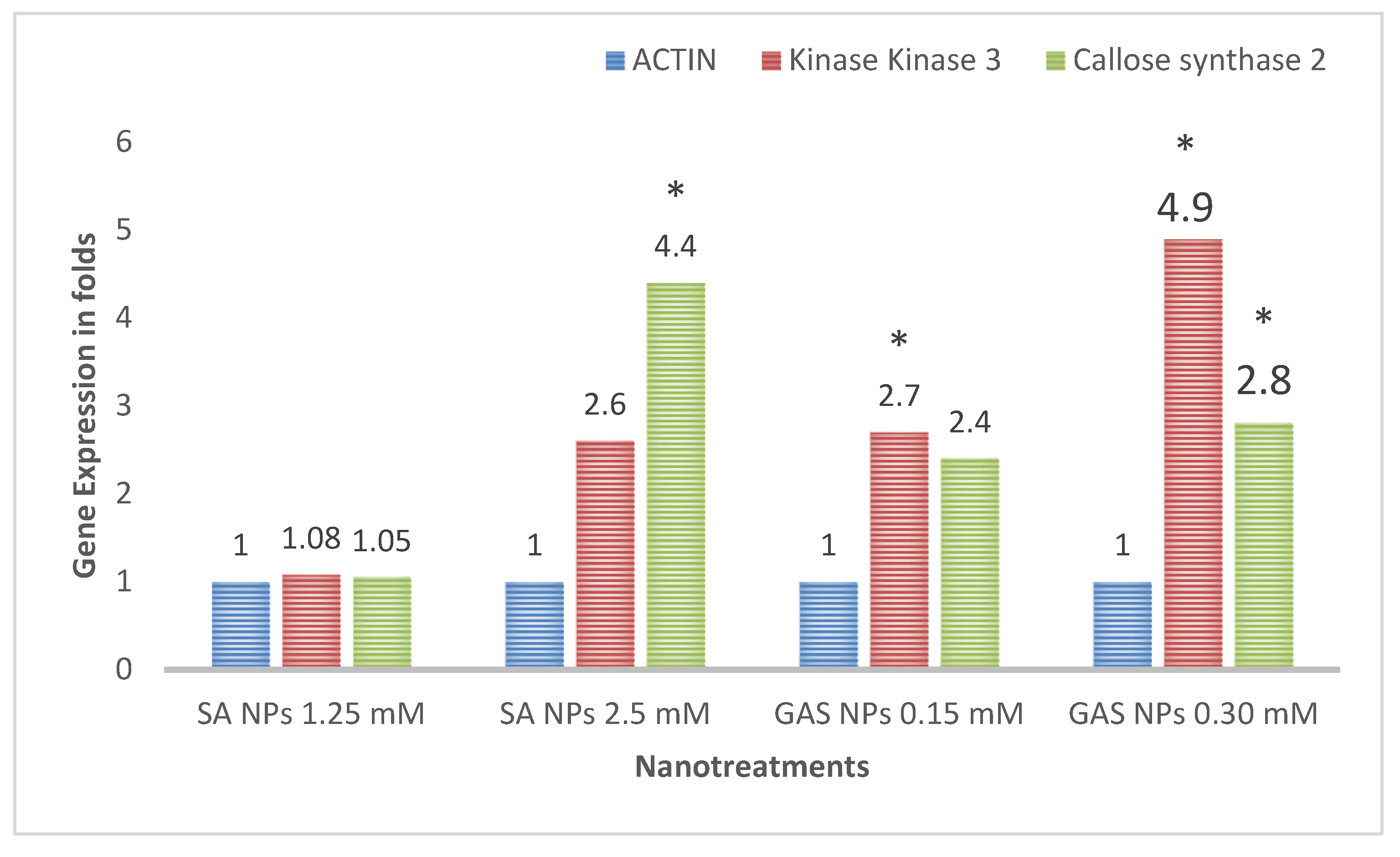
3.5. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kreuze, J.F.; Souza-Dias, J.A.C.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.P.T.; Jones, R.A.C. Viral Diseases in Potato. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Chatzivassiliou, E.K.; Moschos, E.; Gazi, S.; Koutretsis, P.; Tsoukaki, M. Infection of Potato Crops and Seeds with Potato Virus y and Potato Leafroll Virus in Greece. J. Plant. Pathol. 2008, 90, 253–261. [Google Scholar]
- IHIDMA. Pharmacopoeia; Indian Herbal Indian Drug Manufacturers Association: Mumbai, India, 2002. [Google Scholar]
- Pompei, R.; Flore, O.; Marccialis, M.A.; Pani, A.; Loddo, B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef]
- Ates, D.A.; Turgay, O. Antimicrobial activities of various medicinal and commercial plant extracts. Turk. J. Biol. 2003, 27, 157–162. [Google Scholar]
- Alonso, J. Tratado de Fitofármacos y Nutracéuticos, 1st ed.; Editorial Corpus Libros: Rosario, Argentina, 2004. [Google Scholar]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef]
- Amirghofran, Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran. J. Immunol. 2010, 7, 65. [Google Scholar] [PubMed]
- Krausse, R.; Bielenberg, J.; Blaschek, W.; Ullmann, U. In Vitro anti- Helicobacter pylori activity of Extractum liquiritiae, glycyrrhizin and its metabolites. J. Antimicrob. Chemother. 2004, 54, 243–246. [Google Scholar] [CrossRef]
- Li, Y.; Sun, F.; Jing, Z.; Wang, X.; Hua, X.; Wan, L. Glycyrrhizic acid exerts anti-inflammatory effect to improve cerebral vasospasm secondary to subarachnoid hemorrhage in a rat model. Neurol. Res. 2017, 39, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sun, L.; He, F.; Che, H. Anti-allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells. Sci. Rep. 2017, 7, 7222. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kang, H.E.; Lee, M.G.; Hwang, S.J.; Kim, S.C.; Lee, C.H.; Kim, S.G. Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G372–G381. [Google Scholar] [CrossRef]
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. S. Afr. J. Bot. 2011, 77, 36–44. [Google Scholar]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism deferentially in two mungbean cultivars. J. Plant. Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant. Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Maodzeka, A.; Hussain, N.; Shamsi, I.H.; Jiang, L. The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant. Growth Regul. 2014, 75, 641–655. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant. Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shoala, T. Positive Impacts of Nanoparticles in Plant Resistance against Different Stimuli. In Nanobiotechnology Applications in Plant Protection, 1st ed.; Abd-Elsalam, K.A., Prasad, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 267–279. [Google Scholar]
- Richmond, T.A.; Somerville, C.R. The cellulose synthase superfamily. Plant. Physiol. 2020, 124, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Hong, Z. Unplugging the callose plug from sieve pores. Plant. Signal. Behav. 2011, 6, 491–493. [Google Scholar] [CrossRef]
- Barratt, D.H.P.; Kölling, K.; Graf, A.; Pike, M.; Calder, G.; Findlay, K.; Zeeman, S.; Smith, A. Callose synthase GSL7 is necessary for normal phloem transport and inflorescence growth in Arabidopsis. Plant. Physiol. 2011, 155, 328–341. [Google Scholar] [CrossRef]
- Dong, X.; Hong, Z.; Chatterjee, J.; Kim, S.; Verma, D.P.S. Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 2008, 229, 87–98. [Google Scholar] [CrossRef]
- Cui, W.; Lee, J.Y. Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nat. Plants 2016, 2, 16034. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Mayo, M.A.; D’Arcy, C.J. Family Luteoviridae: A reclassification of luteoviruses. In The Luteoviridae; Smith, H.G., Barker, H., Eds.; CABI Publishing: Wallingford, UK, 1999; pp. 15–22. [Google Scholar]
- Miller, W.A.; Dinesh-Kumar, S.P.; Paul, C.P. Luteovirus gene expression. Crit. Rev. Plant. Sci. 1995, 14, 179–211. [Google Scholar] [CrossRef]
- Pfeffer, S.; Dunoyer, P.; Heim, F.; Richards, K.E.; Jonard, G.; Ziegler-Graff, V. P0 of beet western yellows virus is a suppressor of posttranscriptional gene silencing. J. Virol. 2002, 76, 6815–6824. [Google Scholar] [CrossRef]
- Ashoub, A.; Rohde, W.; Prufer, D. In planta transcription of a second subgenomic RNA increases the complexity of the subgroup 2 luteovirus genome. Nucleic Acids Res. 1998, 26, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Massalski, P.R.; Harrison, B.D. Properties of Monoclonal Antibodies to Potato Leafroll Luteovirus and Their Use to Distinguish Virus Isolates Differing in Aphid Transmissibility. J. Gen. Virol. 1987, 68, 1813–1821. [Google Scholar] [CrossRef]
- Syller, J. Potato leafroll virus (PLRV): Its transmission and control. Integr. Pest. Manag. Rev. 1996, 1, 217–227. [Google Scholar] [CrossRef]
- Khattab, M.; Al-Karmalawy, A.A. Revisiting Activity of Some Nocodazole Analogues as a Potential Anticancer Drugs Using Molecular Docking and DFT Calculations. Front. Chem. 2021, 9, 628398. [Google Scholar] [CrossRef] [PubMed]
- Danci, O.; Baciu, A.; Danci, M. Potato (Solanum tuberosum L.) regeneration using the technique of meristem tip culture. J. Biotechnol. Hortic. For. 2011, 15, 175–178. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Danci, O.; Erdei, L.; Vidacs, L.; Danci, M.; Baciu, A.; David, I.; Berbentea, F. Influence of ribavirin on potato plants regeneration and virus eradication. J. Hortic. For. Biotechnol. 2009, 13, 421–425. [Google Scholar]
- Abdel-Rahman, F.A.; Rashid, I.A.; Shoala, T. Nanoactivities of natural nanomaterials rosmarinic acid, glycyrrhizic acid and glycyrrhizic acid ammonium salt against tomato phytopathogenic fungi Alternaria alternata and Penicillium digitatum. J. Plant. Prot. Res. 2020, 60, 150–160. [Google Scholar]
- Abdel-Rahman, F.A.; Khafagi, E.Y.; Soliman, M.S.; Shoala, T.; Ahmed, Y. Preharvest application of salicylic acid induces some resistant genes of sweet pepper against black mold disease. Eur. J. Plant. Pathol. 2021, 159, 755–768. [Google Scholar] [CrossRef]
- Shoala, T.; Eid, K.; EL-Fiki, I. Impact of Chemotherapy and Thermotherapy Treatments on the Presence of Potato Viruses PVY, PVX and PLRV in Tissue-Cultured Shoot Tip Meristem. J. Plant. Prot. Pathol. 2019, 10, 581–585. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritschi, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Wales, S.; Platt, H.W.; Cattlin, N. Diseases, Pests and Disorders of Potatoes: A Colour Handbook, 1st ed.; Manson Publishing Ltd.: London, UK, 2008; pp. 75–76. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Faisal, Z.; Ali, R.; Sayed, M.M.; Jubayer, A.M.; Masayuki, F.; Vasileios, F. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H.; et al. Oxi1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef]
- Petersen, L.N.; Ingle, R.A.; Knight, M.R.; Denby, K.J. OXI1 protein kinase is required for plant immunity against Pseudomonas syringae in Arabidopsis. J. Exp. Bot. 2009, 60, 3727–3735. [Google Scholar] [CrossRef]
| Primer Name | Accession No. | Primer Sequence | bp | T (°C) |
|---|---|---|---|---|
| Kinase Kinase 3 | XM_006351467.2 | F 5′-TTA CAT GTC GCC GGA ACG AA-3′R 5′-CAC TCA AAC AGT GCA AGC CC-3′ | 84 | 58.4 °C 60.5 °C |
| Callose synthase 2 | XM_015314276.1 | F 5′-AGC AGT GAA GTG TAG CTA AGG C-3′R 5′-ATT ACC AGC AGT CTG CGT CC-3′ | 139 | 62.1 °C 60.5 °C |
| ACTIN | X55749 | F 5′-GCT TCC CGA TGG TCA AGT CA-3′R 5′-GGA TTC CAG CTG CTT CCA TTC-3′ | 101 | 60.5 °C 61.3 °C |
| Compound | Score Kcal/Mole | RMSD_Refine | Amino Acid Bond | Distance Å |
|---|---|---|---|---|
| Glycyrrhizic acid ammonium salt | −8.95 | 2.09 | Asp A177/H-acceptor | 2.76 |
| Asp A177/H-acceptor | 2.86 | |||
| Cys A139/H-donor | 3.01 | |||
| Glu A176/H-acceptor | 2.84 | |||
| His A172/H-donor | 2.86 | |||
| Trp A171/H-acceptor | 2.44 | |||
| Lys B140/H-donor | 3.23 | |||
| Glu C170/H-donor | 2.79 | |||
| Asn C167/H-donor | 3.17 | |||
| Salicylic acid | −3.83 | 1.04 | Asp 177/H-acceptor | 2.95 |
| Cys 139/H-donor | 3.06 | |||
| Cys 139/H-donor | 3.07 | |||
| His 172/pi-H | 4.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoala, T.; Al-Karmalawy, A.A.; Germoush, M.O.; ALshamrani, S.M.; Abdein, M.A.; Awad, N.S. Nanobiotechnological Approaches to Enhance Potato Resistance against Potato Leafroll Virus (PLRV) Using Glycyrrhizic Acid Ammonium Salt and Salicylic Acid Nanoparticles. Horticulturae 2021, 7, 402. https://doi.org/10.3390/horticulturae7100402
Shoala T, Al-Karmalawy AA, Germoush MO, ALshamrani SM, Abdein MA, Awad NS. Nanobiotechnological Approaches to Enhance Potato Resistance against Potato Leafroll Virus (PLRV) Using Glycyrrhizic Acid Ammonium Salt and Salicylic Acid Nanoparticles. Horticulturae. 2021; 7(10):402. https://doi.org/10.3390/horticulturae7100402
Chicago/Turabian StyleShoala, Tahsin, Ahmed A. Al-Karmalawy, Mousa O. Germoush, Salha M. ALshamrani, Mohamed A. Abdein, and Nabil S. Awad. 2021. "Nanobiotechnological Approaches to Enhance Potato Resistance against Potato Leafroll Virus (PLRV) Using Glycyrrhizic Acid Ammonium Salt and Salicylic Acid Nanoparticles" Horticulturae 7, no. 10: 402. https://doi.org/10.3390/horticulturae7100402
APA StyleShoala, T., Al-Karmalawy, A. A., Germoush, M. O., ALshamrani, S. M., Abdein, M. A., & Awad, N. S. (2021). Nanobiotechnological Approaches to Enhance Potato Resistance against Potato Leafroll Virus (PLRV) Using Glycyrrhizic Acid Ammonium Salt and Salicylic Acid Nanoparticles. Horticulturae, 7(10), 402. https://doi.org/10.3390/horticulturae7100402








