Development of a Multipurpose Core Collection of New Promising Iranian Pomegranate (Punica granatum L.) Genotypes Based on Morphological and Pomological Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Agro-Morphological Evaluation
2.3. Establishment of the Multipurpose Core Collection
2.4. Statistical Analysis
3. Results
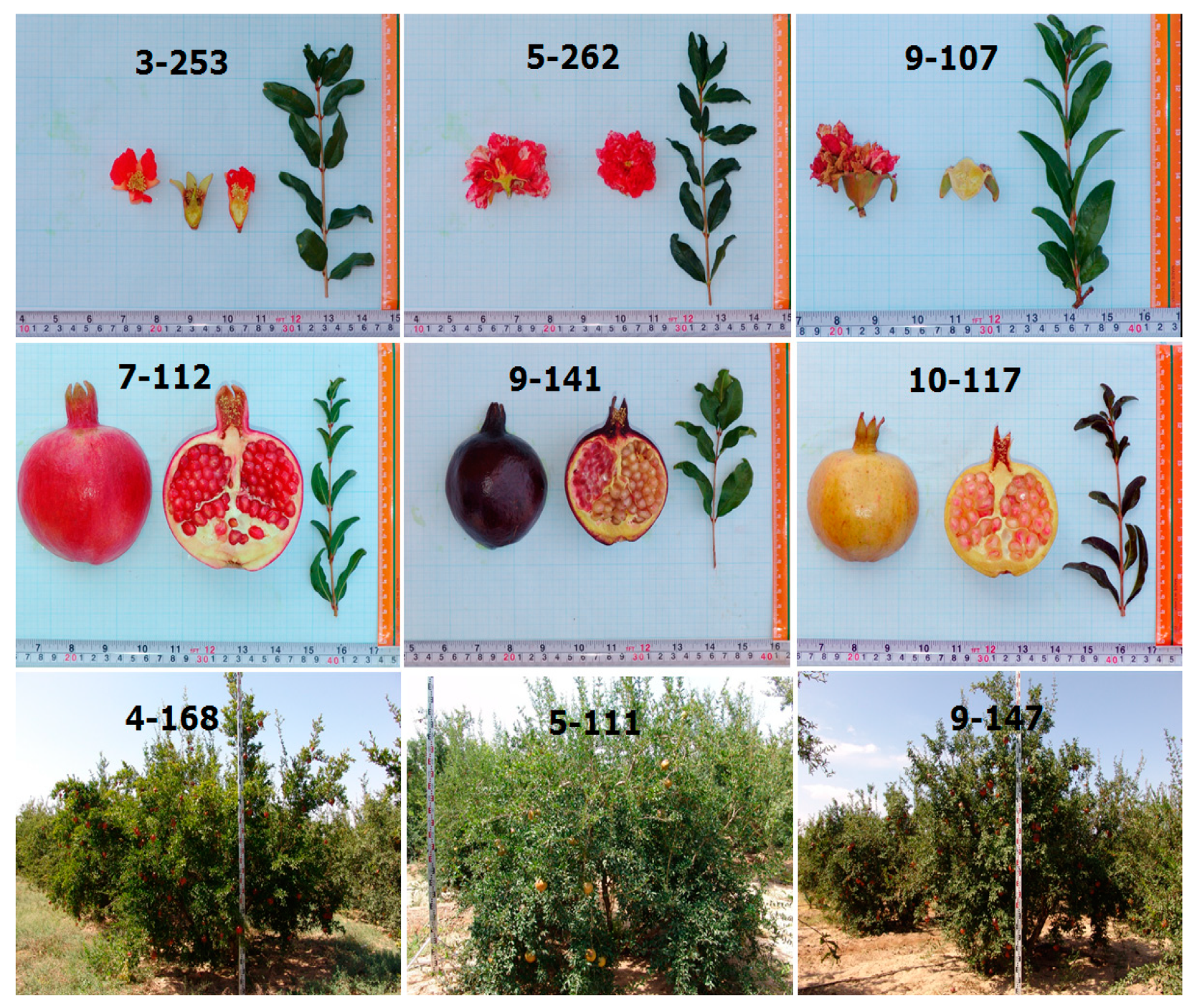
3.1. Description of Morphological and Pomological Traits
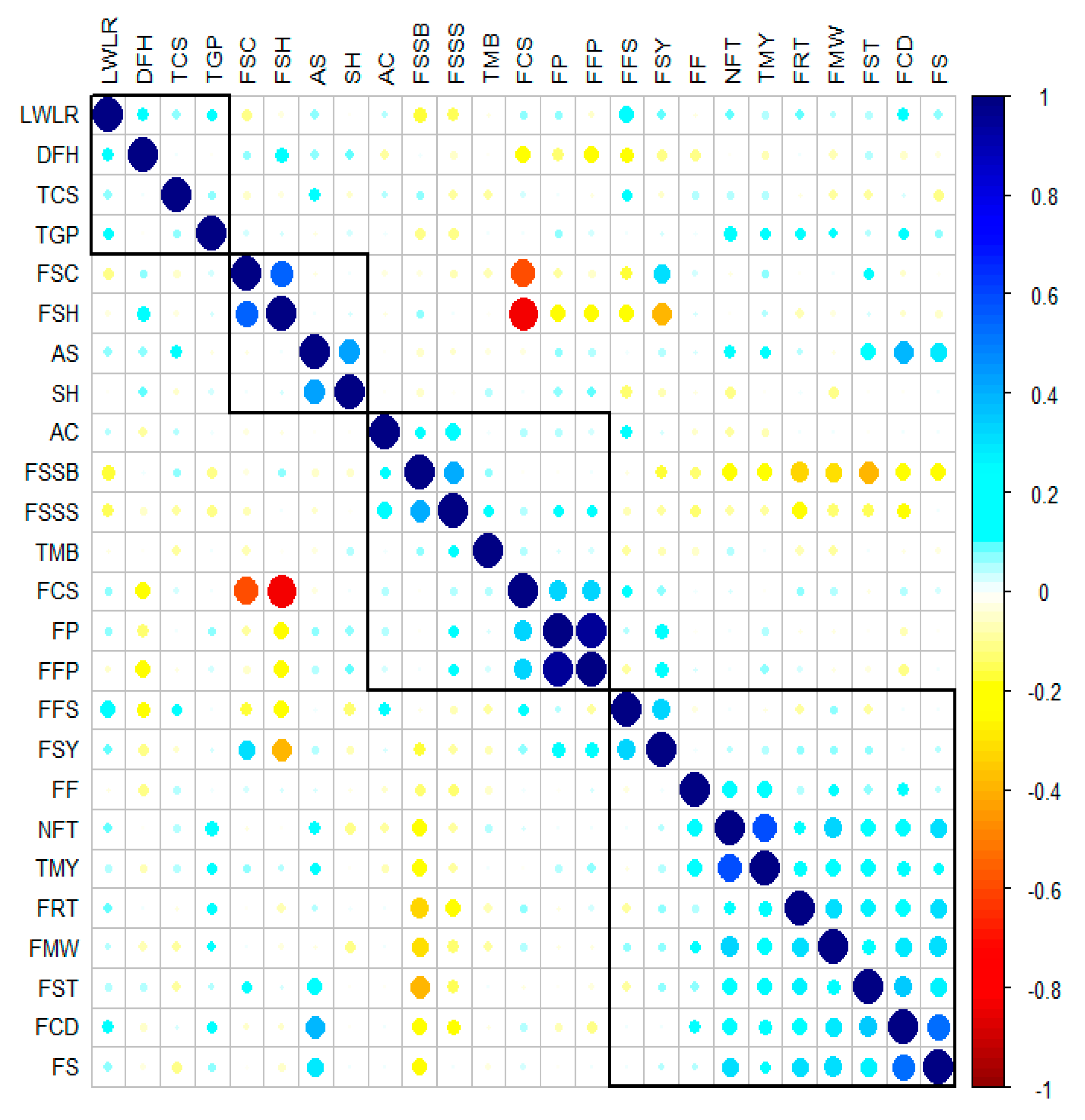
3.2. Correlations between Morphological and Pomological Traits
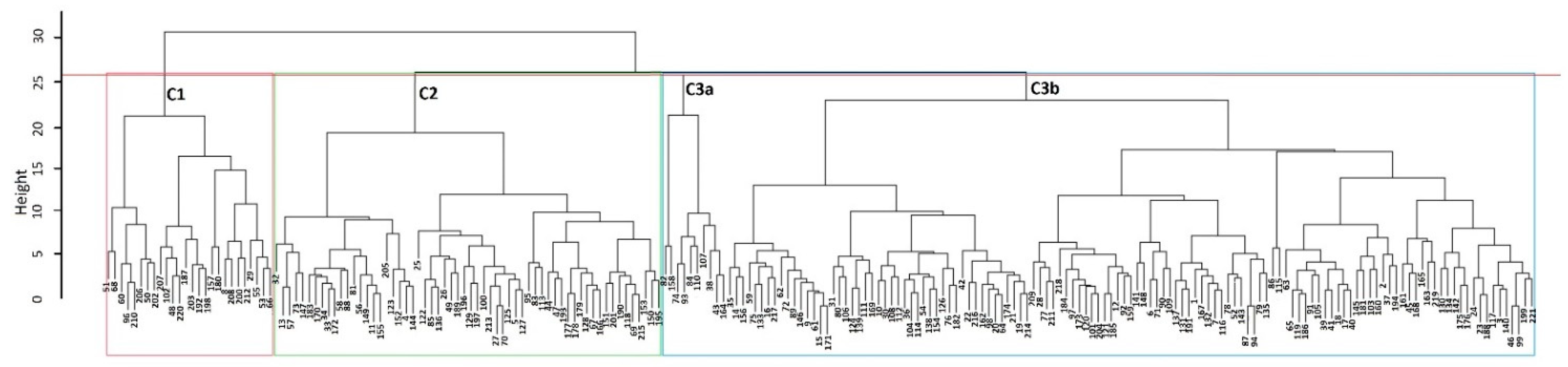
3.3. Multivariate Analysis
3.4. Construction of the Core Collection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Levin, G.M. Pomegranate (Punica granatum) plant genetic resources in Turkmenistan. Plant Genet. Resour. Newslett. 1994, 97, 31–36. [Google Scholar]
- Levin, G.M. Pomegranate Roads: A Soviet Botanist’s Exile from Eden, 1st ed.; Floreant Press: Forestville, CA, USA, 2006; pp. 15–183. [Google Scholar]
- Smartt, J. Evolution of Crop Plants. In Experimental Agriculture; Simmonds, N.W., Ed.; Cambridge University Press: London, UK, 1977; Volume 13, p. 350. [Google Scholar] [CrossRef]
- Verma, N.; Mohanty, A.; Lal, A. Pomegranate genetic resources and germplasm conservation: A review. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 120–125. [Google Scholar]
- Da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef]
- Zaouay, F.; Mena, P.; Garcia-Viguera, C.; Mars, M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind. Crops Prod. 2012, 40, 81–89. [Google Scholar] [CrossRef]
- Ahmadi, K.; Ebadzadeh, H.R.; Hatami, F.; Hoseinpoor, R.; Abdeshah, H. Horticultural Products. In Agricultural Statistics; Ministry of Agriculture, Information and Communication Technology Center: Tehran, Iran, 2020; Volume 3, pp. 13–19. (In Farsi) [Google Scholar]
- Jalikop, S.; Kumar, P.S. Use of a gene marker to study the mode of pollination in pomegranate (Punica granatum L.). Int. J. Hortic. Sci. 1990, 65, 221–223. [Google Scholar] [CrossRef]
- Behzadi Shahrbabaki, H. Distribution and Diversity of Pomegranate Cultivars in Iran; Agriculture Education Publication: Tehran, Iran, 1998; p. 280. (In Farsi) [Google Scholar]
- Zahravi, M.; Vazifeshenas, M.R. Study of genetic diversity in pomegranate germplasm of Yazd province of Iran. Iran. J. Genet. Plant Breed. 2017, 6, 20–35. [Google Scholar] [CrossRef]
- Nemati, Z.; Tehranifar, A.; Farsi, M.; Mirshamsi Kakhki, A.; Nemati, S. Assessment of genetic variation of Punica granatum L. genotypes from seven regions of Iran using AFLP markers. J. Hortic. Sci. 2012, 26, 263–270. [Google Scholar] [CrossRef]
- Caliskan, O.; Bayazit, S. Morpho-pomological and chemical diversity of pomegranate accessions grown in Eastern Mediterranean region of Turkey. J. Agric. Sci. Technol. 2013, 15, 1449–1460. [Google Scholar]
- Dandachi, F.; Hamadeh, B.; Youssef, H.; Chahine, H.; Chalak, L. Diversity assessment of the Lebanese germplasm of pomegranate (Punica granatum L.) by morphological and chemical traits. Ann. Agric. Sci. 2017, 62, 89–98. [Google Scholar] [CrossRef]
- Martínez-Nicolas, J.J.; Melgarejo, P.; Legua, P.; Garcia-Sanchez, F.; Hernández, F. Genetic diversity of pomegranate germplasm collection from Spain determined by fruit, seed, leaf and flower characteristics. PeerJ 2016, 4, e2214. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Yavari, A.M.; Zamani, Z. The Pomegranate: Botany, Production and Uses; CABI: Oxfordshire, UK, 2020. [Google Scholar]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Harel-Beja, R.; Bar-Ya’akov, I.; Hatib, K.; Trainin, T.; Ben-Simhon, Z.; Holland, D.; Eshed, R.; Sharabi, M.; Rubinstein, M.; Ophir, R.; et al. Pomegranate breeding: Utilization of molecular and genetic data for improvement of fruit quality and adaptation to different climatic conditions. Acta Hortic. 2015, 1089, 249–252. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Ren, Y.; Wang, Y.; Yuan, Z. Fruit Breeding in Regard to Color and Seed Hardness: A Genomic View from Pomegranate. Agronomy 2020, 10, 991. [Google Scholar] [CrossRef]
- Huan, Z.J.; Zhu, W.Y.; Yuan, S.H.; Li, Y.; Ning, N. Establishment of core collection from apricot germplasm in China. Afr. J. Biotechnol. 2013, 12, 5577–5587. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Chen, X.S.; Zhang, Y.M.; Yuan, Z.H.; Liu, Z.C.; Wang, Y.L.; Qun, L. Method of constructing core collection for Malus sieversii in Xinjiang, China using molecular markers. Agric. Sci. China 2009, 8, 276–284. [Google Scholar] [CrossRef]
- Gouesnard, B.; Bataillon, T.; Decoux, G.; Rozale, C.; Schoen, D.; David, J. MSTRAT: An algorithm for building germ plasm core collections by maximizing allelic or phenotypic richness. J. Hered. 2001, 92, 93–94. [Google Scholar] [CrossRef]
- Liu, W.; Shahid, M.Q.; Bai, L.; Lu, Z.; Chen, Y.; Jiang, L.; Diao, M.; Liu, X.; Lu, Y. Evaluation of genetic diversity and development of a core collection of wild rice (Oryza rufipogon Griff.) populations in China. PLoS ONE 2015, 10, e0145990. [Google Scholar] [CrossRef]
- Wang, J.C.; Jin, H.; Zhang, C.F.; Zhang, S. Assessment on evaluating parameters of rice core collections constructed by genotypic values and molecular marker information. Rice Sci. 2007, 14, 101–110. [Google Scholar] [CrossRef]
- Brown, A. Core collections: A practical approach to genetic resources management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Sun, H.; Ning, N.; Yang, L. Construction and evaluation of a primary core collection of apricot germplasm in China. Sci. Hortic. 2011, 128, 311–319. [Google Scholar] [CrossRef]
- El Bakkali, A.; Haouane, H.; Moukhli, A.; Costes, E.; Van Damme, P. Construction of Core Collections Suitable for Association Mapping to Optimize. PLoS ONE 2013, 8, e61265. [Google Scholar] [CrossRef]
- Lopez, G.; Pallas, B.; Martinez, S.; Lauri, P.E.; Regnard, J.L.; Durel, C.E.; Costes, E. Genetic variation of morphological traits and transpiration in an apple core collection under well-watered conditions: Towards the identification of morphotypes with high water use efficiency. PLoS ONE 2015, 10, e0145540. [Google Scholar] [CrossRef]
- Mahmoodi, R.; Dadpour, M.R.; Hassani, D.; Zeinalabedini, M.; Vendramin, E.; Micali, S.; Nahandi, F.Z. Development of a core collection in Iranian walnut (Juglans regia L.) germplasm using the phenotypic diversity. Sci. Hortic. 2019, 249, 439–448. [Google Scholar] [CrossRef]
- Pomegranate, UPOV Code: PUNIC_GRA, Punica granatum L. Available online: https://www.upov.int/portal/index.html.en (accessed on 19 June 2012).
- Kim, K.-W.; Chung, H.K.; Cho, G.T.; Ma, K.H.; Chandrabalan, D.; Gwag, J.G.; Kim, T.S.; Cho, E.G.; Park, Y.J. PowerCore: A program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics 2007, 23, 2155–2162. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. ACM SIGMOBILE Mob. Comput. Commun. Rev. 2001, 5, 3–55. [Google Scholar] [CrossRef]
- Tadesse, W.; Bekele, E. Factor analysis of components of yield in grasspea (Lathyrus sativus L.). Lathyrus Lathyrism Newsl. 2001, 2, 91–93. [Google Scholar] [CrossRef]
- Eticha, F.; Belay, G.; Bekele, E. Species diversity in wheat landrace populations from two regions of Ethiopia. Genet. Resour. Crop Evol. 2006, 53, 387–393. [Google Scholar] [CrossRef]
- Jamago, J.M.; Cortes, R.V. Seed diversity and utilization of the upland rice landraces and traditional varieties from selected areas in Bukidnon, Philippines. IAMURE Int. J. Glob. Ecol. Conserv. 2012, 4, 112. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Jie, Z.; Yi, W.; Xinzhong, Z.; Tianzhong, L.; Kun, W.; Xuefeng, X.; Zhenhai, H. Sampling strategy to develop a primary core collection of apple cultivars based on fruit traits. Afr. J. Biotechnol. 2010, 9, 123–127. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, T.H.; Zhang, H.L.; Qi, Y.W. Sampling strategy for a primary core collection of peach (Prunus persica (L.) Batsch.) germplasm. Eur. J. Hortic. Sci. 2007, 72, 268–274. [Google Scholar]
- Schoen, D.J.; Brown, A. Conservation of allelic richness in wild crop relatives is aided by assessment of genetic markers. Proc. Natl. Acad. Sci. USA 1993, 90, 10623–10627. [Google Scholar] [CrossRef]
- Hepaksoy, S.; Aksoy, U.; Can, H.Z.; UI, M.A. Determination of relationship between fruit cracking and some physiological responses, leaf characteristics and nutritional status of some pomegranate varieties. Options Méditerranéennes Ser. A 2000, 42, 87–92. [Google Scholar]
- Rodriguez, J.; Anoruo, A.; Jifon, J.; Simpson, C. Physiological effects of exogenously applied reflectants and anti-transpirants on leaf temperature and fruit sunburn in citrus. Plants 2019, 8, 549. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Bowen, J.; Ferguson, I.; Woolf, A.; McGhie, T. Sunburn on apples-Causes and control mechanisms. Acta Hortic. 2004, 636, 631–636. [Google Scholar] [CrossRef]
- Shakeri, M.; Sadat Akhavi, Y. Pests and Diseases of Pomegranate; Tasbih Publication: Yazd, Iran, 2003; pp. 91–92. (In Farsi) [Google Scholar]
- Wetzstein, H.Y.; Yi, W.; Porter, J.A.; Ravid, N. Flower position and size impact ovule number per flower, fruitset, and fruit size in pomegranate. J. Am. Soc. Hortic. Sci. 2013, 138, 159–166. [Google Scholar] [CrossRef]
- Khadivi-Khub, A.; Kameli, M.; Moshfeghi, N.; Ebrahimi, A. Phenotypic characterization and relatedness among some Iranian pomegranate (Punica granatum L.) accessions. Trees 2015, 29, 893–901. [Google Scholar] [CrossRef]
- Khadivi, A.; Mirheidari, F.; Moradi, Y.; Paryan, S. Morphological variability of wild pomegranate (Punica granatum L.) accessions from natural habitats in the Northern parts of Iran. Sci. Hortic. 2020, 264, 109165. [Google Scholar] [CrossRef]
- Yuan, Z.; Yin, Y.; Qu, J.; Zhu, L.; Li, Y. Population genetic diversity in Chinese pomegranate (Punica granatum L.) cultivars revealed by fluorescent-AFLP markers. J. Genet. Genom. 2007, 34, 1061–1071. [Google Scholar] [CrossRef]
- Jbir, R.; Hasnaoui, N.; Mars, M.; Marrakchi, M.; Trifi, M. Characterization of Tunisian pomegranate (Punica granatum L.) cultivars using amplified fragment length polymorphism analysis. Sci. Hortic. 2008, 115, 231–237. [Google Scholar] [CrossRef]
- Narzary, D.; Mahar, K.S.; Rana, T.; Ranade, S. Analysis of genetic diversity among wild pomegranates in Western Himalayas, using PCR methods. Sci. Hortic. 2009, 121, 237–242. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Buonamici, A.; Sebastiani, F.; Mars, M.; Zhang, D.; Vendramin, G.G. Molecular genetic diversity of Punica granatum L. (pomegranate) as revealed by microsatellite DNA markers (SSR). Gene 2012, 493, 105–112. [Google Scholar] [CrossRef]
- Vazifeshenas, M.R. Identification and Introduction of Some Pomegranate Cultivars Resistant to Early Autumn and Late Spring Cold; Agricultural and Natural Resources Research Center of Yazd Province Publication: Yazd, Iran, 2009; p. 158. (In Farsi) [Google Scholar]
- Hu, J.; Zhu, J.; Xu, H. Methods of constructing core collections by stepwise clustering with three sampling strategies based on the genotypic values of crops. Theor. Appl. Genet. 2000, 101, 264–268. [Google Scholar] [CrossRef]
- Yanfang, Z.; Dechang, H.; Jincheng, Z.; Ping, Z.; Zhaohong, W.; Chuanjie, C. Development of a mulberry core collection originated in China to enhance germplasm conservation. Crop Breed. Appl. Biotechnol. 2019, 19, 55–61. [Google Scholar] [CrossRef]
- Belaj, A.; Dominguez-García, M.D.C.; Atienza, S.G.; Urdiroz, N.M.; De la Rosa, R.; Satovic, Z.; Martin, A.; Kilian, A.; Trujillo, I.; Valpuesta, V.; et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, Y.; Lee, G.A.; Kwon, Y.S.; Kim, S.A.; Kwon, S.I.; Do, Y.S.; Choi, C. Genetic Diversity, Structure, and Core Collection of Korean Apple Germplasm Using Simple Sequence Repeat Markers. Hortic. J. 2019, 88, 329–337. [Google Scholar] [CrossRef]
- Orhan, E.; Ercisli, S.; Esitken, A.; Sengul, M. Molecular and morphological characterization of pomegranate (Punica granatum L.) genotypes sampled from Coruh Valley in Turkey. Genet. Mol. Res. 2014, 13, 6375–6382. [Google Scholar] [CrossRef]




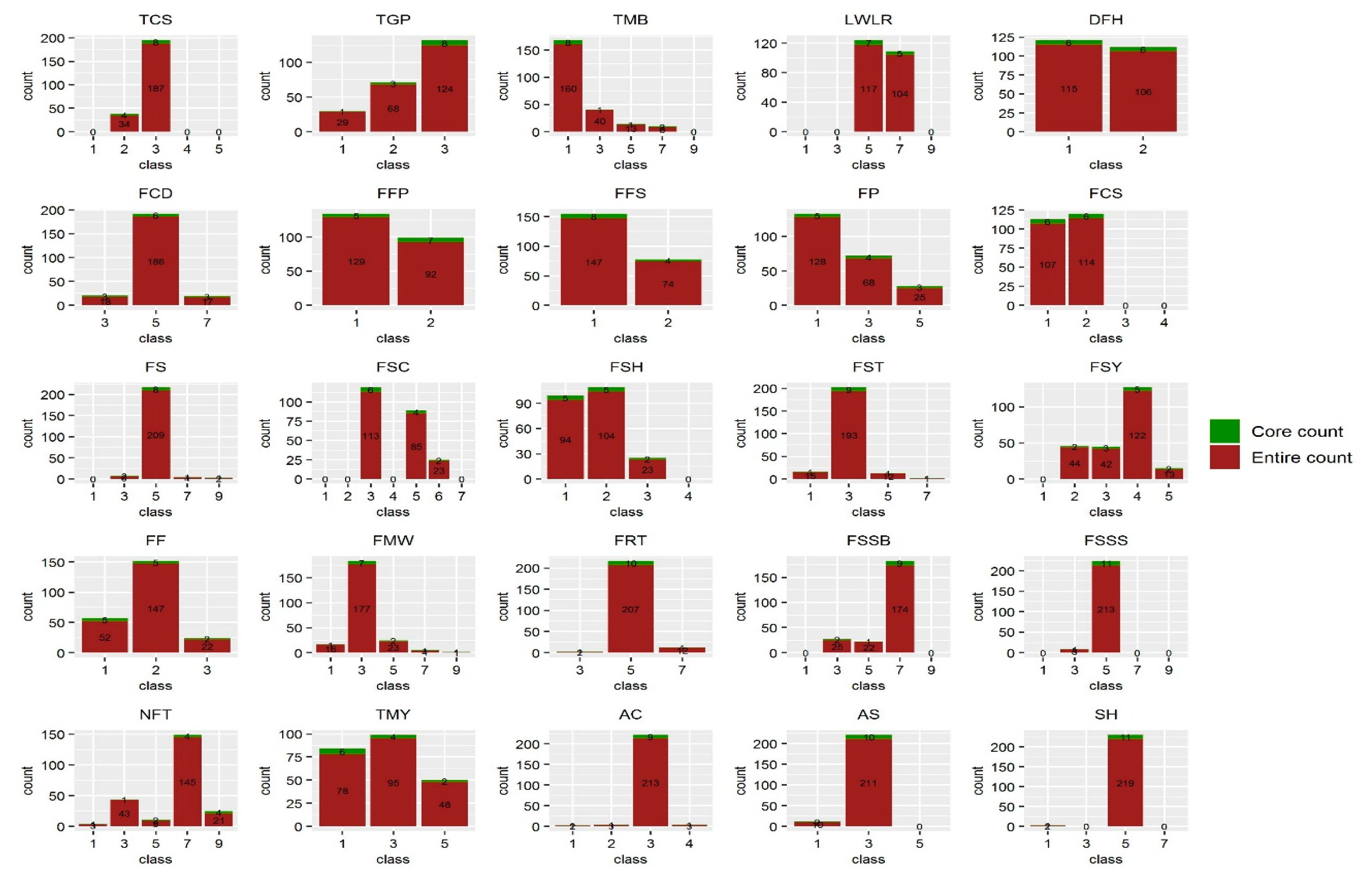
| Trait | Core Collection | Entire Collection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mode | Median | Heterozygosity | Min | Max | Mode | Median | Heterozygosity | |
| TCS | 2 | 3 | 3 | 3 | 0.64 | 2 | 3 | 3 | 3 | 0.43 |
| TGP | 1 | 3 | 3 | 3 | 0.82 | 1 | 3 | 3 | 3 | 0.95 |
| TMB | 1 | 7 | 1 | 1 | 0.98 | 1 | 7 | 1 | 1 | 0.83 |
| LWLR | 5 | 7 | 5 | 5 | 0.68 | 5 | 7 | 5 | 5 | 0.69 |
| FP | 1 | 5 | 1 | 3 | 1.07 | 1 | 5 | 1 | 1 | 0.92 |
| FFS | 1 | 2 | 1 | 1 | 0.64 | 1 | 2 | 1 | 1 | 0.64 |
| DFH | 1 | 2 | 1 | 1.5 | 0.69 | 1 | 2 | 1 | 1 | 0.69 |
| FFP | 1 | 2 | 2 | 2 | 0.68 | 1 | 2 | 1 | 1 | 0.68 |
| FCD | 3 | 7 | 5 | 5 | 1.03 | 3 | 7 | 5 | 5 | 0.55 |
| FS | 3 | 9 | 5 | 5 | 0.98 | 3 | 9 | 5 | 5 | 0.26 |
| FST | 1 | 7 | 3 | 3 | 0.84 | 1 | 7 | 3 | 3 | 0.48 |
| FSSB | 3 | 7 | 7 | 7 | 0.72 | 3 | 7 | 7 | 7 | 0.66 |
| FSSS | 3 | 5 | 5 | 5 | 0.29 | 3 | 5 | 5 | 5 | 0.15 |
| FF | 1 | 3 | 1 | 2 | 1.02 | 1 | 3 | 2 | 2 | 0.84 |
| FRT | 3 | 7 | 5 | 5 | 0.57 | 3 | 7 | 5 | 5 | 0.26 |
| FSC | 3 | 6 | 3 | 4 | 1.01 | 3 | 6 | 3 | 3 | 0.94 |
| FSH | 1 | 3 | 1 | 2 | 1.02 | 1 | 3 | 2 | 2 | 0.95 |
| FCS | 1 | 2 | 2 | 1.5 | 0.69 | 1 | 2 | 2 | 2 | 0.69 |
| FSY | 2 | 5 | 4 | 4 | 1.31 | 2 | 5 | 4 | 4 | 1.13 |
| AS | 1 | 3 | 3 | 3 | 0.45 | 1 | 3 | 3 | 3 | 0.18 |
| AC | 1 | 4 | 3 | 3 | 0.84 | 1 | 4 | 3 | 3 | 0.19 |
| SH | 1 | 5 | 5 | 5 | 0.29 | 1 | 5 | 5 | 5 | 0.05 |
| NFT | 1 | 9 | 9 | 7 | 1.44 | 1 | 9 | 7 | 7 | 1 |
| FMW | 1 | 9 | 3 | 3 | 1.23 | 1 | 9 | 3 | 3 | 0.7 |
| TMY | 1 | 5 | 1 | 2 | 1.01 | 1 | 5 | 3 | 3 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razi, S.; Soleimani, A.; Zeinalabedini, M.; Vazifeshenas, M.R.; Martínez-Gómez, P.; Mohsenzade Kermani, A.; Raiszadeh, A.R.; Tayari, M.; Martínez-García, P.J. Development of a Multipurpose Core Collection of New Promising Iranian Pomegranate (Punica granatum L.) Genotypes Based on Morphological and Pomological Traits. Horticulturae 2021, 7, 350. https://doi.org/10.3390/horticulturae7100350
Razi S, Soleimani A, Zeinalabedini M, Vazifeshenas MR, Martínez-Gómez P, Mohsenzade Kermani A, Raiszadeh AR, Tayari M, Martínez-García PJ. Development of a Multipurpose Core Collection of New Promising Iranian Pomegranate (Punica granatum L.) Genotypes Based on Morphological and Pomological Traits. Horticulturae. 2021; 7(10):350. https://doi.org/10.3390/horticulturae7100350
Chicago/Turabian StyleRazi, Sara, Ali Soleimani, Mehrshad Zeinalabedini, Mohammad Reza Vazifeshenas, Pedro Martínez-Gómez, Asghar Mohsenzade Kermani, Ahmad Reza Raiszadeh, Mostafa Tayari, and Pedro José Martínez-García. 2021. "Development of a Multipurpose Core Collection of New Promising Iranian Pomegranate (Punica granatum L.) Genotypes Based on Morphological and Pomological Traits" Horticulturae 7, no. 10: 350. https://doi.org/10.3390/horticulturae7100350
APA StyleRazi, S., Soleimani, A., Zeinalabedini, M., Vazifeshenas, M. R., Martínez-Gómez, P., Mohsenzade Kermani, A., Raiszadeh, A. R., Tayari, M., & Martínez-García, P. J. (2021). Development of a Multipurpose Core Collection of New Promising Iranian Pomegranate (Punica granatum L.) Genotypes Based on Morphological and Pomological Traits. Horticulturae, 7(10), 350. https://doi.org/10.3390/horticulturae7100350







