Pilot Study of Eco-Physiological Pepper Responses to Starfish-Based Organic Soil Amendments in Open-Field and Greenhouse Cultivations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Layout
2.2. Treatment in Pre-Experiment
2.3. Treatment in the Main Experiment
2.4. Nutrient Analysis in the Main Experiment
2.5. Bacterial Analysis in the Main Experiment
2.6. Pepper Measurement in the Main Experiment
2.7. Data Analysis
3. Results and Discussion
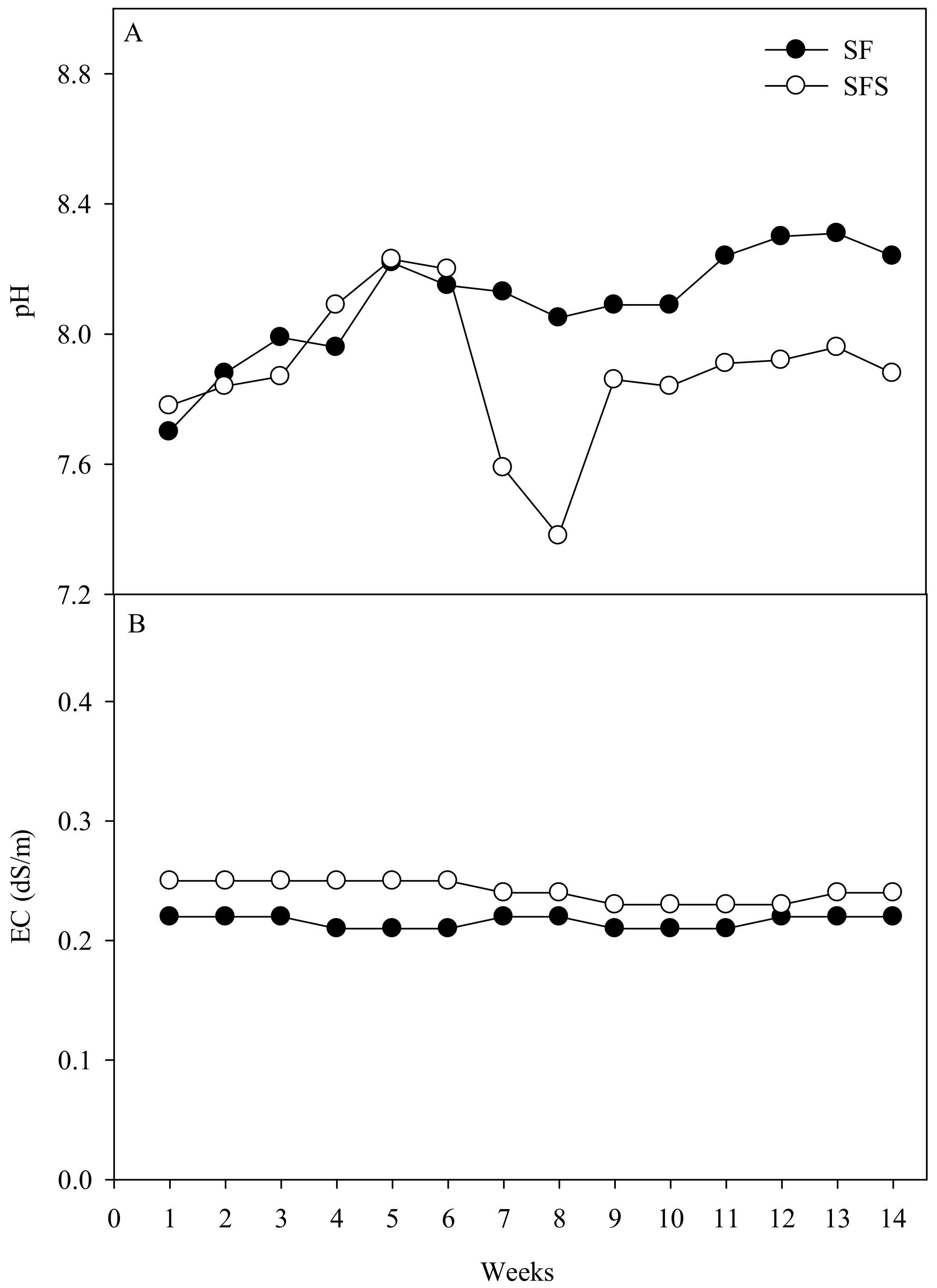
3.1. Pre-Experiment
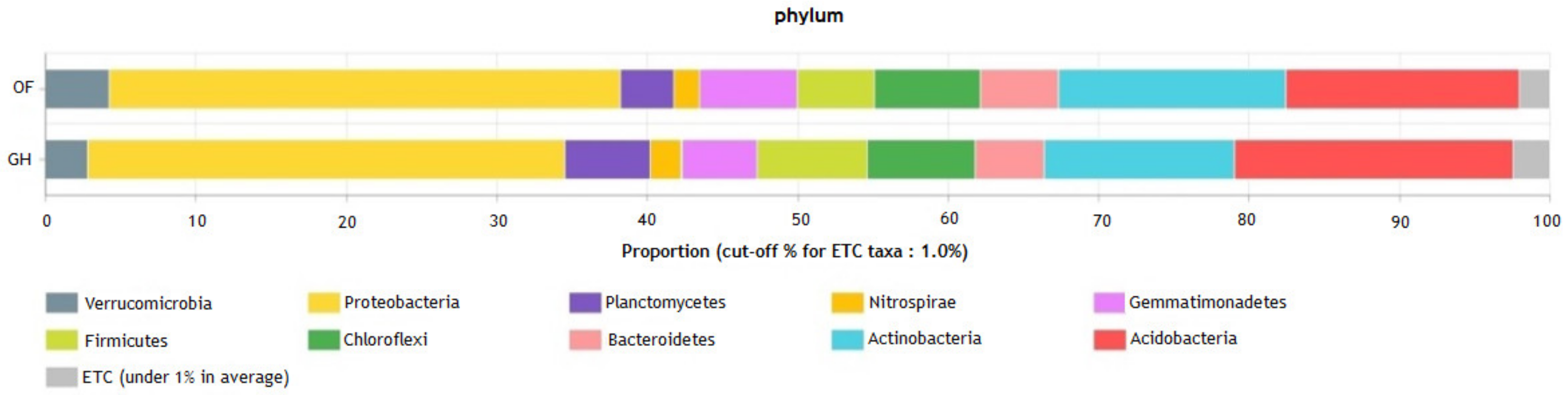
3.2. Nutrients and Bacterial Community in the Main Experiment
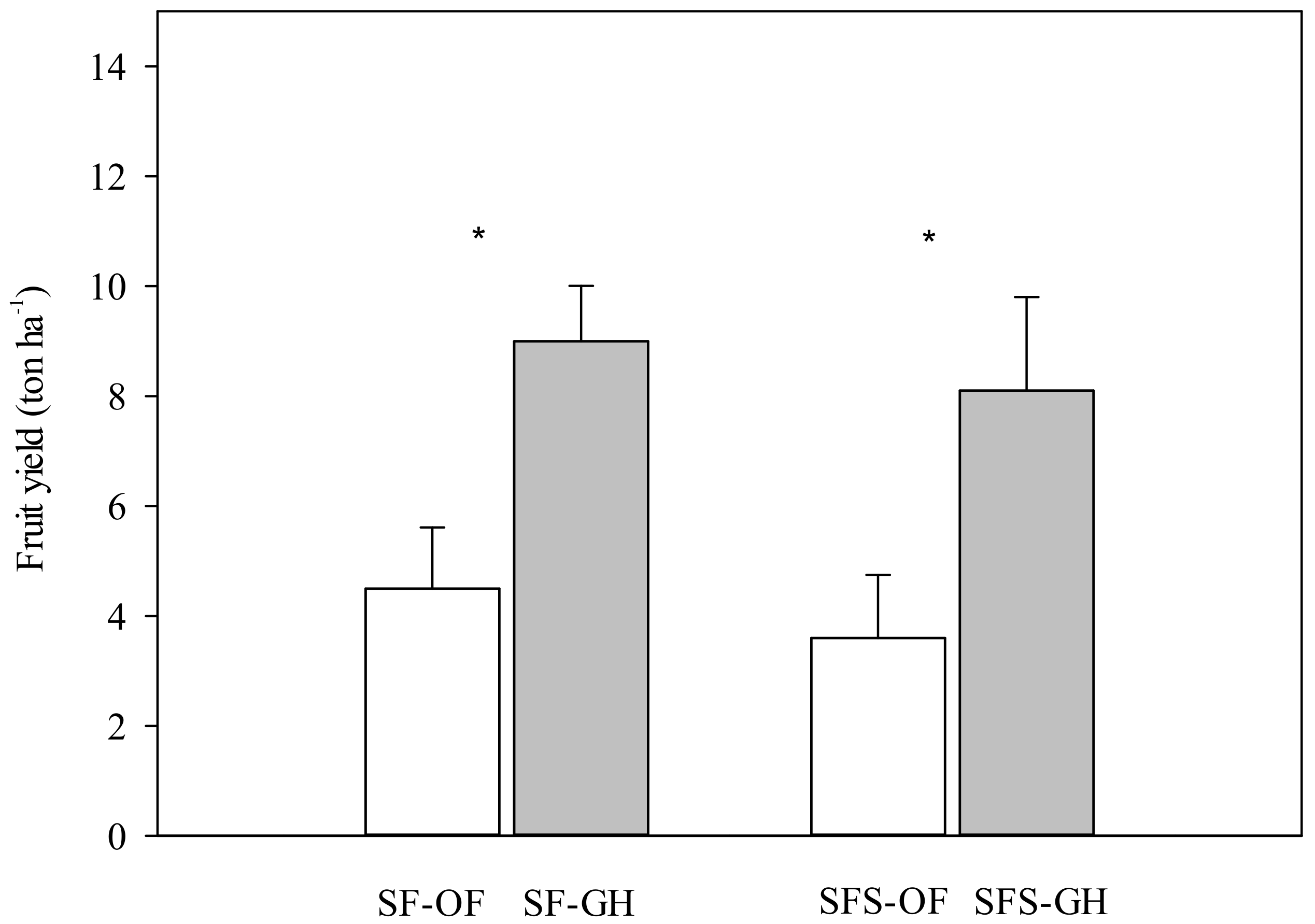
3.3. Plant Growth in Main Experiment
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- RDA. Pepper; Rural Development Administration: Jeonju, Korea, 2017. [Google Scholar]
- Luttikholt, L.W.M. Principles of organic agriculture as formulated by the International Federation of Organic Agriculture Movements. Wagen. J. Life Sci. 2007, 54, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Badgley, C.; Moghtader, J.; Quintero, E.; Zakem, E.; Chappell, M.J.; Avilés-Vázquez, K.; Samulon, A.; Perfecto, I. Organic agriculture and the global food supply. Renew. Agric. Food. Syst. 2007, 22, 86–108. [Google Scholar] [CrossRef]
- Aliyu, L. Effect of organic and mineral fertilizers on growth, yield and composition of pepper (Capsicum annuum L.). Biol. Agric. Hortic. 2000, 18, 29–36. [Google Scholar] [CrossRef]
- Parr, J.F.; Papendick, R.I.; Colacicco, D. Recycling of organic wastes for a sustainable agriculture. Biol. Agric. Hortic. 1986, 3, 115–130. [Google Scholar] [CrossRef]
- Rosen, C.J.; Allan, D.L. Exploring the benefits of organic nutrient sources for crop production and quality. Horttechnology 2006, 17, 422–430. [Google Scholar] [CrossRef]
- An, N.H.; Cho, Y.S.; Cho, J.R.; Kim, Y.K.; Lee, Y.; Jee, H.J.; Lee, S.M.; Park, K.L.; Lee, B.M. The survey of actual using conditions of farm-made liquid fertilizers for cultivating environment-friendly agricultural products. Korean J. Org. Agric. 2012, 20, 345–356. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Kim, J.M.; Roh, A.S.; Choi, S.C.; Kim, E.J.; Choi, M.T.; Ahn, B.K.; Kim, S.K.; Lee, Y.H.; Joh, J.H.; Kang, S.S.; et al. Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J. Microbiol. 2016, 54, 838–845. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Metzger, J.D.; Lucht, C. Effects of vermicomposts produced from cattle manure, food waste and paper waste on the growth and yield of peppers in the field. Pedobiologia 2005, 49, 297–306. [Google Scholar] [CrossRef]
- Nair, A.; Ngouajio, M. Soil microbial biomass, functional microbial diversity, and nematode community structure as affected by cover crops and compost in an organic vegetable production system. Appl. Soil Ecol. 2012, 58, 45–55. [Google Scholar] [CrossRef]
- Nkansah, G.O.; Norman, J.C.; Martey, A. Growth, yield and consumer acceptance of sweet pepper (Capsicum annuum L.) as influenced by open field and greenhouse production systems. J. Hortic. 2017, 4, 216. [Google Scholar]
- KMA. Statistical Analysis of Climate; Korea Meteorological Administration: Seoul, Korea, 2019. [Google Scholar]
- RDA. Comprehensive Test Room Analysis Manual; Rural Development Administration Publishers: Jeonju, Korea, 2017. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inbar, Y.; Chen, Y.; Hadar, Y. Humic substances formed during the composting of organic matter. Soil Sci. Soc. Am. J. 1990, 54, 1316–1323. [Google Scholar] [CrossRef]
- Kuk, Y.I.; Yun, Y.B.; Jang, S.J.; Jeong, J.Y.; Kim, D.S.; Kim, S.S. Evaluation of tomato growth-promoting effect and mineral nutrient of farm-made liquid fertilizers. Korean J. Org. Agric. 2019, 27, 205–224. [Google Scholar]
- Verkleij, F.N. Seaweed extracts in agriculture and horticulture: A review. Biol. Agric. Hortic. 1992, 8, 309–324. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Hou, J.; Li, M.; Mao, X.; Hao, Y.; Ding, J.; Liu, D.; Xi, B.; Liu, H. Response of microbial community of organic-matter-impoverished arable soil to long-term application of soil conditioner derived from dynamic rapid fermentation of food waste. PLoS ONE 2017, 12, e0175715. [Google Scholar] [CrossRef]
- RDA. Criteria of Fertilizer Application in Crops; Rural Development Administration, Sammi Press: Wanju, Korea, 2011. [Google Scholar]
- Han, G.; Lan, J.; Chen, Q.; Yu, C.; Bie, S. Response of soil microbial community to application of biochar in cotton soils with different continuous cropping years. Sci. Rep. 2017, 7, 101184. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S. Effects of organic liquid fertilizers on biological activities and fruit productivity in open-field cherry tomato. Bragantia 2020, 79, 447–457. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils; Person Publishers: Columbus, OH, USA, 2016. [Google Scholar]
- El-Jaoual, T.; Cox, D.A. Manganese toxicity in plants. J. Plant Nutr. 1998, 21, 353–386. [Google Scholar] [CrossRef]
- León, J.J.; Elías, J.L.; López, M.A.H.; López, A.M.G.; Ortiz, R.S.; García, L.F.E. Postharvest quality and shelf life of green pepper (Capsicum annuum L.) grown under open-field and greenhouse conditions. Idesia 2013, 31, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.C.; Lee, K.H.; Suh, W.M. Water requirement of twisted sweet peppers in greenhouse. J. Korean Soc. Agric. Eng. 2000, 42, 59–66. [Google Scholar]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
| Treatment | T-N | P | K | Ca | Mg | Fe | Mn | Zn | Cu | B | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | |||||||||||
| SF | 20 | 70 | 600 | 250 | 30 | 3.9 | 0.08 | 0.77 | 0.35 | 8.0 | 0.002 |
| SFS | 20 | 70 | 610 | 380 | 30 | 2.6 | 0.06 | 0.49 | 0.35 | 7.4 | 568 |
| Significance | ns | ns | ns | * | ns | * | * | * | ns | * | *** |
| Treatment | Shoot Length | Root Length | Total Length | Stem Diameter | Shoot (S) DW | Root (R) DW | S:R Ratio | SPAD | No. Fruit |
|---|---|---|---|---|---|---|---|---|---|
| (cm) | (g) | ||||||||
| SF | 79.8 | 24.8 | 105 | 0.6 | 6.6 | 1.2 | 5.5 | 33.2 | 2.0 |
| SFS | 88.5 | 24.0 | 113 | 0.6 | 8.1 | 1.2 | 6.6 | 34.8 | 1.8 |
| Significance | * | ns | * | ns | * | ns | ns | ns | ns |
| Treatment | pH | EC (dS m−1) | OM | T-N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | (cmolc kg−1) | |||||||
| SF-OF | 7.3 | 0.9 | 14.1 | 8000 | 108 | 117 | 680 | 156 |
| SF-GH | 7.2 | 2.1 | 27.1 | 1600 | 768 | 704 | 1160 | 288 |
| Significance | ns | ns | * | * | * | * | ns | ns |
| SFS-OF | 7.4 | 1.1 | 19.5 | 1100 | 545 | 782 | 2540 | 276 |
| SFS-GH | 7.1 | 3.7 | 41.9 | 2400 | 1301 | 1369 | 2400 | 696 |
| Significance | ns | * | * | * | * | * | ns | * |
| Fertilizer significance | ns | ns | ns | ns | * | ns | *** | ns |
| Desired level | 6.0–7.0 | 0.5–1.5 | 20.0–30.0 | − | 400–500 | 274–313 | 1000–1200 | 180–240 |
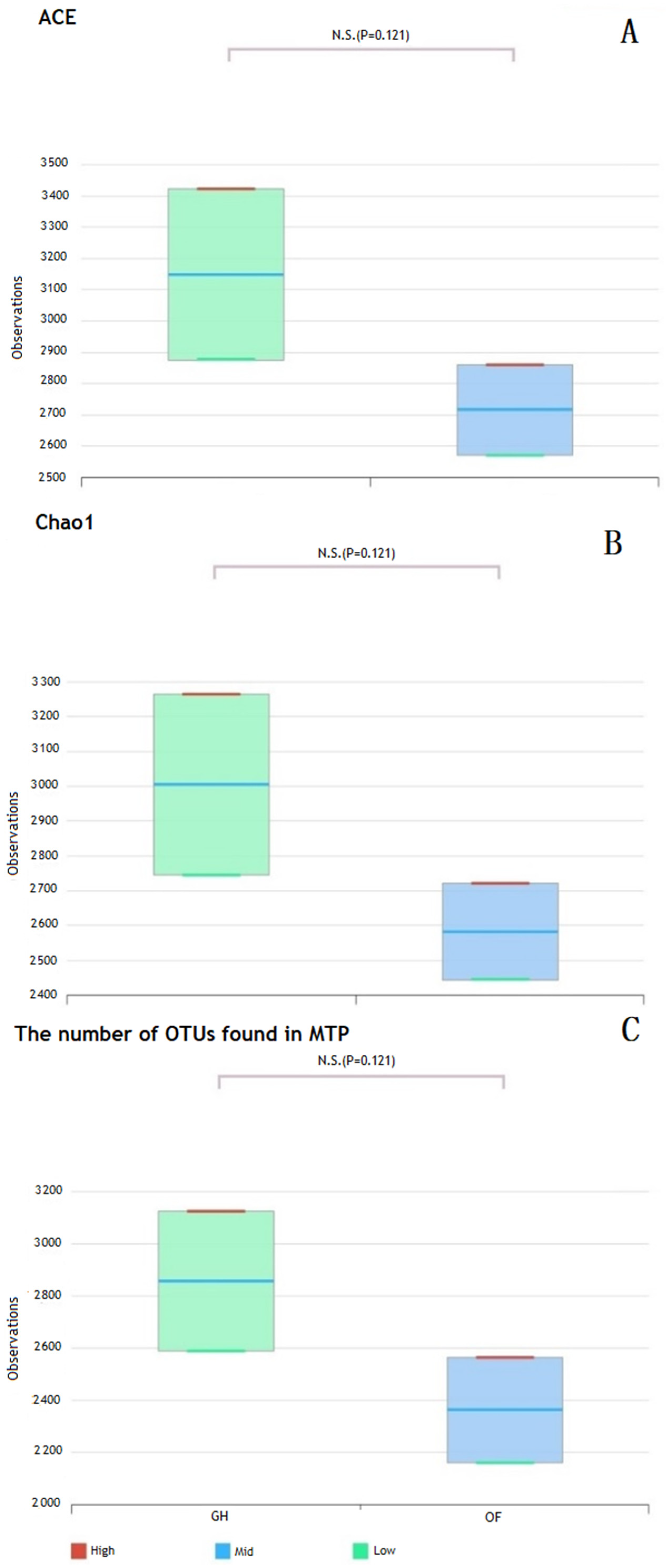
| LF | Number of OTUs | Good’s Coverage | Richness Estimator (Chao1) | Diversity Index | |
|---|---|---|---|---|---|
| Shannon | Inverse Simpson | ||||
| SF-OF | 2132 | 95.7 | 2445 | 6.4 | 0.012 |
| SF-GH | 3126 | 98.0 | 3265 | 6.9 | 0.003 |
| Significance | * | * | * | * | * |
| SFS-OF | 2564 | 97.6 | 2722 | 6.8 | 0.004 |
| SFS-GH | 2589 | 97.1 | 2746 | 6.9 | 0.003 |
| Significance | ns | ns | ns | ns | ns |
| Fertilizer significance | ns | ns | ns | ns | ns |
| Treatment | T-N | P | K | Ca | Mg | Fe | Mn | Zn | Cu | B |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | ||||||||||
| SF-OF | 43,000 | 500 | 50,000 | 31,000 | 6000 | 201 | 1948 | 225 | 33 | 186 |
| SF-GH | 43,000 | 400 | 50,000 | 34,000 | 9000 | 223 | 2108 | 62 | 21 | 125 |
| Significance | ns | ns | ns | ns | * | ns | ns | * | * | * |
| SFS-OF | 47,000 | 400 | 60,000 | 34,000 | 6000 | 147 | 1523 | 56 | 17 | 274 |
| SFS-GH | 47,000 | 300 | 55,000 | 37,000 | 11,000 | 224 | 2446 | 47 | 9 | 252 |
| Significance | ns | ns | ns | ns | * | * | * | ns | * | ns |
| Fertilizer significance | *** | ns | ** | * | ns | ns | ns | ns | ns | ** |
| Desired level | 21,000–54,000 | 300–400 | 44,000–56,000 | 11,000–27,000 | 3000–9000 | 55–174 | 16–53 | 21–74 | − | 17–68 |
| Treatment | Leaf (cm) | Canopy Width | Stem Diameter | Plant Height | |
|---|---|---|---|---|---|
| Width | Length | ||||
| (cm) | |||||
| SF-OF | 4.8 | 9.4 | 80.8 | 2.2 | 120 |
| SF-GH | 5.5 | 11.8 | 70.0 | 1.7 | 160 |
| Significance | * | * | * | * | * |
| SFS-OF | 4.5 | 9.5 | 85.8 | 2.3 | 140 |
| SFS-GH | 5.3 | 11.3 | 71.3 | 1.7 | 158 |
| Significance | * | * | * | * | * |
| Fertilizer significance | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-S. Pilot Study of Eco-Physiological Pepper Responses to Starfish-Based Organic Soil Amendments in Open-Field and Greenhouse Cultivations. Horticulturae 2021, 7, 344. https://doi.org/10.3390/horticulturae7100344
Choi H-S. Pilot Study of Eco-Physiological Pepper Responses to Starfish-Based Organic Soil Amendments in Open-Field and Greenhouse Cultivations. Horticulturae. 2021; 7(10):344. https://doi.org/10.3390/horticulturae7100344
Chicago/Turabian StyleChoi, Hyun-Sug. 2021. "Pilot Study of Eco-Physiological Pepper Responses to Starfish-Based Organic Soil Amendments in Open-Field and Greenhouse Cultivations" Horticulturae 7, no. 10: 344. https://doi.org/10.3390/horticulturae7100344
APA StyleChoi, H.-S. (2021). Pilot Study of Eco-Physiological Pepper Responses to Starfish-Based Organic Soil Amendments in Open-Field and Greenhouse Cultivations. Horticulturae, 7(10), 344. https://doi.org/10.3390/horticulturae7100344





