Detection of Above-Ground Physiological Indices of an Apple Rootstock Superior Line 12-2 with Improved Apple Replant Disease (ARD) Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatments
2.2. Determining Plant Height and Stem Thickness
2.3. Determining Relative Chlorophyll Content
2.4. Determining the Leaf Photosynthetic Parameters
2.5. Determining the Leaf Fluorescence Parameters
2.6. Determining Leaf Antioxidant Enzyme Activities and Malondialdehyde (MDA) Content
2.7. Determining Mineral Nutrient Element Contents in Leaves
2.8. Data Analysis
3. Results
3.1. Plant Height and Stem Thickness Analysis
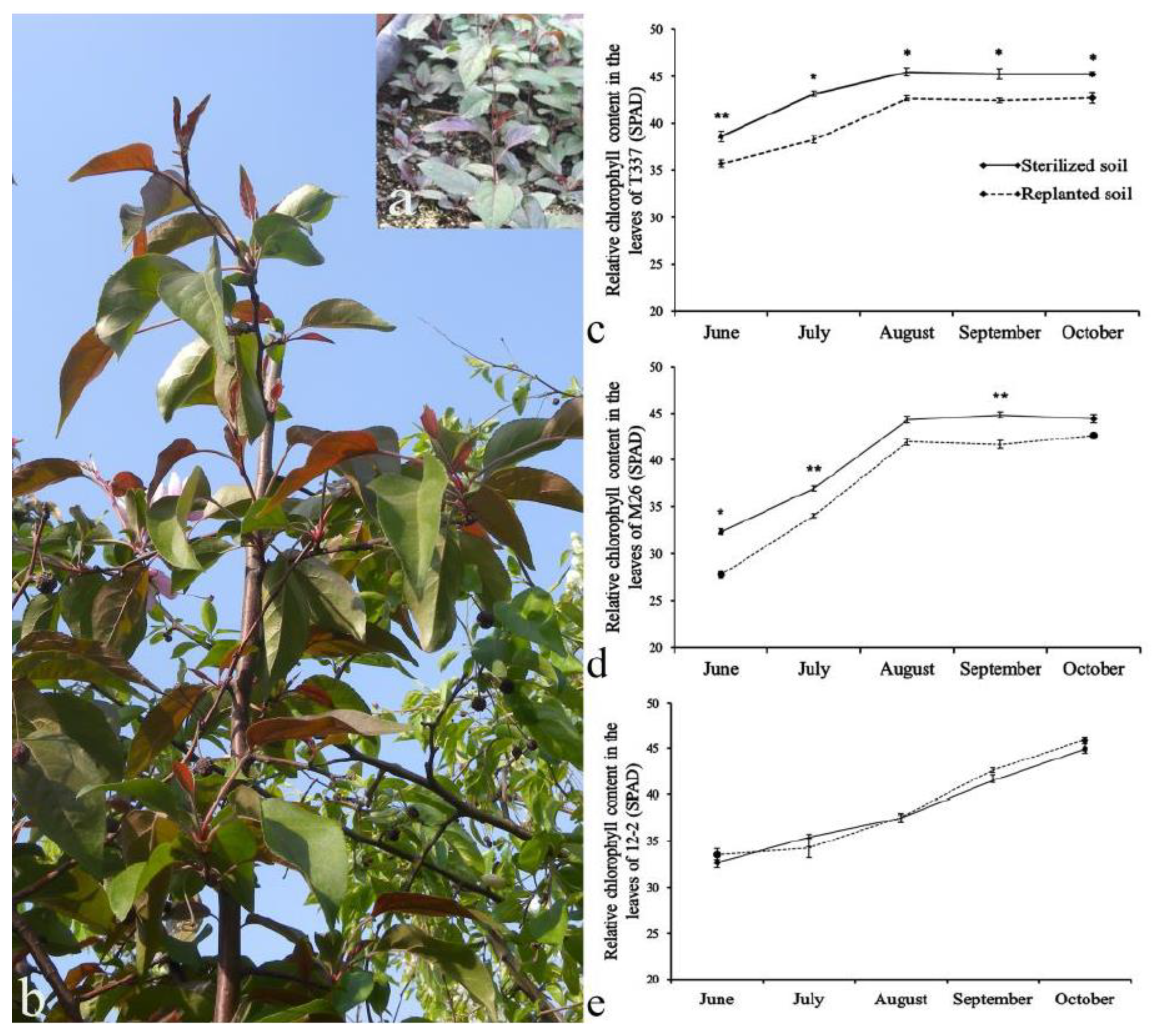
3.2. Analysis of Relative Chlorophyll Content in Plant Leaves
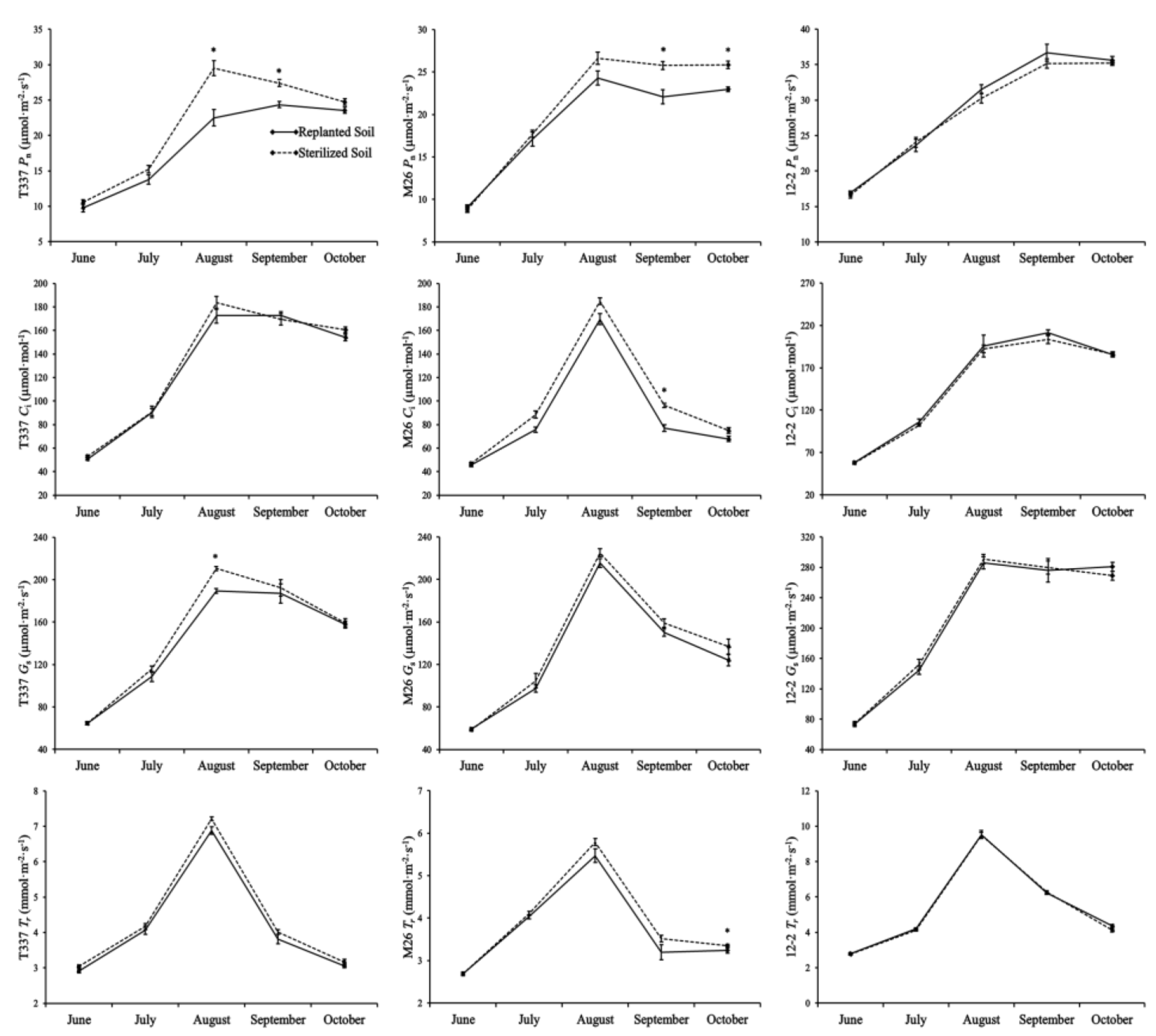
3.3. Analysis of Plant Leaf Photosynthetic Parameters
3.4. Analysis of Fluorescence Parameters and Fluorescence Imaging of Plant Leaves
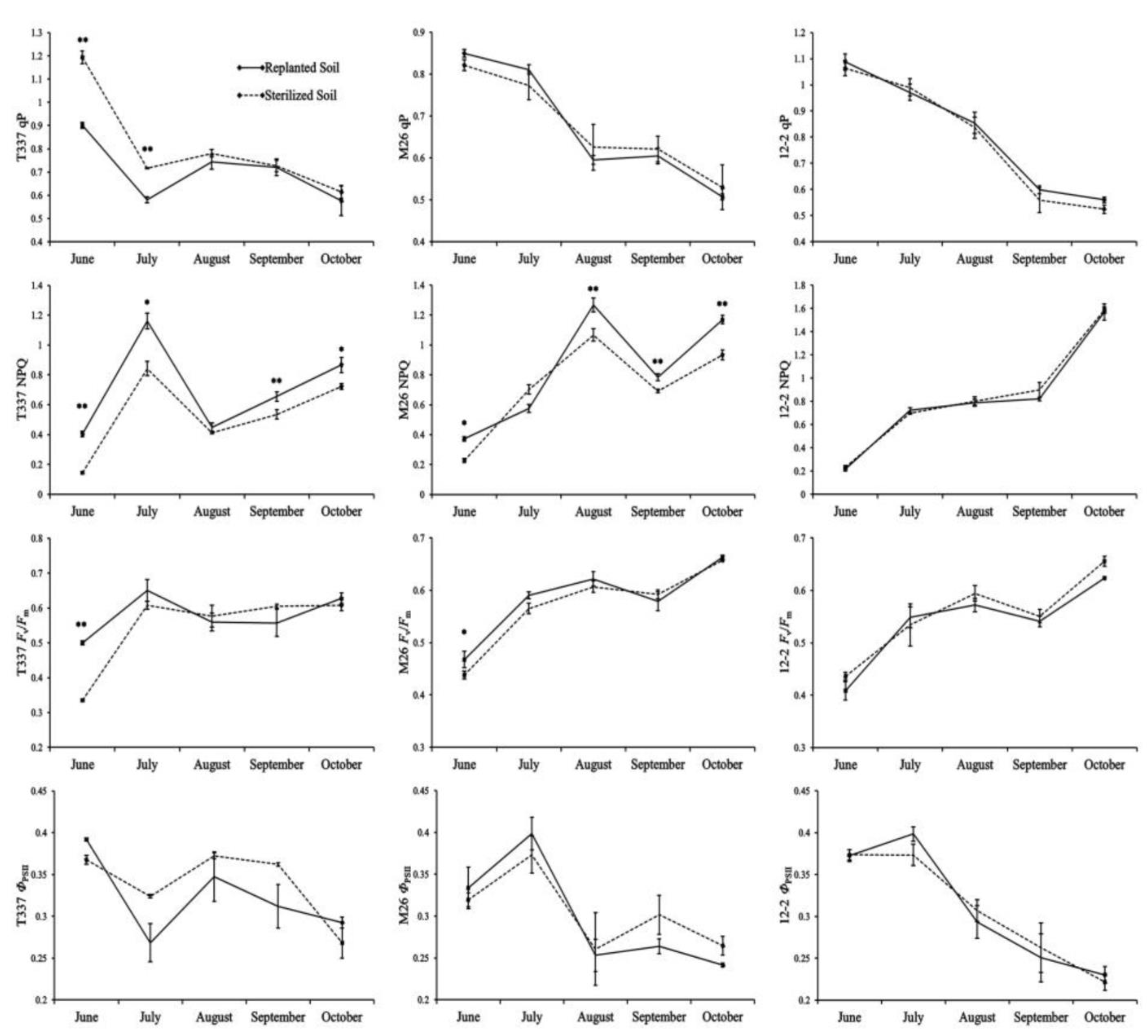
3.4.1. Analysis of Fluorescence Parameters
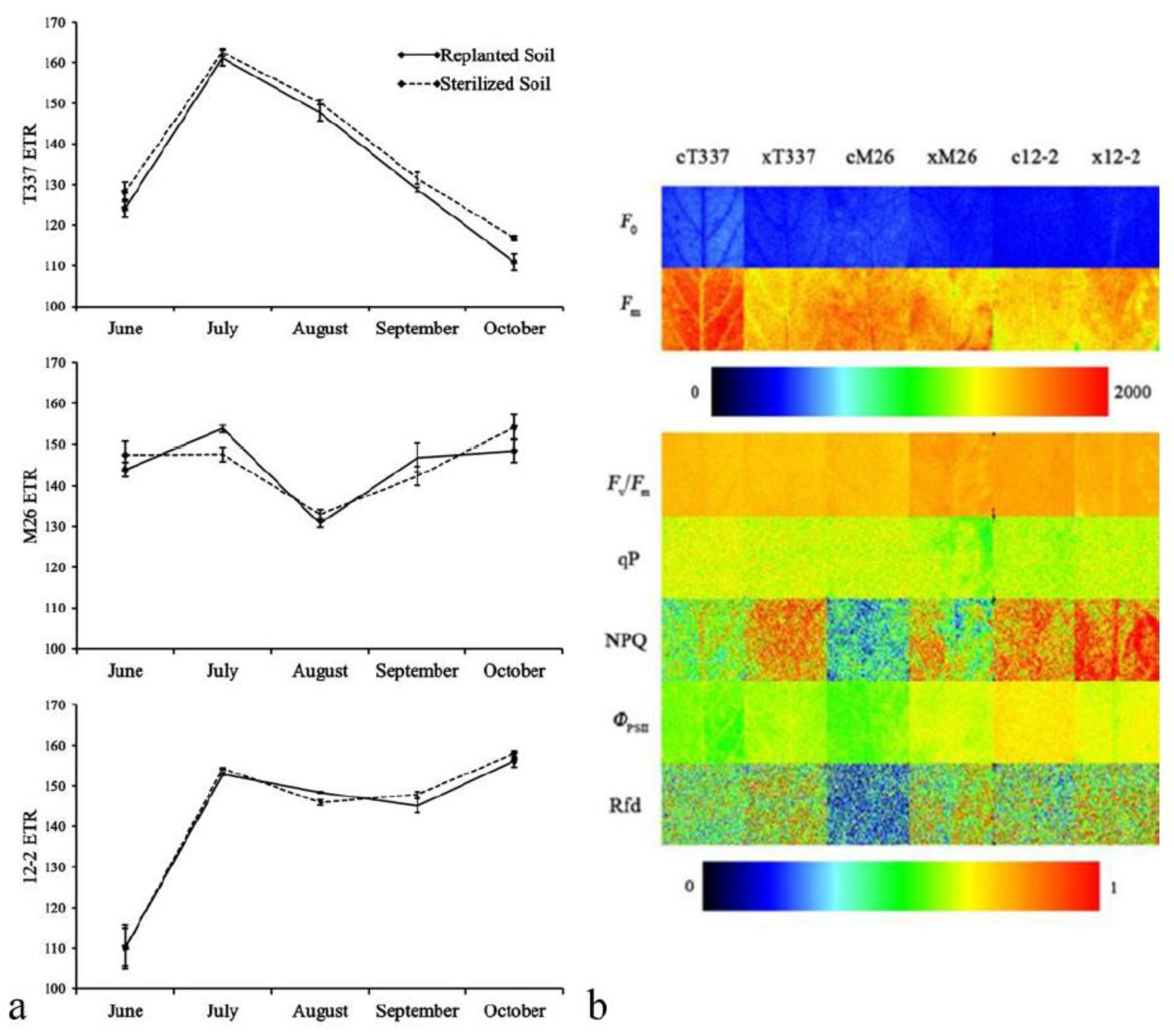
3.4.2. Analysis of Fluorescence Imaging
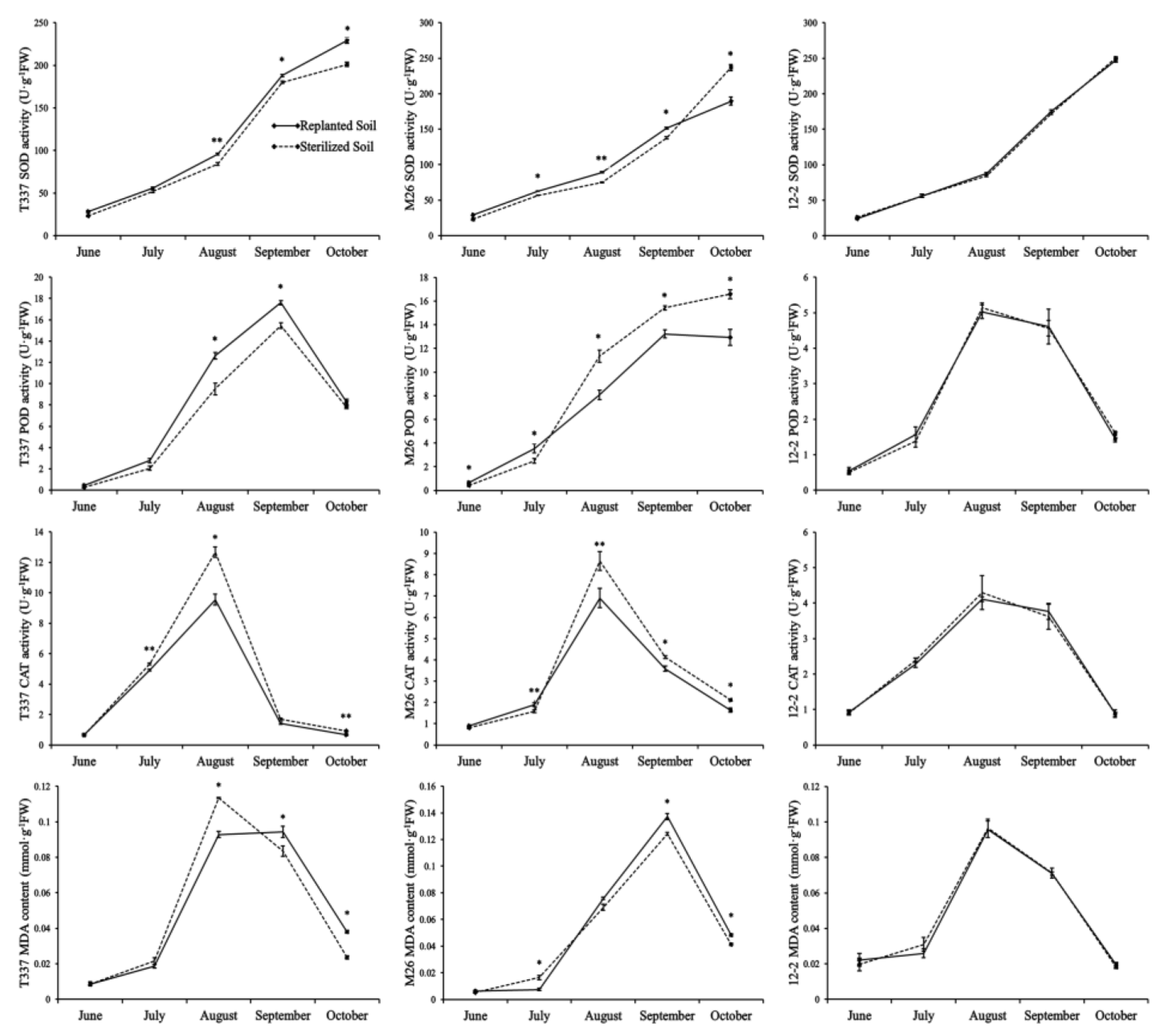
3.5. Analysis of Antioxidant Enzyme Activities and MDA Content in Leaves
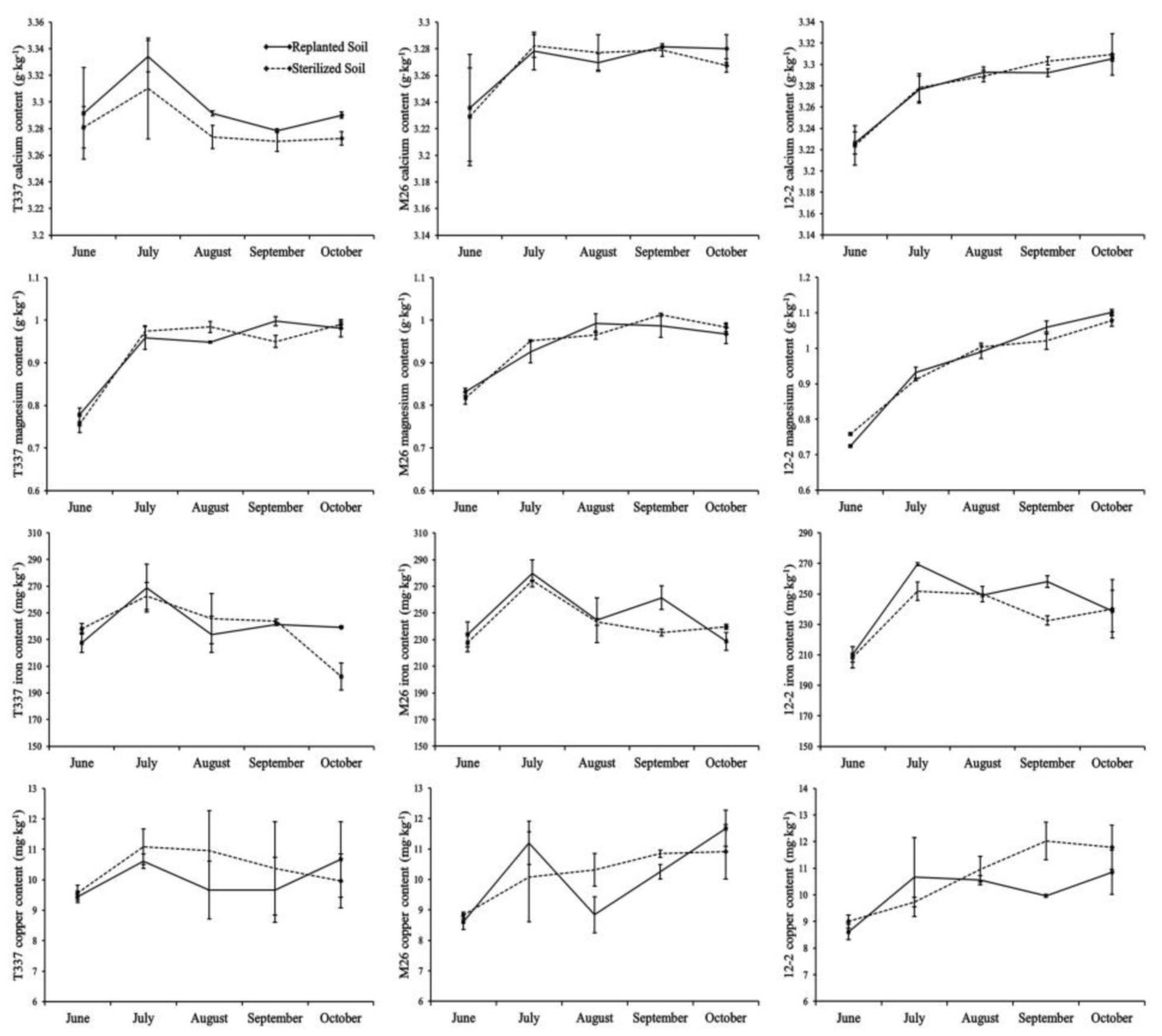
3.6. Analysis of Mineral Elements in the Leaves
4. Discussion
4.1. Effect of ARD on Plant Height, Stem Diameter
4.2. Effect of ARD on Relative Leaf Chlorophyll Content
4.3. Effect of ARD on Plant Leaf Photosynthetic Parameters
4.4. Effect of ARD on Fluorescence Parameters and Fluorescence Imaging of Plant Leaves
4.5. Effect of ARD on Antioxidant Enzyme Activities and MDA Content in Leaves
4.6. Effect of ARD on Mineral Elements in the Leaves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Polverigiani, S.; Kelderer, M.; Neri, D. Growth of ‘M9’ apple root in five Central Europe replanted soils. Plant Root 2014, 8, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Polverigiani, S.; Franzina, M.; Neri, D. Effect of soil condition on apple root development and plant resilience in intensive orchards. Appl. Soil Ecol. 2018, 123, 787–792. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.Z.; Liu, Q.Z.; Zhang, Y.T.; Li, X.Y.; Li, H.Q.; Li, W.H. Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Appl. Soil Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Mao, Y.F.; Zhang, L.L.; Wang, Y.Y.; Yang, L.; Yin, Y.J.; Su, X.F.; Liu, Y.P.; Pang, H.L.; Xu, J.; Hu, Y.L.; et al. Effects of polycyclic aromatic hydrocarbons (PAHs) from different sources on soil enzymes and microorganisms of Malus prunifolia var. Ringo. Arch. Agron. Soil Sci. 2020, 1–15. [Google Scholar] [CrossRef]
- Mazzola, M.; Manici, L.M. Apple replant disease: Role of microbial ecology in cause and control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Kelderer, M.; Franke-Whittle, I.H.; Rühmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Topp, A.; Insam, H.; Naef, A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Gangatharan, R.; Neri, D. Can Biodiversity Improve Soil Fertility Resilience in Agroecosystems? Spec. Issue New Medit. N 2012, 11, 11–18. [Google Scholar]
- Mia, M.J.; Furmanczyk, E.M.; Golian, J.; Kwiatkowska, J.; Malusá, E.; Neri, D. Living Mulch with Selected Herbs for Soil Management in Organic Apple Orchards. Horticulturae 2021, 7, 59. [Google Scholar] [CrossRef]
- St Laurent, A.; Merwin, I.A.; Thies, J.E. Long-term orchard groundcover management systems affect soil microbial communities and apple replant disease severity. Plant Soil 2008, 304, 209–225. [Google Scholar] [CrossRef]
- Narwal, S.S. Allelopathy in ecological sustainable organic agriculture. Allelopath. J. 2010, 25, 51–72. [Google Scholar]
- Pan, F.B.; Xiang, L.; Wang, S.; Li, J.J.; Shen, X.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Effects of short-term rotation and Trichoderma application on the soil environment and physiological characteristics of Malus hupehensis Rehd. seedlings under replant conditions. Acta Ecol. Sin. 2017, 37, 315–321. [Google Scholar] [CrossRef]
- Sheng, Y.F.; Wang, H.Y.; Wang, M.; Li, H.H.; Xiang, L.; Pan, F.B.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. Effects of Soil Texture on the Growth of Young Apple Trees and Soil Microbial Community Structure Under Replanted Conditions. Hortic. Plant J. 2020, 6, 123–131. [Google Scholar] [CrossRef]
- Li, H.; Yuan, G.L.; Zhu, C.W.; Zhao, T.T.; Zhang, R.M.; Wang, X.L.; Yang, J.Q.; Ma, J.X.; Zhang, Y.; Zhang, X. Soil fumigation with ammonium bicarbonate or metam sodium under high temperature alleviates continuous cropping-induced Fusarium wilt in watermelon. Sci. Hortic. 2019, 246, 979–986. [Google Scholar] [CrossRef]
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The causal agent of apple scab. Mol. Plant. Pathol. 2010, 12, 105–122. [Google Scholar] [CrossRef]
- Rivard, C.L.; O’Connell, S.; Peet, M.M.; Welker, R.M. Grafting Tomato to Manage Bacterial Wilt Caused by Ralstonia solanacearum in the Southeastern United States. Plant Dis. 2012, 96, 973–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.M.; Saltzgiver, M. A systematic analysis of apple root resistance traits to Pythium ultimum infection and the underpinned molecular regulations of defense activation. Hortic. Res. 2020, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Leinfelder, M.M.; Merwin, I.A. Rootstock selection, replant soil treatments, and tree planting positions as factors in managing apple replant disease. HortScience 2006, 41, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Rumberger, A.; Yao, S.R.; Merwin, I.A.; Nelson, E.B.; Thies, J.E. Rootstock genotype and orchard replant position rather than soil fumigation or compost amendment determine tree growth and rhizosphere bacterial community composition in an apple replant soil. Plant Soil 2004, 264, 247–260. [Google Scholar] [CrossRef]
- Mazzola, M.; Zhao, X.W. Brassica juncea seed meal particle size influences chemistry but not soilbiology-based suppression of individual agents inciting apple replant disease. Plant Soil 2010, 337, 313–324. [Google Scholar] [CrossRef]
- Fallahi, E.; Colt, W.M.; Fallahi, B.; Chun, I.J. The Importance of Apple Rootstocks on Tree Growth, Yield, Fruit Quality, Leaf Nutrition, and Photosynthesis with an Emphasis on ‘Fuji’. Horttechnology 2001, 12, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, W.; Xu, X.F.; Qiu, C.P.; Wu, T.; Wei, Q.P.; Ma, F.W.; Han, Z.H. Progress of Apple Rootstock Breeding and Its Use. Hortic. Plant J. 2019, 5, 183–191. [Google Scholar] [CrossRef]
- Shen, X.; Mao, Z.Q.; Ni, W.R.; Wang, R.; Hu, Y.L.; Chen, X.S.; Wu, S.J.; Han, T.T.; Zhang, W.H. In Situ Breeding Method for Three-Stage Selection of Apple Rootstock Tolerant to ARD. Chinese Patent No. CN104488645A, 8 April 2015. (In Chinese). [Google Scholar]
- Gao, F.F. The Continuous Cropping Testing and Rapid Propagation of Primary Apple Rootstocks Superior Lines. Bachelor’s Thesis, Shandong Agricultural University, Tai’an, Shandong, China, 2018. [Google Scholar]
- Su, X.F.; Chai, S.S.; Mao, Y.F.; Zhang, L.L.; Yin, Y.J.; Liu, Y.P.; Pang, H.L.; Hu, Y.L. Polyploid Induction and Identification of Apple Rootstocks with Tolerance to Continuous Cropping. Mol. Plant Breed. 2021, 1–12. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20210521.1458.010.html (accessed on 16 September 2021). (In Chinese).
- Atucha, A.; Emmett, B.; Bauerle, T.L. Growth rate of fine root systems influences rootstock tolerance to replant disease. Plant Soil 2014, 376, 337–346. [Google Scholar] [CrossRef]
- Sun, D.Q.; Sun, X.M.; Xu, Y.Y.; Wu, T.J.; Tao, L.X. Superoxide dismutase activity and risk of cognitive decline in older adults: Findings from the Chinese Longitudinal Healthy Longevity Survey. Exp. Gerontol. 2019, 118, 72–77. [Google Scholar] [CrossRef]
- Omran, R.G. Peroxide levels and the activities of catalase, peroxidase, and indoleacetic acid oxidase during and after chilling cucumber seedlings. Plant Physiol. 1980, 65, 407–408. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Sharma, S.R.; Singh, B. Antioxidant enzymes in cabbage:variability and inheritance of superoxide dismutase, peroxidase and catalase. Sci. Hortic. 2010, 124, 9–13. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Determination of Malondialdehyde as Dithiobarbituric Acid Adduct in Biological Samples by HPLC with Fluorescence Detection: Comparison with Ultraviolet-Visible Spectrophotometry. Clin. Chem. 2001, 47, 1725–1727. [Google Scholar] [CrossRef]
- Długaszek, M.; Kaszczuk, M.; Mularczyk-Oliwa, M. Magnesium, calcium, and trace elements excretion in 24-h urine. Biol. Trace Elem. Res. 2011, 142, 1–10. [Google Scholar] [CrossRef]
- Udoh, A.P. Atomic absorption spectrometric determination of calcium and other metallic elements in some animal protein sources. Talanta 2000, 52, 749–754. [Google Scholar] [CrossRef]
- Li, S.Y.; Jiang, H.L.; Wang, J.J.; Wang, Y.D.; Pan, S.G.; Tian, H.; Duan, M.Y.; Wang, S.L.; Tang, X.R.; Mo, Z.W. Responses of plant growth, physiological, gas exchange parameters of super and non-super rice to rhizosphere temperature at the tillering stage. Sci. Rep. 2019, 9, 10618. [Google Scholar] [CrossRef]
- Mahnkopp, F.; Simon, M.; Lehndorff, E.; Pätzold, S.; Wrede, A.; Winkelmann, T. Induction and diagnosis of apple replant disease (ARD): A matter of heterogeneous soil properties? Sci. Hortic. 2018, 241, 167–177. [Google Scholar] [CrossRef]
- Winkelmann, T.; Smalla, K.; Amelung, W.; Baab, G.; Grunewaldt-Stöcker, G.; Kanfra, X.; Meyhöfer, R.; Reim, S.; Schmitz, M.; Vetterlein, D.; et al. Apple Replant Disease: Causes and Mitigation Strategies. Curr Issues Mol. Biol. 2019, 30, 89–106. [Google Scholar] [CrossRef] [Green Version]
- Reim, S.; Siewert, C.; Winkelmann, T.; Wöhner, T.; Hanke, M.V.; Flachowsky, H. Evaluation of Malus genetic resources for tolerance to apple replant disease (ARD). Sci. Hortic. 2019, 256, 108517. [Google Scholar] [CrossRef]
- Rohr, A.D.; Staudt, J.; Cziborra, K.; Fritz, A.; Schmitz, M.; Winkelmann, T. Split-root approach reveals localized root responses towards apple replant disease (ARD) in terms of ARD biomarker gene expression and content of phenolic compounds. Sci. Hortic. 2021, 286, 110117. [Google Scholar] [CrossRef]
- Guo, X.J.; Han, T.T.; Wang, R.; Zhang, Z.; Ge, R.; Mao, Z.Q.; Chen, X.S.; Shen, X. Differences of growth and root absorption of different apple rootstock seedlings in replant stress. J. Plant Nutr. Fertil. 2015, 21, 1312–1319. (In Chinese) [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.D.; Shi, X.B.; Wang, B.L.; Zheng, X.C.; Wang, H.B.; Liu, F.Z. Red and Blue Lights Significantly Affect Photosynthetic Properties and Ultrastructure of Mesophyll Cells in Senescing Grape Leaves. Hortic. Plant J. 2016, 2, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Botyanszka, L.; Zivcak, M.; Chovancek, E.; Sytar, O.; Barek, V.; Hauptvogel, P.; Halabuk, A.; Brestic, M. Chlorophyll Fluorescence Kinetics May Be Useful to Identify Early Drought and Irrigation Effects on Photosynthetic Apparatus in Field-Grown Wheat. Agronomy 2020, 10, 1275. [Google Scholar] [CrossRef]
- Li, Y.; He, N.P.; Huo, J.H.; Xu, L.; Liu, C.C.; Zhang, J.H.; Wang, Q.F.; Zhang, X.M.; Wu, X.Q. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 44090951. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Lin, K.D.; Hou, Z.A.; Richardson, B.; Gan, J. Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J. Soil Sediment 2010, 10, 283–289. [Google Scholar] [CrossRef]
- Gago, J.; Daloso, D.M.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of Leaf Net Photosynthesis, Stomatal Conductance, and Mesophyll Conductance to Primary Metabolism: A Multispecies Meta-Analysis Approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Uzunova, A.N.; Popova, L.P. Effects of short-term soil flooding on stomata behaviour and leaf gas exchange in barley plants. Biol. Plant. 2005, 49, 317–319. [Google Scholar] [CrossRef]
- Wright, H.; Delong, J.; Lada, R.; Prange, R. The relationship between water status and chlorophyll a fluorescence in grapes (Vitis spp.). Postharvest Biol. Tec. 2009, 51, 193–199. [Google Scholar] [CrossRef]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of Chlorophyll a Fluorescence in Natural Compound-Induced Stress Detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Rungrat, T.; Awlia, M.; Brown, T.; Cheng, R.Y.; Sirault, X.; Fajkus, J.; Trtilek, M.; Furbank, B.; Badger, M.; Tester, M.; et al. Using Phenomic Analysis of Photosynthetic Function for Abiotic Stress Response Gene Discovery. Arab. Book 2016, 14, e0185. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, B.; Chakraborty, S.; Crawford, M.; Taylor, J.M.; Blackmon, L.E.; Biswas, L.E.; Kramer, W.H. Characterization of Diadzein-Hemoglobin Binding using Optical Spectroscopy and Molecular Dynamics Simulations. Int. J. Biol. Macromol. 2012, 51, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.X.; Zhou, X.; Wei, B.D.; Cheng, S.C.; Zhou, Q.; Ji, S.J. GABA application improves the mitochondrial antioxidant system and reduces peel browning in ‘Nanguo’ pears after removal from cold storage. Food Chem. 2019, 297, 124903. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M.; Polle, A. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive Oxygen Species, Oxidative Damage and Cell Death. Immun. Inflamm. Health Dis. 2018, 45–55. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, Y.; Han, M.; Yan, L.M. Changes in the physiological characteristics of Panax ginseng embryogenic calli and molecular mechanism of ginsenoside biosynthesis under cold stress. Planta 2021, 253, 79. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.Z.; Tang, W.Y.; An, Q.R.; Liu, Y.X.; Tian, J.Q.; Zhao, N.; Zhu, S.N. Physiological effects of the combined stresses of freezing-thawing, acid precipitation and deicing salt on alfalfa seedlings. BMC Plant Biol. 2020, 20, 204. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Li, T.T.; Hao, Z.J.; Cheng, M.L.; Tao, J. Exogenous trehalose confers high temperature stress tolerance to herbaceous peony by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. Cell Stress Chaperon 2019, 24, 247–257. [Google Scholar] [CrossRef]
- Song, X.H.; Xie, K.; Zhao, H.B.; Li, Y.L.; Xu, C.Y.; Dong, C.X. Investigation of the Mineral Nutrients Status of Pear Leaves in Main Orchards Around the Bohai Bay Region. Acta Hortic. Sin. 2011, 38, 2049–2058. (In Chinese) [Google Scholar] [CrossRef]
- Li, Z.Y.; Xu, B.; Du, T.H.; Ma, Y.K.; Tian, X.H.; Wang, F.L.; Wang, W.K. Excessive Nitrogen Fertilization Favors the Colonization, Survival, and Development of Sogatella furcifera via Bottom-Up Effects. Plants 2021, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Kavanová, M.; Lattanzi, F.A.; Grimoldi, A.A.; Schnyder, H. Phosphorus Deficiency Decreases Cell Division and Elongation in Grass Leaves. Plant Physiol. 2006, 141, 766–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zheng, Q.S.; Shen, Q.R.; Guo, S.W. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.J.; Wu, L.Q.; Chai, T.Y.; Zhang, Y.X.; Tan, J.J.; Ma, S.W. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J. Plant Physiol. 2012, 169, 1243–1252. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Q.P.; Jiang, R.S.; Liu, X.D.; Liu, H.P.; Wang, X.W. Correlation Analysis of Fruit Mineral Nutrition Contents with Several Key Quality Indicators in ‘Fuji’ Apple. Acta Hortic. Sin. 2011, 38, 1963–1968. (In Chinese) [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. -Lond. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Blake-Hedges, J.M.; Pereira, J.H.; Hangasky, J.A.; Belcher, M.S.; Moore, W.M.; Barajas, J.F.; Cruz-Morales, P.; Washington, L.J.; Haushalter, R.W.; et al. An iron (II) dependent oxygenase performs the last missing step of plant lysine catabolism. Nat. Commun. 2020, 11, 2931. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.R.; Liu, J.Z.; Lu, C.H.; Qu, X.H.; Luo, K.K.; Li, C.M.; He, M.L.; Zhang, H.Y.; Yan, H.L. Intercropping With Turmeric or Ginger Reduce the Continuous Cropping Obstacles That Affect Pogostemon cablin (Patchouli). Front. Microbiol. 2020, 11, 579719. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [Green Version]

| Treatment | June | July | August | September | October | |
|---|---|---|---|---|---|---|
| T337 | Replanted soil | 43.2 ± 1.0 | 47.3 ± 1.9 | 47.2 ± 2.6 | 54.1 ± 1.5 | 56.3 ± 2.4 |
| Sterilized soil | 52.1 ± 0.7 * | 61.4 ± 0.5 * | 68.9 ± 3.1 * | 77.5 ± 2.1 ** | 79.7 ± 1.2 * | |
| M26 | Replanted soil | 43.0 ± 1.7 | 54.7 ± 3.0 | 55.0 ± 3.9 | 63.2 ± 0.9 | 67.3 ± 2.9 |
| Sterilized soil | 56.3 ± 0.9 * | 76.7 ± 2.2 * | 78.4 ± 2.5 ** | 86.3 ± 1.9 ** | 90.9 ± 1.3 * | |
| 12-2 | Replanted soil | 79.4 ± 2.0 | 94.8 ± 1.3 | 94.7 ± 2.7 | 96.3 ± 2.1 | 99.7 ± 1.2 |
| Sterilized soil | 79.7 ± 1.5 | 90.3 ± 2.7 | 99.1 ± 1.4 | 99.3 ± 2.4 | 97.5 ± 1.3 | |
| Treatment | June | July | August | September | October | |
|---|---|---|---|---|---|---|
| T337 | Replanted soil | 4.8 ± 0.2 | 5.0 ± 0.1 | 6.6 ± 0.1 | 7.2 ± 0.2 | 7.2 ± 0.0 |
| Sterilized soil | 5.7 ± 0.0 * | 5.9 ± 0. 1 ** | 8.1 ± 0.2 ** | 8.2 ± 0.1 ** | 8.2 ± 0.1 * | |
| M26 | Replanted soil | 4.1 ± 0.1 | 4.5 ± 0.2 | 5.9 ± 0.1 | 6.4 ± 0.1 | 6.9 ± 0.1 |
| Sterilized soil | 4.9 ± 0.1 * | 5.9 ± 0.1 ** | 7.2 ± 0.1 * | 7.7 ± 0.1 * | 8.1 ± 0.1 * | |
| 12-2 | Replanted soil | 5.9 ± 0.1 | 6.4 ± 0.3 | 8.0 ± 0.1 | 8.2 ± 0.1 | 8.3 ± 0.1 |
| Sterilized soil | 5.9 ± 0.2 | 6.4 ± 0.2 | 8.1 ± 0.1 | 8.2 ± 0.1 | 8.2 ± 0.1 | |
| Treatment | F0 | Fm | Fv/Fm | qP | NPQ | ΦPSII | ETR | |
|---|---|---|---|---|---|---|---|---|
| T337 | Replanted soil | 383.0 ± 8.4 | 1537.1 ± 32.7 | 0.8 ± 0.0 | 1.0 ± 0.0 | 0.3 ± 0.0 | 1.1 ± 0.0 | 0.8 ± 0.0 |
| Sterilized soil | 375.4 ± 13.7 | 1461.4 ± 22.0 | 0.7 ± 0.0 | 1.0 ± 0.0 | 0.3 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.0 | |
| M26 | Replanted soil | 369.5 ± 16.1 | 1455.7 ± 75.9 | 0.8 ± 0.0 | 1.0 ± 0.0 | 0.3 ± 0.0 ** | 0.8 ± 0.1 | 0.8 ± 0.0 |
| Sterilized soil | 338.3 ± 6.0 | 1497.2 ± 42.9 | 0.8 ± 0.0 | 1.0 ± 0.0 | 0.2 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.0 | |
| 12-2 | Replanted soil | 284.5 ± 10.4 | 1271.8 ± 60.6 | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.5 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.0 |
| Sterilized soil | 300.7 ± 11.0 | 1324.4 ± 45.1 | 0.8 ± 0.0 | 1.0 ± 0.0 | 0.5 ± 0.0 | 1.1 ± 0.1 | 0.8 ± 0.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Yin, Y.; Cui, X.; Wang, H.; Su, X.; Qin, X.; Liu, Y.; Hu, Y.; Shen, X. Detection of Above-Ground Physiological Indices of an Apple Rootstock Superior Line 12-2 with Improved Apple Replant Disease (ARD) Resistance. Horticulturae 2021, 7, 337. https://doi.org/10.3390/horticulturae7100337
Mao Y, Yin Y, Cui X, Wang H, Su X, Qin X, Liu Y, Hu Y, Shen X. Detection of Above-Ground Physiological Indices of an Apple Rootstock Superior Line 12-2 with Improved Apple Replant Disease (ARD) Resistance. Horticulturae. 2021; 7(10):337. https://doi.org/10.3390/horticulturae7100337
Chicago/Turabian StyleMao, Yunfei, Yijun Yin, Xueli Cui, Haiyan Wang, Xiafei Su, Xin Qin, Yangbo Liu, Yanli Hu, and Xiang Shen. 2021. "Detection of Above-Ground Physiological Indices of an Apple Rootstock Superior Line 12-2 with Improved Apple Replant Disease (ARD) Resistance" Horticulturae 7, no. 10: 337. https://doi.org/10.3390/horticulturae7100337
APA StyleMao, Y., Yin, Y., Cui, X., Wang, H., Su, X., Qin, X., Liu, Y., Hu, Y., & Shen, X. (2021). Detection of Above-Ground Physiological Indices of an Apple Rootstock Superior Line 12-2 with Improved Apple Replant Disease (ARD) Resistance. Horticulturae, 7(10), 337. https://doi.org/10.3390/horticulturae7100337






