1. Introduction
Cycads are dioecious gymnosperms that are of horticultural and conservation interest [
1,
2], and are widely considered the most threatened plant group worldwide [
3] primarily due to habitat destruction and the unsustainable trade of wild-collected plants. The unsustainable harvesting of cycads is a major concern at the local level but also internationally, where their trade is regulated by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) [
4,
5]. Adaptive management of threatened cycad species requires the co-production of knowledge during conservation projects [
6]. During horticultural projects in cultivated settings and biology or ecology projects in habitat, research results may provide crucial information for improving management decisions [
7].
In wild cycad populations, only a certain proportion of plants may exhibit strobili at a given time. Consequently, most short-term field studies of these populations record sex ratio estimates that represent only what was observable at the time of fieldwork. This estimate is variously known as the coning [
8], operational [
9], or observed sex ratio [
10], and it may differ greatly from the true genotypic sex ratio of the population and may vary widely depending on the time of year when observations were made. Therefore, the development of any protocol that would inform how to improve on the methods for determining the sex of cycads may improve decision-making.
Three publications indicated the use of stem branching of a cycad plant might be useful as secondary sex characteristics [
11] for predicting sex of a plant. First, Ornduff [
12] followed a cultivated group of
Zamia integrifolia L.f. plants in Florida for 9 y to determine growth traits. The male plants produced an average of 5.7 branches per plant and the female plants produced 2.7 branches per plant. Male plants began producing cones at an earlier age and produced more cones throughout the observational period than female plants. These reproductive behavior differences were discussed as the probable cause of increased branching in male plants.
Second, Norstog and Nicholls [
1] (p. 141) studied a fifty-year-old planting of
Z. integrifolia and found marked sexual dimorphism in the cultivated cohort, with the males carrying more branches, more cones, and more leaves than female plants. The males held an average of 27 branches per plant whereas the females held an average of 8 branches per plant. The more frequent branching in male plants was attributed to the males experiencing more frequent coning events and producing more cones per reproductive episode than the female plants.
Third, Niklas and Marler [
13] determined the branching traits of 483
C. micronesica adult plants in four research locations in Guam. The male trees exhibited a mean of 3.4 branches per tree and the female trees exhibited a mean of 1.5 branches per tree. The difference in reproductive behavior of male and female
Cycas plants was discussed as the probable cause of increased branching in male trees.
Throughout our field work we recorded the number of stem apices on plants within numerous in situ populations as part of the plant metrics obtained to more fully understand the biology of several species. Our objectives herein were to look closely at these data to more fully understand branching behavior of four cycad species. Moreover, we observed branching behavior of
C. micronesica before the invasions of several non-native insect herbivore species that have led to 96% mortality of Guam’s population [
14]. We exploited this phenomenon to look closely at branching behavior of the tree population that has survived the biological threats. Finally, we explore how this knowledge may improve horticultural and conservation decisions.
2. Materials and Methods
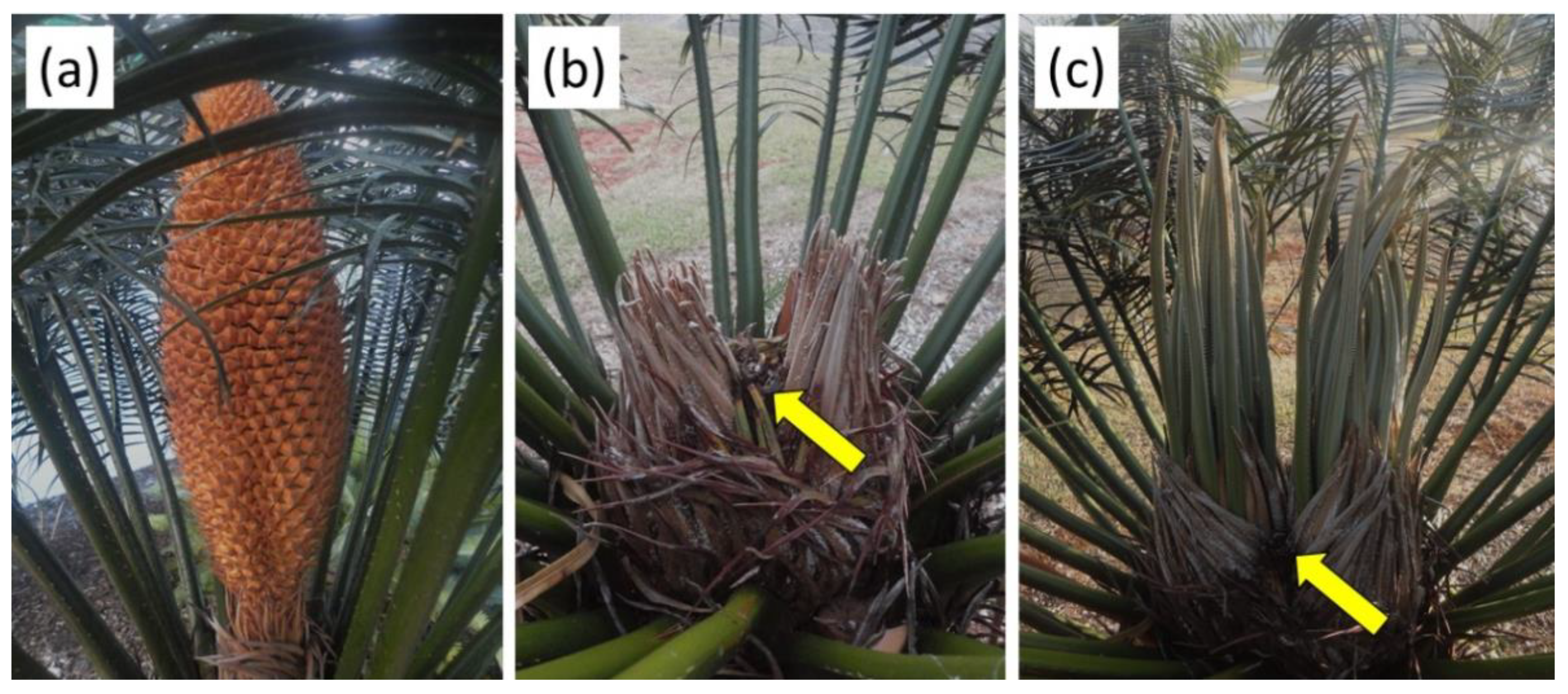
We collected data from numerous areas of occupancy for four cycad species in Colombia, Guam, and Philippines. These data included sympatric species, plant height and basal stem diameter, number of branches, number of leaves per plant and leaflets per leaf, length of petiole and rachis, and size of strobili if present. For the purpose of this study, we focused on the number of stem apices on each plant that we could identify as male or female. The general appearance of male and female
Cycas trees in Guam and Philippines contrasted sharply with the appearance of male and female
Z. encephalartoides plants in Colombia, the latter species had much shorter stems with a higher prevalence of stem branching (
Figure 1).
2.1. Cycas micronesica
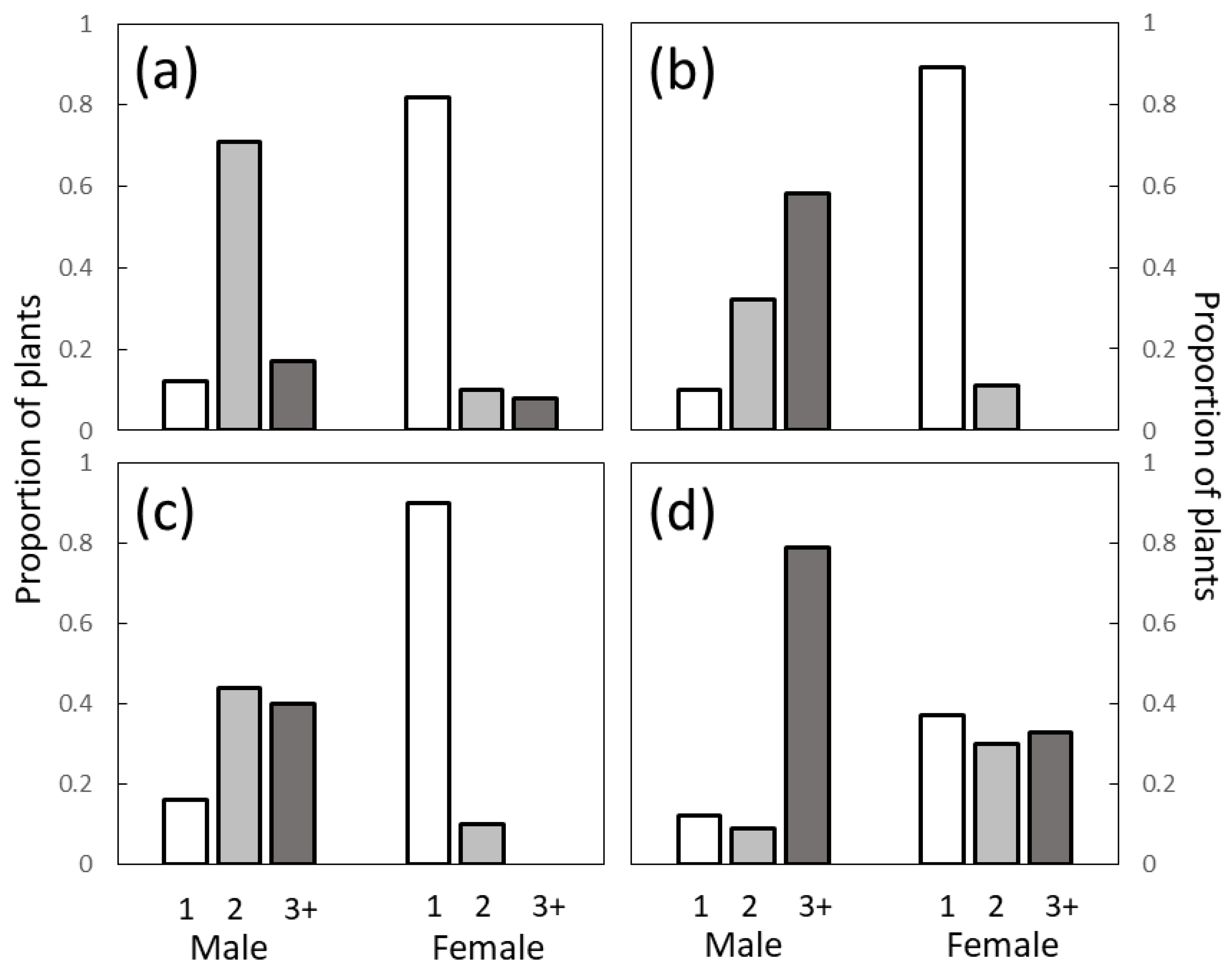
The observations on C. micronesica trees were conducted in 2004 in Guam. Ten areas of occupancy throughout Guam were used for biochemistry studies designed to determine the biological and habitat factors that influenced neurotoxin concentrations in seeds. The plant traits were recorded in conjunction with these methods. Several non-native insect herbivore species invaded Guam during this time period, but their entry into our study sites occurred in 2005. Therefore, the 2004 data captured the population traits prior to any non-native insect herbivore damage. The number of branches on every tree that was identified as male or female was recorded. These data were combined to define traits of 1041 male and 754 female trees.
2.2. Cycas edentata
We observed 23 areas of occupancy of C. edentata among numerous islands within a latitudinal gradient of 9°10.655′–13°32.145′ N and longitudinal gradient of 119°52.788′–126°7.122′ E. The field work was conducted from August 2008 to March 2019. These data were combined to obtain measurements on 178 male and 234 female trees.
2.3. Cycas wadei
The only known endemic population of C. wadei on Culion Island was observed in March 2019 to obtain measurements on 104 male and 153 female trees.
2.4. Zamia encephalartoides
A single large grouping of
Z. encephalartoides, restricted to a single plateau and consisting of 287 adult-sized individuals was studied in Santander, Colombia in February 2009 as part of a conservation survey for this species [
15]. This population consisted of monopodial individuals or clumps containing up to 40 stems. As the stems of this cycad species may be partly subterranean and some branching may occur underground, it was sometimes difficult to ascertain with certainty whether clumps of stems belonged to the same individual plants. For the purpose of this study, only clumps exclusively containing strobili of a single sex and where all stems exhibited similar phenological stages were counted as individual plants. As the species occurs in a hot and dry environment, cone remnants can remain on plants for several months, allowing the sexual identity to be determined for over 80% of the observed plants. Measurements were obtained from 143 male and 88 female plants.
2.5. Unbiased Demography of Cycas micronesica
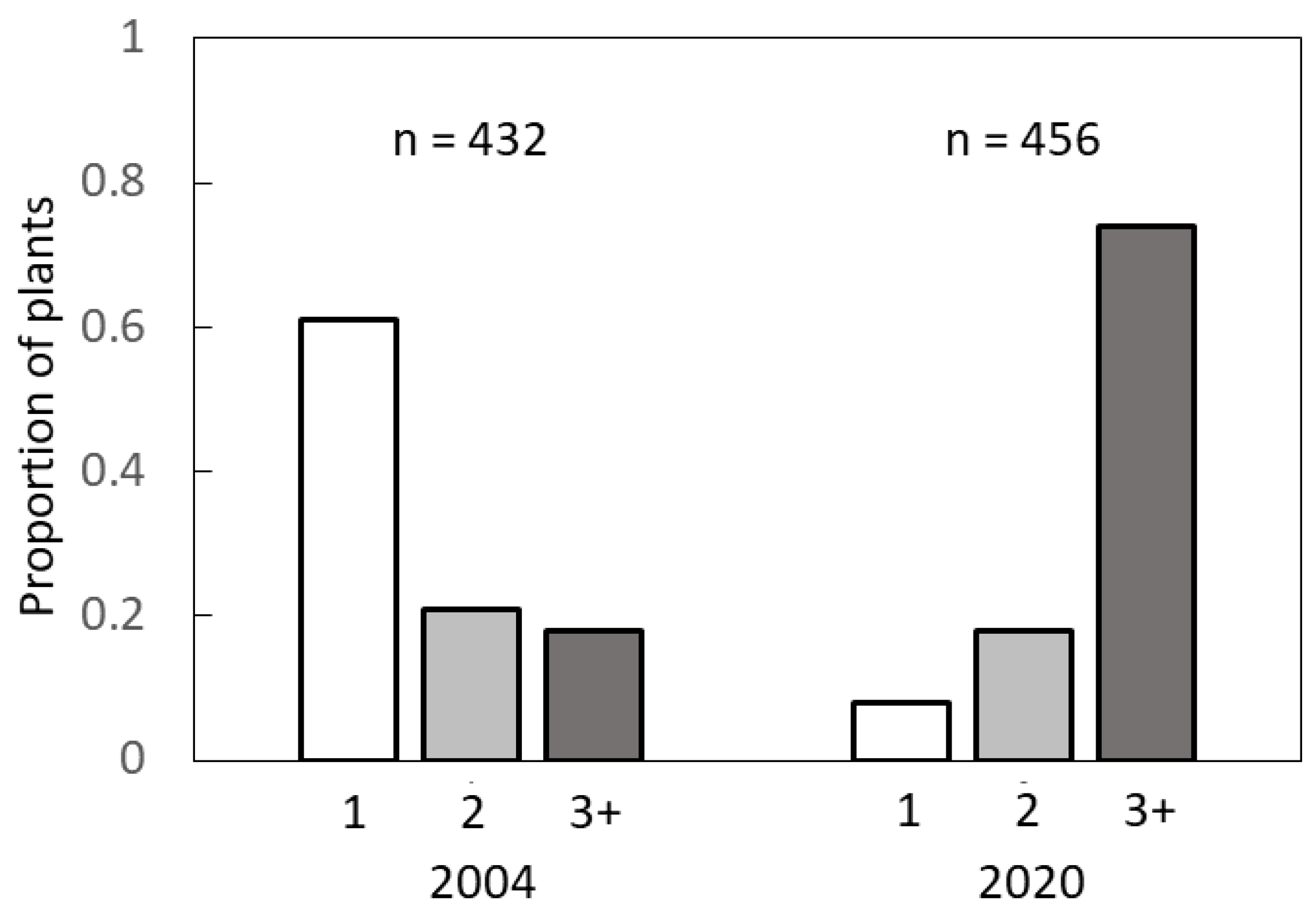
The methods described in the previous sub-sections employed biased methods where sexual identity of a plant was verified prior to collection of data. These methods did not allow an understanding of the branch patterns of the entire population, including plants that were mature in stature but exhibited no evidence of a strobilus. Therefore, we collected data from three high density C. micronesica localities with 900+ mature plants per ha along the east coast of Guam in January 2004 (13°25.674′–13°29.847′). We used four 4 × 100 m transects per locality and observed every plant greater than 100 cm in height and recorded the number of branches. A total of 432 mature plants were observed. We returned to these same localities to repeat the measurements in January 2020. By this time the density was less than 200 plants per ha. We repeated the methods with 4 × 100 m transects, but because of the plant density we increased the number of transects in each locality until we reached ≈150 plants per locality. A total of 456 mature plants were observed.
2.6. Analyses
The male versus female branch number data for each species were analyzed separately. The data did not meet parametric prerequisites, so we used the Mann–Whitney U test to determine significance (SPSS Statistics, IBM Corp., Armonk, NY, USA).
4. Discussion
The higher propensity for branching in male versus female cycad plants reported for cultivated
Z. integrifolia [
2,
12] was also documented for in situ populations of
Cycas micronesica [
13]. The present study confirms these findings with robust in situ data sets of three
Cycas species and one
Zamia species. We believe the higher branch number found in male plants of the species studied may be the consequence of isotomous branching associated with the more frequent coning of male cycad plants [
12,
16]. Vegetative branching in cycads occurs via two known mechanisms: isotomous branching of the shoot apex or adventitious branching associated with damaged stem regions or leaf bases [
17]. Adventitious branching is the most common form of branching in cycads, occurring in the ten living cycad genera and producing the offsets (known as suckers or pups) that are typically used for vegetative propagation of cycads. Isotomous vegetative branching, where the shoot apex divides dichotomously at the stem axis to form two equal branches, appears to be much rarer in cycads and has only been reported in
Cycas,
Dioon, and
Zamia [
1,
12,
17,
18,
19]. However, another dichotomous branching process, termed anisotomous branching [
17] occurs in cycad genera that produce terminal strobili.
In anisotomous branching, the original apex dichotomizes and produces a fertile apex that will develop a cone (or set of cones) and another apex that goes dormant and resumes vegetative growth after the strobili mature [
17]. Anisotomous branching is detectable in the anatomy of cycads stems by the presence of dome-like profiles of vascular tissue known as cone domes [
1] (p. 45) but is not readily apparent externally because the vegetative apex resumes upward growth of the stem in a seemingly continuous fashion.
All cycad genera, with the exception of members of the Tribe Encephalartae (sensu [
20]) produce terminal cones and therefore undergo anisotomous branching. In Encephalartae, comprised of the three related genera
Encephalartos Lehm.,
Macrozamia Miq., and
Lepidozamia Regel [
21], cones are produced to the side of the vegetative apex with no associated apex dichotomy [
22]. In
Cycas, anisotomous branching is restricted to male plants, as female plants produce indeterminate crowns of individual sporophylls (
Figure 4) that are produced similarly and alternately with crowns of leaves [
23,
24].
Multiple studies have associated coning events with vegetative branching, suggesting a close association between anisotomous and isotomous branching. Gorelick [
19] reported isotomous branching in
Zamia furfuracea L.f. immediately following a coning event. In this case, the vegetative branches occurred on both sides of the cone axis with both branches producing simultaneous vegetative flushes. Ornduff [
12] observed branching was closely associated with cone production in a cultivated cohort of
Z. integrifolia. He found a significantly higher number of branches were produced by male plants and attributed this phenomenon to the male plants experiencing more coning events due to their reproduction starting at an earlier age than the females. Similarly, in a cultivated group of
Z. integrifolia plants of similar age, Norstog and Nicholls [
1] (p. 141) found a significantly larger number of branches on males than females. This phenomenon was attributed to increased apex subdivisions due to their more frequent cone production. Additionally, male plants were generally more robust and produced more leaves than females.
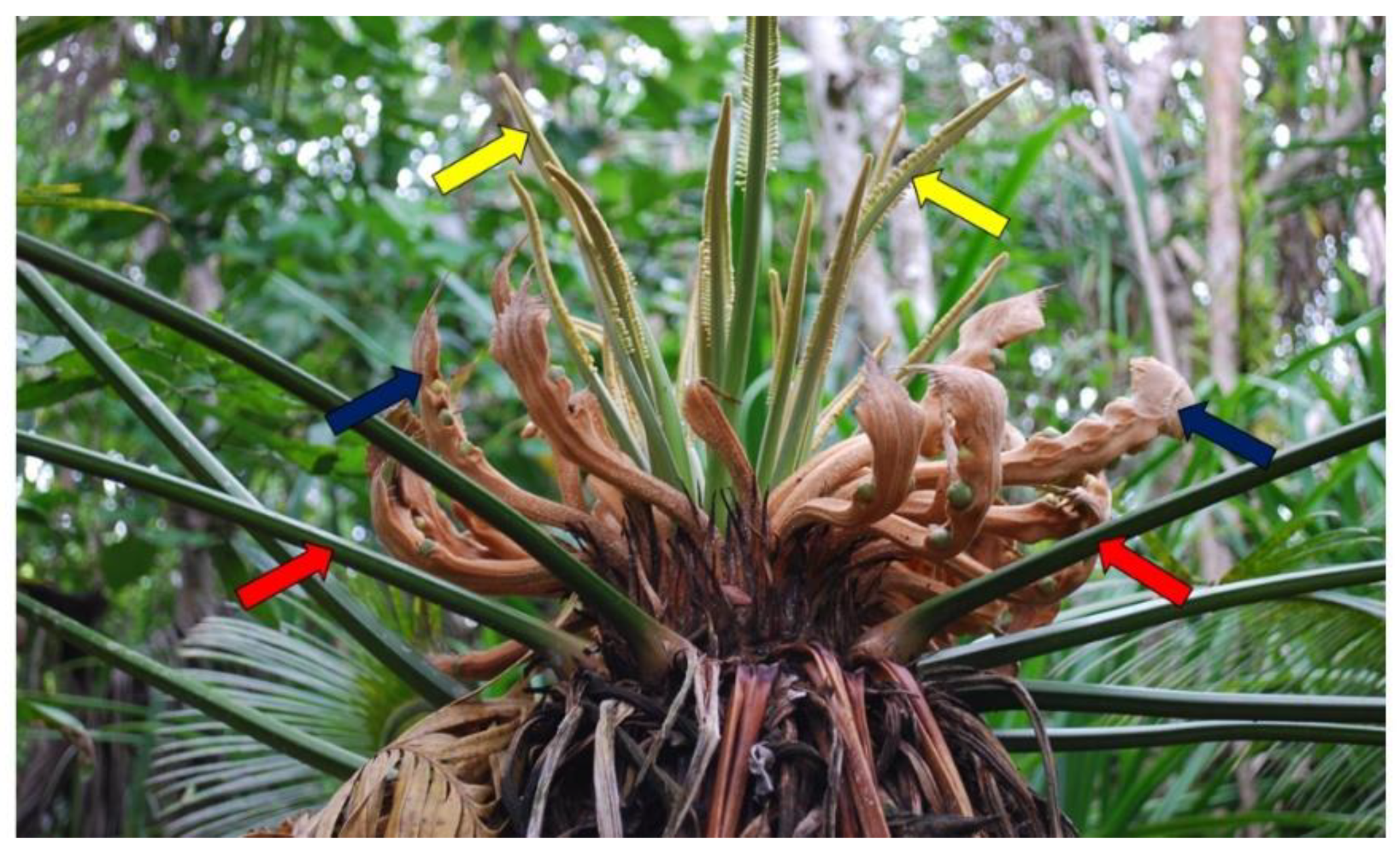
The process of isotomous vegetative branching associated with a coning event is illustrated in male
Cycas micronesica (
Figure 5). In a similar manner as described by Gorelick [
19] with
Z. furfuracea, following the production of a terminal cone, two branches of similar size are formed at either side of the cone axis and produce vegetative flushes simultaneously. In this study, isotomous vegetative branching was observed only on male
Cycas plants, but on both sexes of
Zamia.
The branching behaviors of the three
Cycas species were remarkably similar, with less than 20% of male trees and more than 80% of the female trees exhibiting unbranched trunks. Most female trees were unbranched for all three
Cycas species, but the mean and maximum number of branches observed in female
C. micronesica trees exceeded those in the other two species. We note two factors that may explain these differences. First, the differences could be due to inter-specific genetic differences where
C. micronesica is more prone to branching. The addition of many more
Cycas species to this research agenda would aid in understanding the role of genetics on branching of female trees. Second, the greater branching from Guam’s trees may be due to greater tropical cyclone (TC) activity. The Philippine and Guam habitats are within the most active TC basin worldwide [
25]. Guam’s propensity for TC occurrence must be considered when making horticultural decisions for perennial crops [
26]. A major TC in 1997 caused an estimated 10% of Guam’s
C. micronesica population to become decapitated with the entire stem apex snapped off [
27]. Guam’s trees that suffer this form of damage during TCs develop numerous adventitious buds at positions on the stem that are proximal to the break ([
28], Figure 1). After almost two years of regrowth, the trees that withstood the 1997 TC with intact apices exhibited only two vegetative growth events, but the decapitated trees exhibited as many as 25 vegetative growth events over the experimental period as a result of the numerous adventitious branches [
29]. Observations since the invasions of several non-native insect herbivores indicate this new biotic threat has weakened the
C. micronesica stems such that they are more vulnerable to TC damage [
30]. This was confirmed during a 2015 TC when damage to the
C. micronesica population was greater than expected based on historical observations of damage [
31]. The third possible explanation for the greater number of apices for
C. micronesica than for the other two
Cycas species could be an interaction of genetic differences and TC propensity. This could be tested by observing branching patterns of mature specimens of the three
Cycas species in a common garden setting.
4.1. Refinements
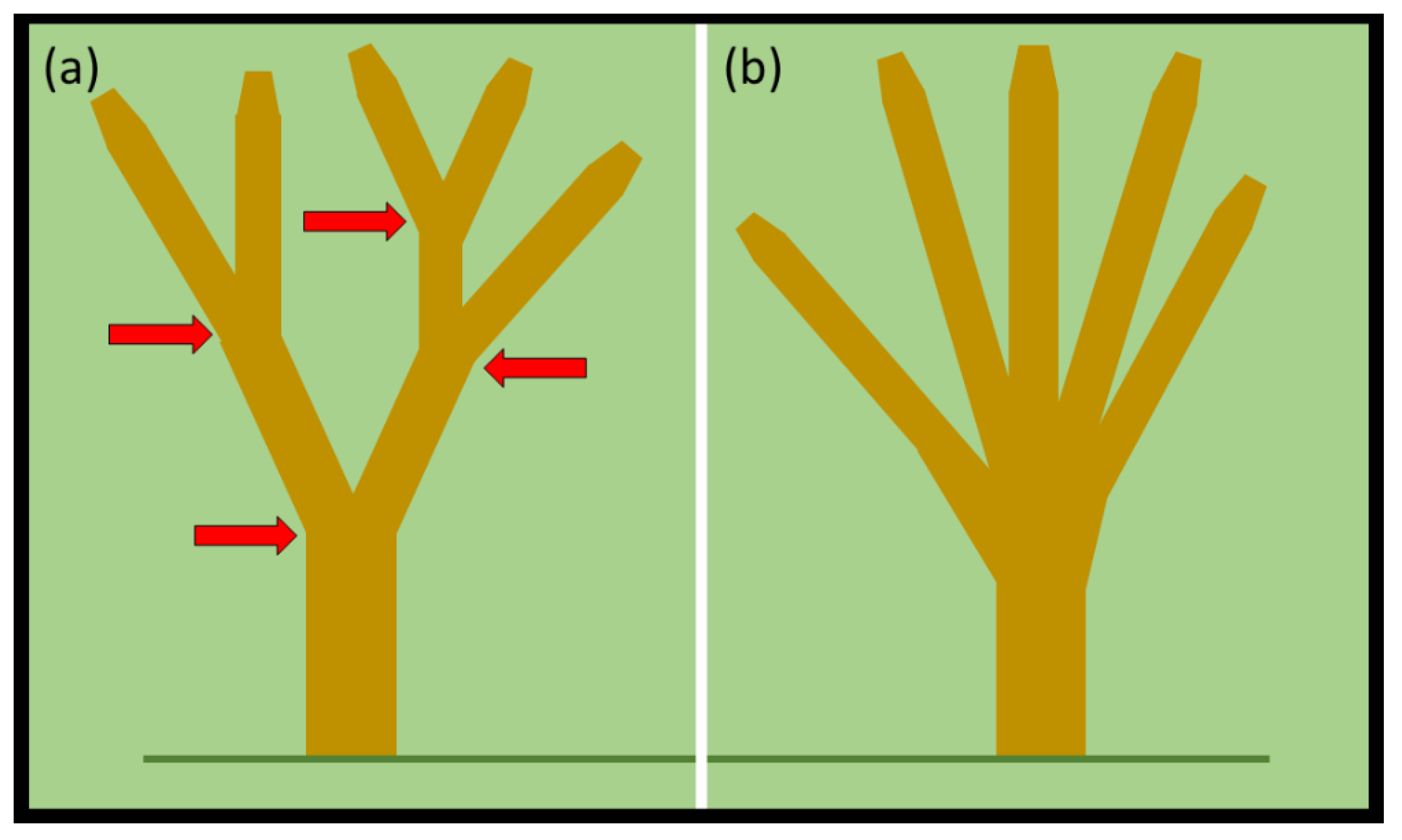
A look at the gestalt appearance of the entire branched
Cycas canopy offers more refined diagnostics by using topology. The first tenet that can be employed to interpret a tree’s canopy is that trees with multiple sites of bifurcation are likely males. Consider two model trees with five apices. One tree exhibits four bifurcation events leading to four stratified dichotomous branching components of the canopy (
Figure 6a). The second tree exhibits five stems that converge to unite at one stratum of the basal stem (
Figure 6b). The first tree is likely a male tree because of the multiple examples of dichotomous branching. The second tree could be either sex because the branching connotes adventitious branching in response to a mechanical injury at the stratum of the manifold branching. The number of
C. micronesica trees that develop a canopy similar to
Figure 6b may increase in the future due to the issues described in
Section 4.1. The stems that have been weakened by non-native insect herbivory may break more often in future TCs such that more trees on average develop regrowth with multiple adventitious branches emerging from one stratum.
A second tenet that may aid in interpretation of branching is that the isotomous branching following a determinate strobilus generates two branches of a similar size, as can be seen in
Figure 1c and
Figure 6a. If a cycad stem reveals branching into two stems, and one secondary stem is much larger than the other second secondary stem, this is not likely the result of a post-strobilus bifurcation event. Sometimes, two equal sized branches may develop from adventitious branching after an injury, a phenomenon referred to as pseudoisotomous branching by Stevenson [
17]. Pseudoisotomous branching is distinguishable macromorphologically from isotomous branching. In isotomous branching, the leaf base pattern is continuous between the stem and the branches and no constriction is observed at the base of the branches, whereas in pseudoisotomous branching, the leaf base pattern is discontinuous and constriction is observed at the base of the branches [
17]. These tenets may be most easily condensed to the simple rule that multiple examples of dichotomous branching on a
Cycas tree likely signifies a male tree and unbranched stems likely signify a female tree. Our observations of
Cycas trees in the field suggest that isotomous branching is most common in male plants, and that it is either very rare or inexistent in female plants. In contrast, isotomous branching was observed on both sexes in
Z. encephalartoides, suggesting that this type of branching may be associated with the capability of producing terminal cones.
4.2. Horticulture and Conservation
Our unambiguous results revealing sexual dimorphism in branch number may be used by horticulturists to inform decisions. Dioecious species offer choices to horticulturists that are not available for monoecious species. For example, if a property owner has a preference for an unbranched
Cycas tree that appears more palm-like (
Figure 1b), the new knowledge that a female tree will meet that preference more often than a male tree can be exploited. Conversely, when considering highly branching, shrub-like geophilous species of
Zamia for landscape design, male plants may make more robust specimens with a higher number of branches, leaves and cones, as demonstrated with
Z. integrifolia by Norstog and Nicholls [
1] (p. 141). Shrub-like
Zamia species are often used in landscape designs as stand-alone specimens or in mass plantings as foreground layers or hedges. This shrub-like habit can be found in several
Zamia species throughout the geographic range of the genus [
32] but is most common in Caribbean species such as
Z. integrifolia, and Mexican species, such as
Z. furfuracea, one of the most abundantly cultivated cycads in the world. Small basal stem cuttings of cycad plants are highly successful in producing adventitious roots [
33,
34,
35]. This knowledge may be exploited to clonally propagate a female or a male tree to obtain the desired landscape objective.
Our results also inform conservation decisions. Cycads form the most threatened group of plants worldwide [
3]. For example,
C. wadei is listed as critically endangered [
36],
C. micronesica is listed as endangered [
37],
Z. encephalartoides is listed as vulnerable [
38], and
C. edentata is listed as near threatened [
39]. Our repeated visits to numerous areas of occupancy for all four of our model species have documented extensive loss of habitat due to anthropogenic land conversion actions.
This is happening in Guam due to expansive construction projects, and federally funded rescue projects have employed the collection of cuttings or excavated transplants of
C. micronesica to rescue some of the genetic diversity of the destroyed populations. Much has been learned about the capture of in situ genetic diversity while collecting for ex situ conservation of cycad species [
40,
41]. The collection of clones instead of seeds improves the conservation of genetic diversity in ex situ collections [
42]. The pilot study for the Guam rescue projects [
43] utilized the knowledge of branch differences to ensure ≈60% of the rescued trees were unbranched, ≈20% contained two apices, and ≈20% contained three or more apices as an approach that would ensure fairly equal representation of male and female trees in the rescued population. In contrast the most recent large-scale salvage project, which employed excavation and transplantation of mature trees, did not utilize this branching knowledge, as the rescued population was heavily represented by specimens with multiple apices (personal observation, T.E.M.). This oversight ensured that most of the rescued population would be male trees, thereby reducing future recruitment and regeneration potential of the restoration site in which the plants were transplanted. These case studies illuminate the need to ensure that appropriate species expertise is guiding the decisions in conservation projects, especially when public funding is involved. Indeed, that male cycad plants produce more stem apices than female plants has been known since the 1980s [
12], so if a cycad biologist had been consulted in the conservation decisions of the transplant project this mistake would not have been made.
The use of stem branching traits to inform cycad conservation decisions adds to a growing body of evidence that illuminates the importance of including conservation practitioners with a verifiable understanding of plant behavior. Other plant traits that have been discussed for use in cycad conservation decisions included stem carbon dioxide efflux [
44] and stem height increment [
45].
4.3. Future Directions
The collective results point toward several areas of needed research. Continued work in these directions would contribute greatly to cycad biology and conservation.
First, long-term observational studies are needed to determine the branching behavior for many cycad species. Although we believe the higher number of branches found on male plants in this study is a consequence of isotomous branching associated with more frequent terminal cone production, this is based on indirect observations, and the relative contribution of adventitious vs. isotomous branching to the stem architecture of these and other cycad species remains obscure. Direct observations over time will provide the data needed to quantify the nature of branching on different cycad species and to better understand the process of stem bifurcations following coning events. The collective data to date include only two
Zamia and three
Cycas species, and little is known about the branching behavior of the remaining 358 species of cycads [
46]. Clearly, observations from the other eight genera and more species are required to improve our understanding of branching processes in cycads, and botanic gardens are ideal settings for this demanding long-term endeavor.
Second, future research should explore the relationship between reproductive anisotomous branching and the isotomous vegetative branching that often follows it and attempt to understand the mechanisms underlying these phenomena. While we observed this in both sexes of Zamia, we only observed it on male Cycas plants. As female Cycas do not form terminal cones, this observation suggests that there may be a link between the ability of cycads to produce terminal cones via anisotomous branching and their ability to produce isotomous vegetative branches. A more detailed survey of female Cycas plants and the plants from the three genera that do not produce terminal cones (Encephalartos, Lepidozamia, and Macrozamia) may confirm whether this is the case. Additionally, anatomical studies are needed in order to understand the mechanism underlying isotomous vegetative branching following cone production. For example, it is unclear if this phenomenon could be the result of trichotomous branching where the apex divides into one fertile and two vegetative apices, or of two dichotomous branching events occurring in quick succession, or of some other unknown mechanism. Careful sectioning of cycad stems undergoing this process could help resolve this conundrum.
Third, our results indicating that the recent invasions of non-native insect herbivores have killed more unbranched trees than multi-branched trees in Guam deserve further study. The results indicate more female trees have been killed than male trees, which will exert negative consequences for species recovery if future conservation interventions become successful in mitigating the ubiquitous biological threats. This disparity in vulnerability to the biological threats is likely mediated by non-structural carbohydrate relations. The greatest threat to the
C. micronesica plants has been the armored scale
Aulacaspis yasumatsui Takagi, and plant mortality from this herbivore is preceded by a gradual depletion of plant non-structural carbohydrates [
47]. Cumulative traditional knowledge that guides the exploitation of cycad stems for the production of starch for human consumption indicates male stems yield more starch than female stems [
48]. These issues collectively indicate the limited pool of non-structural carbohydrates in female trees at the time of the insect invasions may have caused the female trees to be more vulnerable than the male trees. Further studies of stem non-structural carbohydrate relations in male and female trees may improve our understanding of these historical dynamics and inform future conservation decisions for species recovery.
Fourth, illuminating clarity of how subsets of plants inform these issues may improve the predictive usage of the information in horticulture. For example, our three
Cycas arborescent species exhibited less difference in total number of branches between male and female plants than the
Zamia species with partly subterranean stems. Do the male-female differences in branching behave differently based on life form, rather than based on taxonomy, such that the arborescent species as a group behave differently than the subterranean species as a group? Moreover, most of our female
Cycas individuals were unbranched. Do the indeterminate traits of
Cycas megastrobili versus the determinate traits of the megastrobili of the other arborescent genera fully explain the increased proportion of monopodial
Cycas individuals? For arborescent species, tree height increases with the number of primary growth events which accumulate with age [
1]. Does tree height correlate positively with the number of branches for all species?