Impact of Different Growing Substrates on Growth, Yield and Cannabinoid Content of Two Cannabis sativa L. Genotypes in a Pot Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Plant Material
2.3. Measurements
2.4. Plant Samples
2.5. Extraction and Quantification of Cannabinoids by HPLC Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fournier, G.; Richez-Dumanois, C.; Duvezin, J.; Mathieu, J.-P.; Paris, M. Identification of a New Chemotype in Cannabis sativa: Cannabigerol—Dominant Plants, Biogenetic and Agronomic Prospects. Planta Med. 1987, 53, 277–280. [Google Scholar] [CrossRef] [PubMed]
- de Meijer, E. The Chemical Phenotypes (Chemotypes) of Cannabis. In Handbook of Cannabis; Oxford University Press: Oxford, UK, 2014; pp. 89–110. [Google Scholar]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids. Progress in the Chemestry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbson, S., Kobayashi, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 103, pp. 1–36. [Google Scholar]
- Mandolino, G.; Carboni, A. Potential of marker-assisted selection in hemp genetic improvement. Euphytica 2004, 140, 107–120. [Google Scholar] [CrossRef]
- Pacifico, D.; Miselli, F.; Carboni, A.; Moschella, A.; Mandolino, G. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 2008, 160, 231–240. [Google Scholar] [CrossRef]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Tavčar Benković, E. Cannabinoid content in industrial hemp (Cannabis sativa L.) varieties grown in Slovenia. Ind. Crops Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Campbell, B.J.; Berrada, A.F.; Hudalla, C.; Amaducci, S.; McKay, J.K. Genotype × Environment Interactions of Industrial Hemp Cultivars Highlight Diverse Responses to Environmental Factors. Agrosyst. Geosci. Environ. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Laginha, A.M. High-Tech-Anbau von Cannabis. Dtsch. Apoth. Ztg. 2018, 158, 36–43. [Google Scholar]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef]
- Bunt, A.C. Media and Mixes for Container-Growth Plants; Unwin Hyman: London, UK, 1988. [Google Scholar]
- Nelson, P.V. Greenhouse Operation & Mangement; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Evans, M.R.; Gachukia, M. Fresh Parboiled Rice Hulls Serve as an Alternative to Perlite in Greenhouse Crop Substrates. HortScience 2004, 39, 232–235. [Google Scholar] [CrossRef]
- Raviv, M.; Chen, Y.; Inbar, Y. The use of peat and composts as growth media for container-grown plants. In The Role of Organic Matter in Modern Agriculture; Chen, Y., Avnimelech, Y., Eds.; Martinus Nijhoff Publishers: Dordrecht, Germany, 1986; pp. 257–287. [Google Scholar]
- Barkham, J.P. For peat’s sake: Conservation or exploitation? Biodivers. Conserv. 1993, 2, 556–566. [Google Scholar] [CrossRef]
- Robertson, R.A. Peat, horticulture and environment. Biodivers. Conserv. 1993, 2, 541–547. [Google Scholar] [CrossRef]
- Blok, C.; Verhagen, J.B.G.M. Trends in rooting media in dutch horticulture during the period 2001–2005: The new growing media project. Acta Hortic. 2009, 819, 47–58. [Google Scholar] [CrossRef]
- Fonteno, W.C. Problems & considerations in determining physical properties of horticultural substrates. In International Symposium on Horticultural Substrates Other than Soil In Situ; Acta Horticulturae: Leuven, Belgium, 1993; Volume 342, pp. 197–204. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Bar-Tal, A. Substrates and their Analysis. In Hydroponic Prod. Veg. Ornam; Sawas, D., Passam, H., Eds.; Embryo Publications: Athens, Greece, 2002; pp. 25–105. [Google Scholar]
- Ritz, K.; Corstanje, R.; Deeks, L. Final Report; Department for Environment, Food and Rural Affairs: London, UK, 2010. [Google Scholar]
- Schmilewski, G. The role of peat in assuring the quality of growing media. Mires Peat 2008, 3, 1–8. [Google Scholar]
- Krucker, M.; Hummel, R.L.; Cogger, C. Chrysanthemum production in composted and noncomposted organic waste substrates fertilized with nitrogen at two rates using surface and subirrigation. HortScience 2010, 45, 1695–1701. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Evans, M.; Stamps, R. Growth of bedding plants in sphagnum peat and coir dust-based substrates. J. Environ. Hortic. 1996, 14, 187–190. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Holzfasersubstrate als eine torfalternative für die gemüseproduktion. Holz Roh Werkst. 2006, 64, 347–350. [Google Scholar] [CrossRef]
- Maher, M.; Prasad, M.; Raviv, M. Organic soilless media components. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2008; pp. 459–504. [Google Scholar]
- Rodriguez, I.R.; Miller, G.L. Using a chlorophyll meter to determine the chlorophyll concentration, nitrogen concentration, and visual quality of St. Augustinegrass. HortScience 2000, 35, 751–754. [Google Scholar] [CrossRef]
- Burgel, L.; Hartung, J.; Schibano, D.; Graeff-Hönninger, S. Impact of Different Phytohormones on Morphology, Yield and Cannabinoid Content of Cannabis sativa L. Plants 2020, 9, 725. [Google Scholar] [CrossRef]
- Lehmann, T.; Brenneisen, R. High Performance Liquid Chromatographic Profiling of Cannabis Products. J. Liq. Chromatogr. 1995, 18, 689–700. [Google Scholar] [CrossRef]
- Burgel, L.; Hartung, J.; Pflugfelder, A.; Graeff-Hönninger, S. Impact of growth stage and biomass fractions on cannabinoid content and yield of different hemp (Cannabis sativa L.) genotypes. Agronomy 2020, 10, 372. [Google Scholar] [CrossRef]
- Wolfinger, R. Covariance structure selection in general mixed models. Commun. Stat. Simul. Comput. 1993, 22, 1079–1106. [Google Scholar] [CrossRef]
- Piepho, H.P. An algorithm for a letter-based representation of all-pairwise comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Abad, M.; Fornes, F.; Carrión, C.; Noguera, V.; Noguera, P.; Maquieira, Á.; Puchades, R. Physical properties of various coconut coir dusts compared to peat. HortScience 2005, 40, 2138–2144. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrate for production of vegetable transplants: I. Physical properties of wood fiber substrates. Sci. Hortic. 2004, 100, 309–322. [Google Scholar] [CrossRef]
- Domeño, I.; Irigoyen, I.; Muro, J. New wood fibre substrates characterization and evaluation in hydroponic tomato culture. Eur. J. Hortic. Sci. 2010, 75, 89–94. [Google Scholar]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J. 2012, 71, 353–365. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Coir-based growing substrates for indoor cannabis production. Acta Hortic. 2019, 1266, 55–61. [Google Scholar] [CrossRef]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High added-value compounds from Cannabis threshing residues. Ind. Crops Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
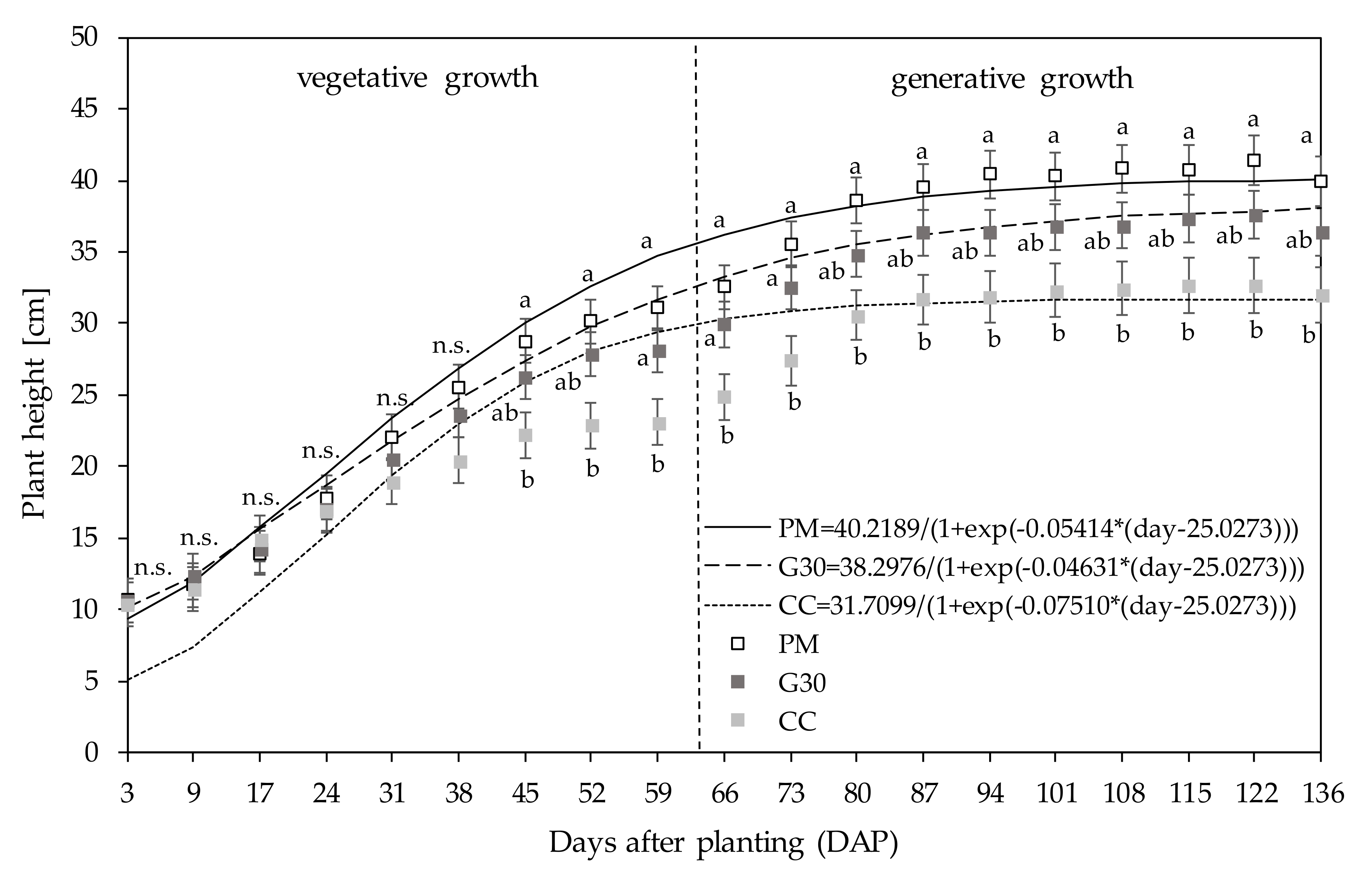
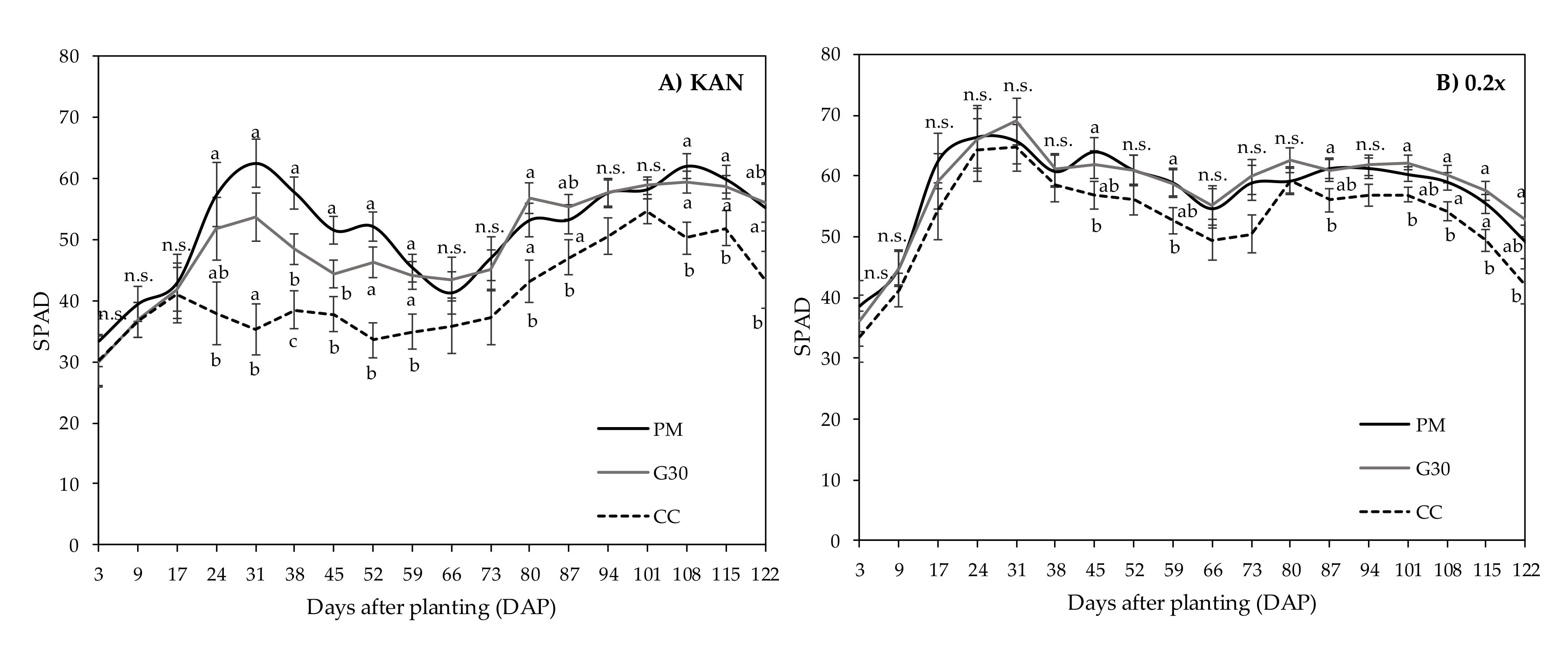
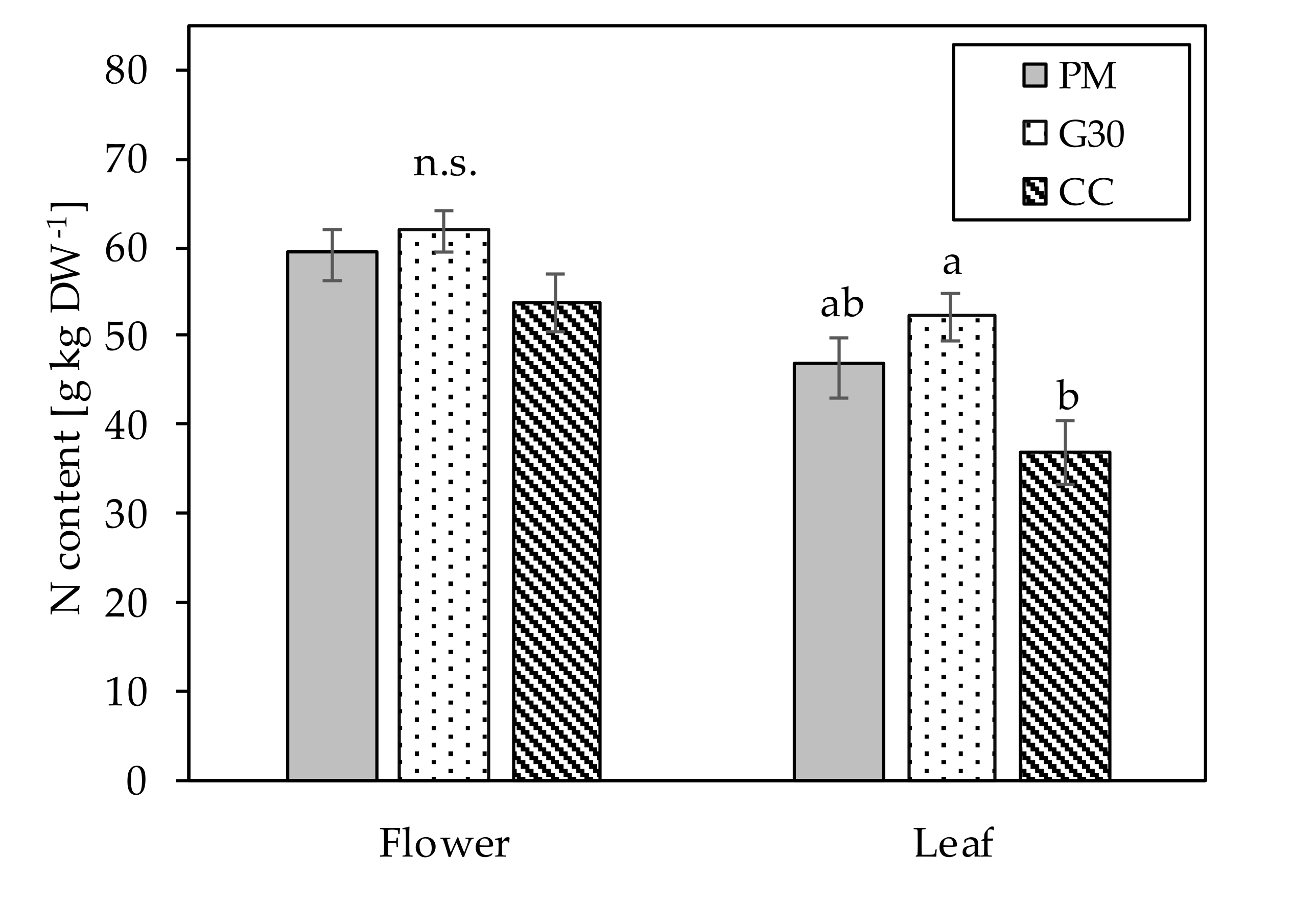

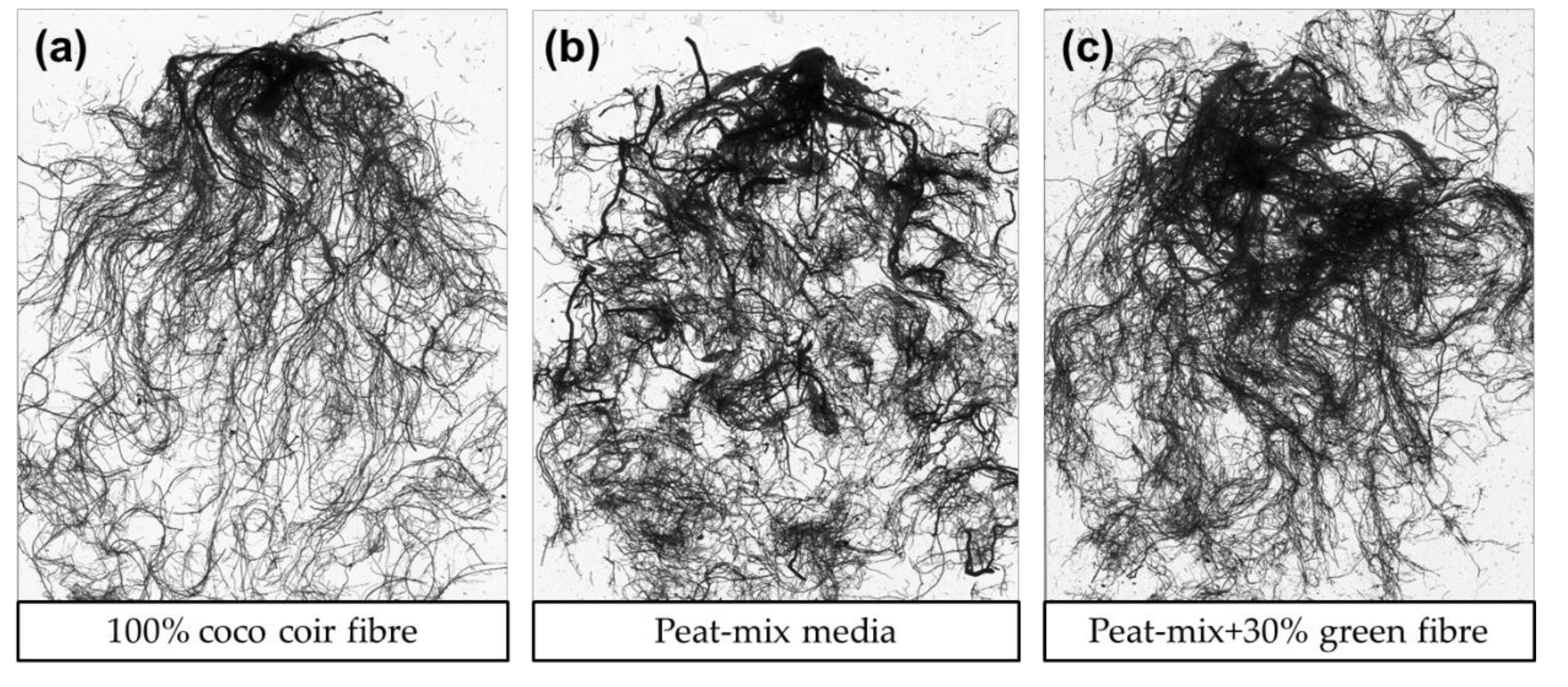
| DAP (Pot Size in cm) | PM | G30 | CC |
|---|---|---|---|
| 1-35 (9) | 70% milled peat 15% sod cut peat fraction 1 15% perlite | 70% milled peat 15% sod cut peat fraction 1 15% perlite | 100% coco coir fibre |
| 36-70 (12) | 45% sod cut peat fraction 2 40% milled peat medium 15% peat fibre | 45% sod cut peat fraction 2 25% milled peat medium 30% green fibre | 100% coco coir fibre |
| 71-154 (15) | 55% sod cut peat fraction 3 15% milled peat 30% green fibre | 55% sod cut peat fraction 3 15% milled peat 30% green fibre | 100% coco coir fibre |
| p-Values | Parameter s | Parameter max | Parameter day0 |
|---|---|---|---|
| Genotype [G] | 0.0280 | 0.1802 | 0.0002 |
| Substrate [S] | 0.0209 | 0.0294 | 0.5794 |
| G × S Interaction | 0.1155 | 0.2382 | 0.4690 |
| Trait | Substrate | Genotype | |
|---|---|---|---|
| KAN | 0.2x | ||
| Flower DW [g plant−1] | PM | 8.56 ± 0.74 aA | 8.68 ± 0.94 aA |
| G30 | 4.94 ± 0.66 bB | 9.19 ± 0.94 aA | |
| CC | 3.84 ± 0.74 bB | 7.90 ± 0.94 aA | |
| p-values | |||
| Genotype [G] | 0.0002 | ||
| Substrate [S] | 0.0081 | ||
| G S Interaction | 0.0251 | ||
| Trait | Substrate | Genotype |
|---|---|---|
| Leaf DW [g plant−1] | PM | 5.78 ± 0.47 a |
| G30 | 5.66 ± 0.44 a | |
| CC | 3.30 ± 0.47 b | |
| RLD [cm cm−3] | PM | 4.32 ± 0.48 ab |
| G30 | 4.63 ± 0.46 a | |
| CC | 3.09 ± 0.48 b | |
| p-values Leaf | ||
| Genotype [G] | 0.0002 | |
| Substrate [S] | 0.0010 | |
| G × S Interaction | 0.2994 | |
| p-values RLD | ||
| Genotype [G] | <0.0001 | |
| Substrate [S] | 0.0279 | |
| G × S Interaction | 0.4819 | |
| Trait | Genotype | |
|---|---|---|
| KAN | 0.2x | |
| Root DW [g plant−1] | 0.55 ± 0.10 b | 1.52 ± 0.09 a |
| Flower CBD/A [mg plant−1] | 335.28 ± 98.67 b | 549.66 ± 79.25 a |
| Leaves CBD/A [mg plant−1] | 135.91 ± 24.02 b | 224.16 ± 18.63 a |
| p-values Root | ||
| Genotype [G] | <0.0001 | |
| Substrate [S] | 0.1479 | |
| G S Interaction | 0.2398 | |
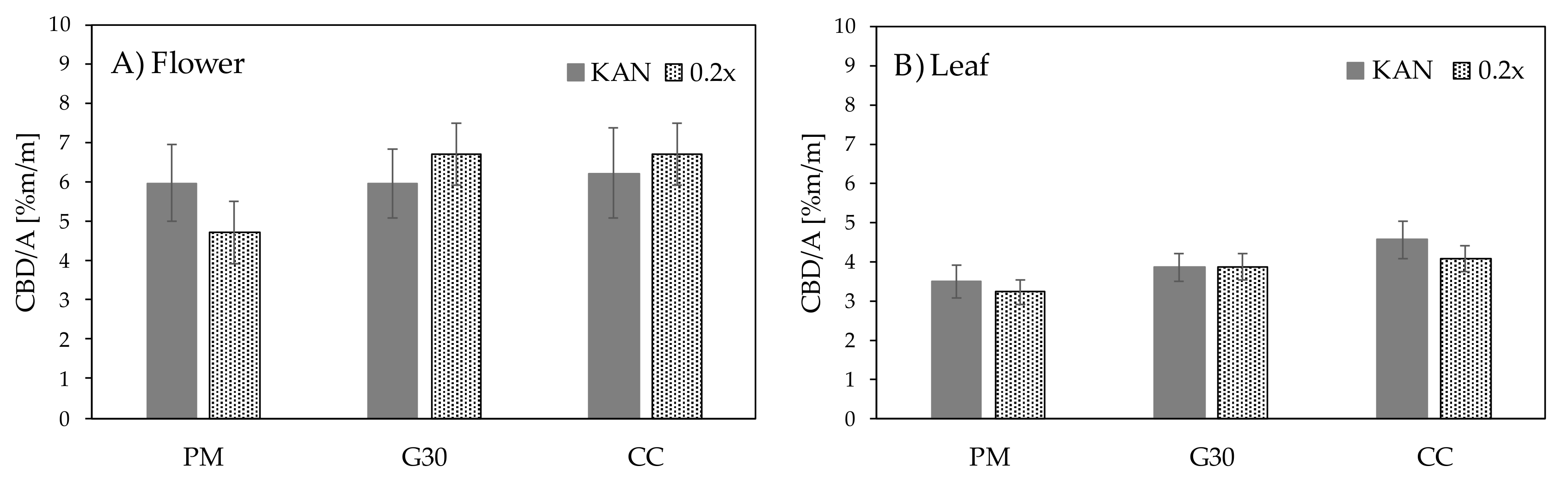
| p-values Flower | ||
| Genotype [G] | 0.0412 | |
| Substrate [S] | 0.7144 | |
| G S Interaction | 0.0937 | |
| p-values Leaves | ||
| Genotype [G] | 0.005 | |
| Substrate [S] | 0.057 | |
| G S Interaction | 0.3947 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgel, L.; Hartung, J.; Graeff-Hönninger, S. Impact of Different Growing Substrates on Growth, Yield and Cannabinoid Content of Two Cannabis sativa L. Genotypes in a Pot Culture. Horticulturae 2020, 6, 62. https://doi.org/10.3390/horticulturae6040062
Burgel L, Hartung J, Graeff-Hönninger S. Impact of Different Growing Substrates on Growth, Yield and Cannabinoid Content of Two Cannabis sativa L. Genotypes in a Pot Culture. Horticulturae. 2020; 6(4):62. https://doi.org/10.3390/horticulturae6040062
Chicago/Turabian StyleBurgel, Lisa, Jens Hartung, and Simone Graeff-Hönninger. 2020. "Impact of Different Growing Substrates on Growth, Yield and Cannabinoid Content of Two Cannabis sativa L. Genotypes in a Pot Culture" Horticulturae 6, no. 4: 62. https://doi.org/10.3390/horticulturae6040062
APA StyleBurgel, L., Hartung, J., & Graeff-Hönninger, S. (2020). Impact of Different Growing Substrates on Growth, Yield and Cannabinoid Content of Two Cannabis sativa L. Genotypes in a Pot Culture. Horticulturae, 6(4), 62. https://doi.org/10.3390/horticulturae6040062





