Soil-Biodegradable Plastic Mulches Undergo Minimal in-Soil Degradation in a Perennial Raspberry System after 18 Months
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Design
2.2. Weather and Soil Characteristics
2.3. Field History, Mulch Laying, and Field Maintenance
2.4. Mulch Deterioration
2.5. Mesh Bag Samples and Burial
2.6. Mesh Bag Collection, Cleaning, and Imaging
2.7. Statistical Analysis
3. Results
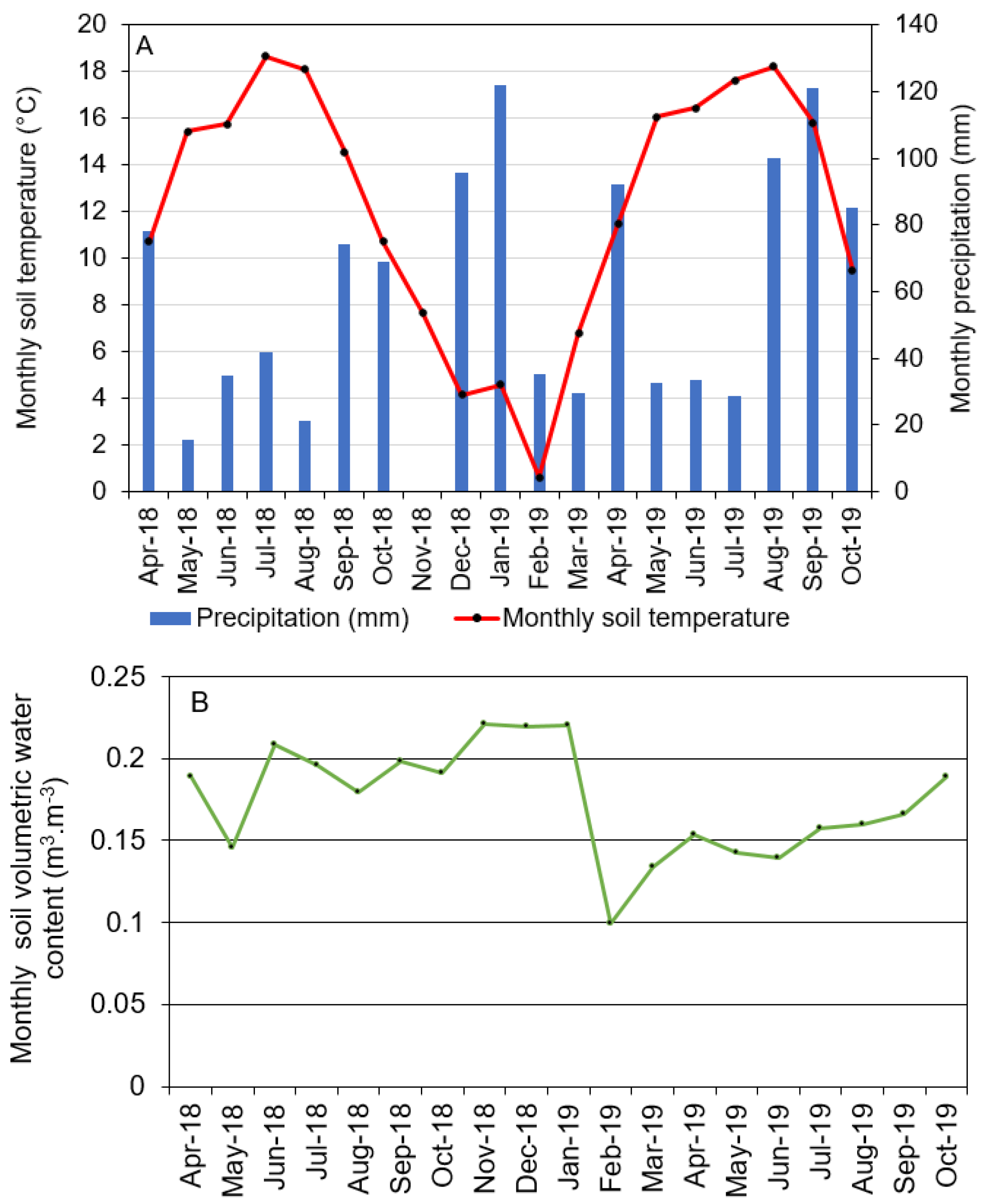
3.1. Environmental Data
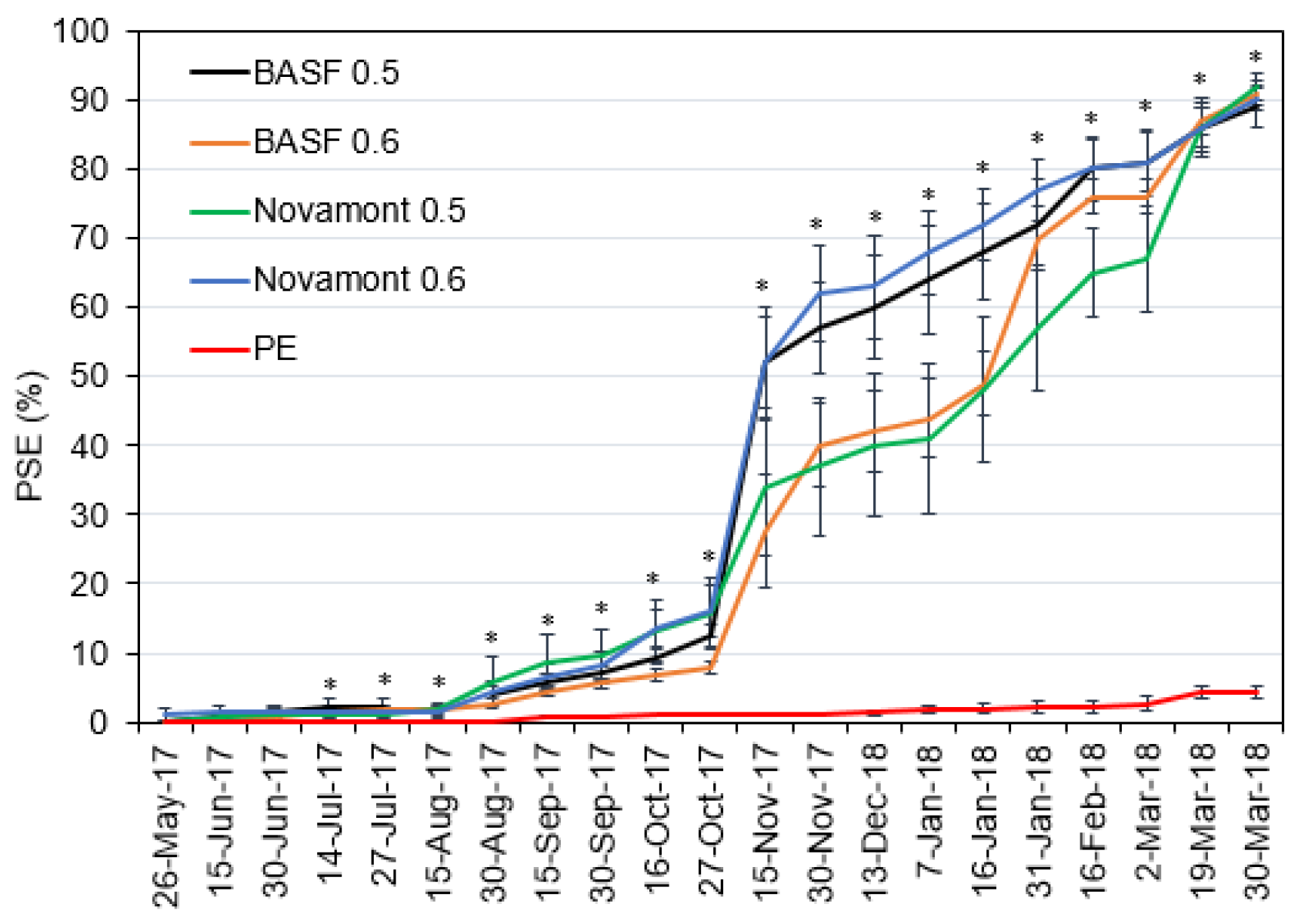
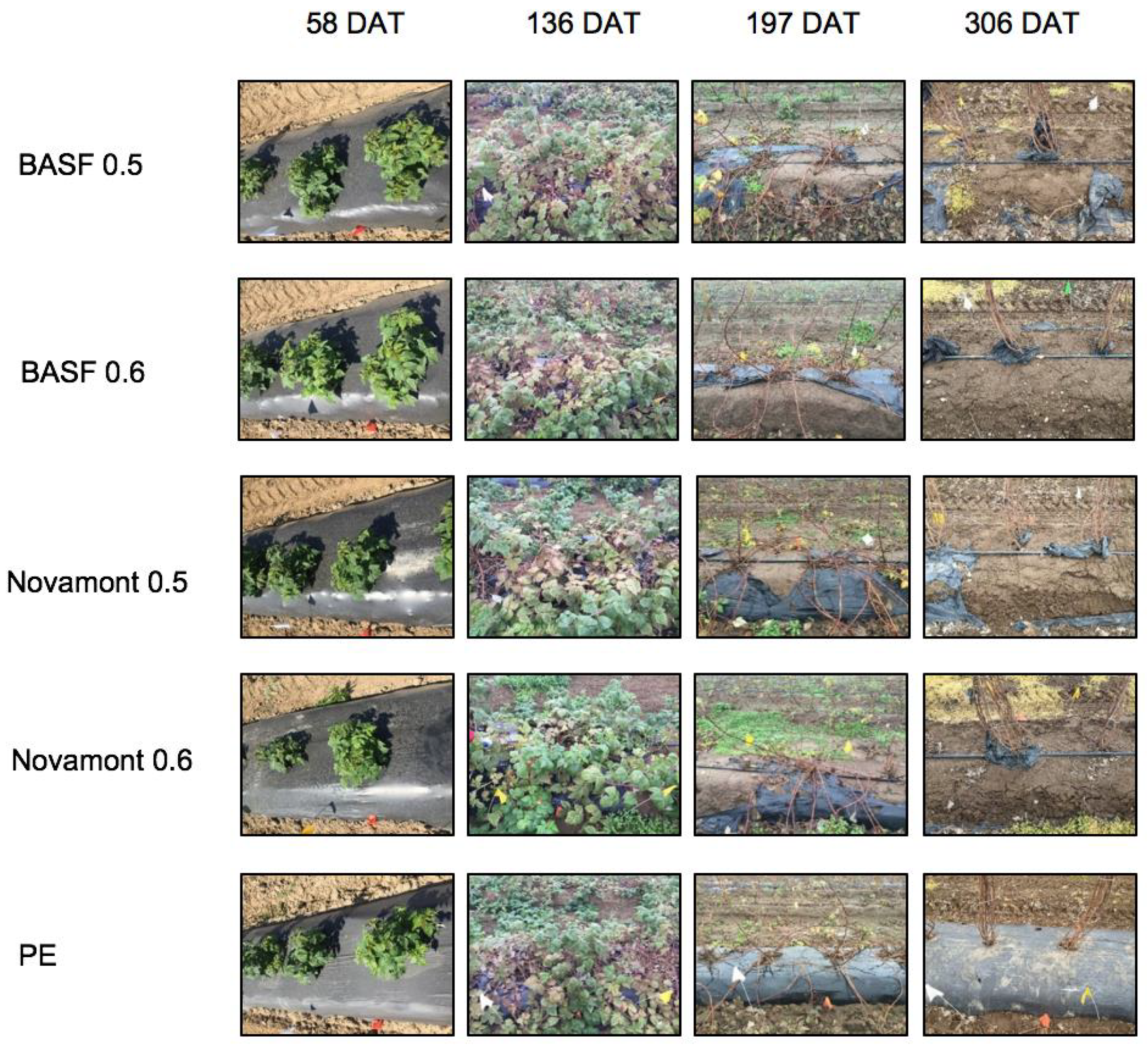
3.2. Mulch Deterioration
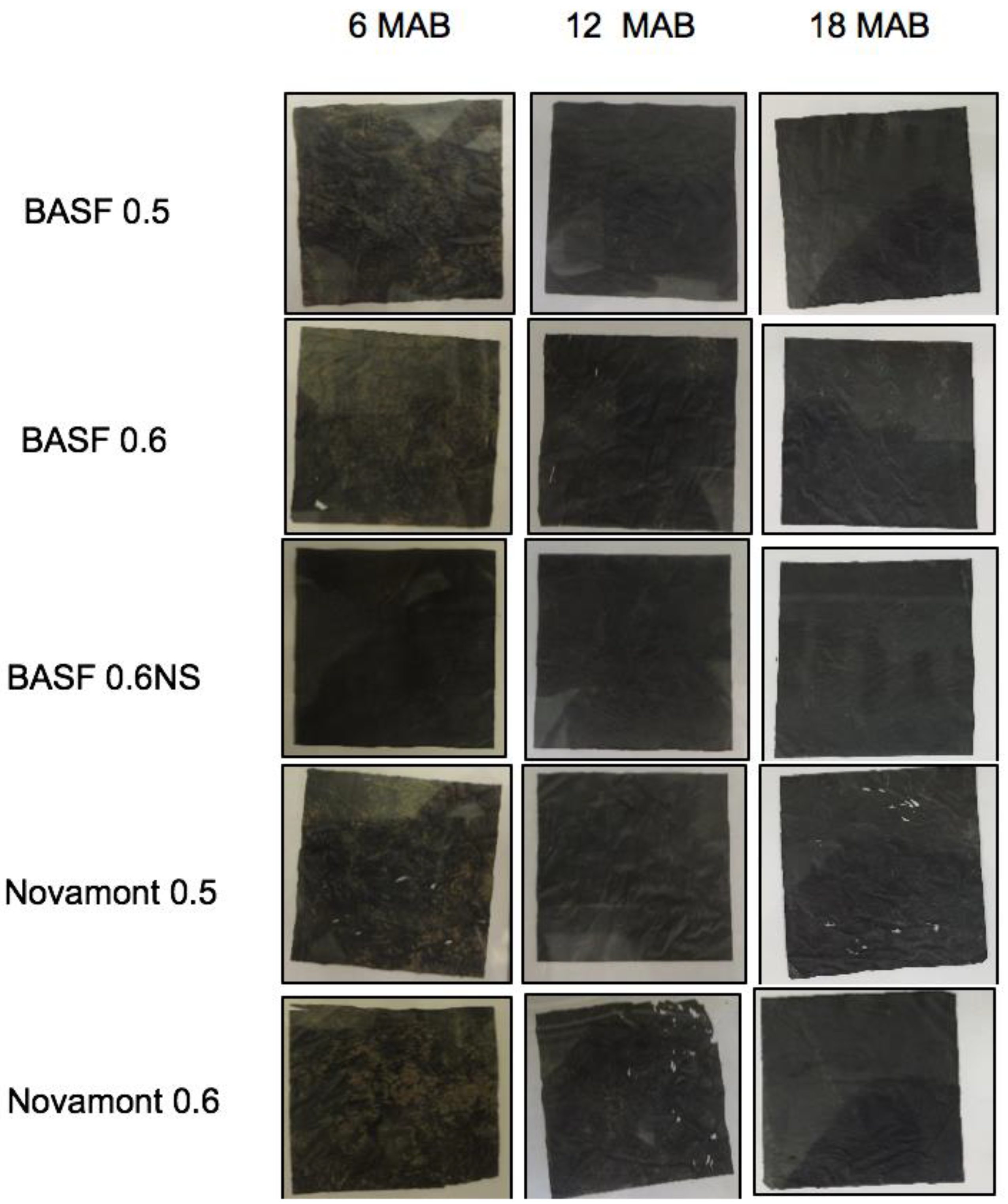
3.3. Mesh Bag Mulch Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Transparency Market Research Analysis. Agricultural Films Market. 2019. Available online: https://www.transparencymarketresearch.com/mulch-films-market.html (accessed on 15 March 2020).
- Liu, E.K.; He, H.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’: Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- MarketsandMarkets. Agricultural Films Market by Applications and Polymers—Global Trends and Forecasts to 2022; MarketsandMarkets Research Private Ltd.: Maharashtra, India, 2017. [Google Scholar]
- Briassoulis, D.; Hiskakis, M.; Babou, E.; Antiohos, S.K.; Papadi, C. Experimental investigation of the quality characteristics of agricultural plastic wastes regarding their recycling and energy recovery potential. Waste Manag. 2012, 32, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E. How can Agriculture Solve Its $5.87 Billion Plastic Problem? 2015. Available online: https://www.greenbiz.com/article/how-can-agriculture-solve-its-1-billion-plastic-problem (accessed on 17 June 2015).
- Miles, C.; DeVetter, L.; Ghimire, S.; Hayes, D.G. Suitability of biodegradable plastic mulches for organic and sustainable agricultural production systems. HortScience 2017, 52, 10–15. [Google Scholar] [CrossRef]
- Ohtake, Y.; Kobayashi, T.; Asabe, H.; Murakami, N. Studies on biodegradation of LDPE-observation of LDPE films scattered in agricultural fields or in garden soil. Polym. Degrad. Stab. 1998, 60, 79–84. [Google Scholar] [CrossRef]
- Goldberger, J.R.; Jones, R.E.; Miles, C.A.; Wallace, R.W.; Inglis, D.A. Barriers and bridges to the adoption of biodegradable plastic mulches for US specialty crop production. Renew. Agric. Food Syst. 2015, 30, 143–153. [Google Scholar] [CrossRef]
- Chang-Rong, Y.; En-Ke, L.; Fan, S.; Liu, Q.; Liu, S.; Wen-Qing, H. Review of agricultural plastic mulching and its residual pollution and prevention measures in China. JARE 2014, 31, 95. [Google Scholar]
- European Norms (EN). Plastics–Biodegradable Mulch Films for Use in Agriculture and Horticulture–Requirements and Test Methods. European Standard 17033; European Committee for Standardization: Brussels, Belgium, 2018. [Google Scholar]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. [Google Scholar] [CrossRef]
- Andrady, A.L. Assessment of environmental biodegradation of synthetic polymers. Macromol. J. Sci. Rev. Macromol. Chem. Phys. 1994, 3434, 25–76. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Hayes, D.G.; Wadsworth, L.C.; Sintim, H.Y.; Flury, M.; English, M.; Schaeffer, S.; Saxton, A.M. Effect of diverse weathering conditions on the physicochemical properties of biodegradable plastic mulches. Polym. Test. 2017, 62, 454–467. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Wiles, D.M. Oxo-biodegradable polymers: Present status and future perspectives. In Handbook of Biodegradable Polymers: Synthesis, Characterization and Applications; Lendlein, A., Sisson, A., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2011; pp. 379–398. [Google Scholar]
- American Society for Testing and Materials (ASTM) D5988-18. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Cowan, J.S.; Saxton, A.; Liu, H.; Leonas, K.; Inglis, D.; Miles, C. Visual assessments of biodegradable mulch deterioration are not indicative of mulch degradation. HortScience 2016, 51, 245–254. [Google Scholar] [CrossRef]
- DeVetter, L.W.; Zhang, H.; Ghimire, S.; Watkinson, S.; Miles, C.A. Plastic biodegradable mulches reduce weeds and promote crop growth in day-neutral strawberry in western Washington. HortScience 2017, 52, 1700–1706. [Google Scholar] [CrossRef]
- Ghimire, S.; Wszelaki, A.L.; Moore, J.C.; Inglis, D.A.; Miles, C. The use of biodegradable mulches in pie pumpkin crop production in two diverse climates. HortScience 2018, 53, 288–294. [Google Scholar] [CrossRef]
- Miles, C.; Wallace, R.; Wszelaki, A.; Martin, J.; Cowan, J.; Walters, T.; Inglis, D. Deterioration of potentially biodegradable alternatives to black plastic mulch in three tomato production regions. HortScience 2012, 47, 1270–1277. [Google Scholar] [CrossRef]
- Moreno, M.M.; González-Mora, S.; Villena, J.; Campos, J.A.; Moreno, C. Deterioration pattern of six biodegradable, potentially low-environmental impact mulches in field conditions. J. Environ. Manag. 2017, 200, 490–501. [Google Scholar] [CrossRef]
- Zhang, H.; Miles, C.; Ghimire, S.; Benedict, C.; Zasada, I.; Liu, H.; DeVetter, L.W. Plastic mulches improved plant growth and suppressed weeds in late summer-planted floricane-fruiting raspberry. HortScience 2020, 55, 565–572. [Google Scholar] [CrossRef]
- Calmon, A.; Guillaume, S.; Bellon-Maurel, V.; Feuilloley, P.; Silvestre, F. Evaluation of material biodegradability in real conditions–development of a burial test and an analysis methodology based on numerical vision. J. Polym. Environ. 1999, 7, 157–166. [Google Scholar] [CrossRef]
- Li, C.; Moore-Kucera, J.; Miles, C.; Leonas, K.; Lee, J.; Corbin, A.; Inglis, D. Degradation of potentially biodegradable plastic mulch films at three diverse U.S. locations. Agroecol. Sustain. Food Syst. 2014, 38, 861–889. [Google Scholar] [CrossRef]
- Tachibana, Y.; Maeda, T.; Ito, O.; Maeda, Y.; Kunioka, M. Utilization of a biodegradable mulch sheet produced from poly(lactic acid)/Ecoflex®/modified starch in Mandarin orange groves. Int. J. Mol. Sci. 2009, 10, 3599–3615. [Google Scholar] [CrossRef]
- Zhang, H.; Miles, C.; Ghimire, S.; Benedict, C.; Zasada, I.; DeVetter, L. Polyethylene and biodegradable plastic mulches improve growth, yield, and weed management in floricane red raspberry. Sci. Hortic. 2019, 250, 371–379. [Google Scholar] [CrossRef]
- Goldin, A. Soil Survey of Whatcom County Area, Washington; United States Department of Agriculture, Soil Conservation Service: Washington, DC, USA, 1992.
- U.S. Department of Agriculture. The National List of Allowed and Prohibited Substances. U.S. Department of Agriculture Washington, DC. 2020. Available online: http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&SID=9874504b6f1025eb0e6b67cadf9d3b40&rgn=div6&view=text&node=7:3.1.1.9.32.7&idno=7#sg7.3.205.g.sg0 (accessed on 29 March 2020).
- Hayes, D.G.; Dharmalingam, S.; Wadsworth, L.C.; Leonas, K.K.; Miles, C.; Inglis, D.A. Biodegradable agricultural mulches derived from biopolymers. In Degradable Polymers and Materials: Principles and Practice, 2nd ed.; Khemani, K., Scholz, C., Eds.; American Chemical Society. Oxford Univ. Press, Inc.: Oxford, UK, 2012; pp. 201–223. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- ASTM D5305-11. Standard Test Method for Breaking Force and Elongation of Textile Fabrics (Strip Method); ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Feuilloley, P.; Cesar, G.; Benguigui, L.; Grohens, Y.; Pillin, I.; Bewa, H.; Lefaux, S.; Jamal, M. Degradation of polyethylene designed for agricultural purposes. J. Polym. Environ. 2005, 13, 349–355. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; English, M.E.; Sean, M.; Miles, C.A.; Flury, M. Release of micro- and nanoparticles from biodegradable plastic during in situ composting. Sci. Total Environ. 2019, 675, 686–693. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ. U.S. National Institutes of Health, Bethesda, Maryland. Available online: http://www.imagej.nih.gov/ij2014 (accessed on 15 December 2019).
- DeVetter, L.W.; Strik, B.C.; Moore, P.; Finn, C.; Dossett, M.; Sagili, R.; Miller, T.; Benedict, C.; Bryla, D.R.; Zasada, I.; et al. Commercial Red Raspberry Production in the Pacific Northwest; Washington State University Extension Publishing: Pullman, WA, USA, 2020. [Google Scholar]
- Dharmalingam, S.; Hayes, D.G.; Wadsworth, L.C.; Dunlap, R.N. Analysis of the time course of degradation for fully biobased nonwoven agricultural mulches in compost-enriched soil. Text. Res. J. 2016, 86, 1343–1355. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.; x Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Oliveira, J.E. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef]
- Thompson, A.A.; Samuelson, M.B.; Kadoma, I.; Soto-Cantu, E.; Drijber, R.; Wortman, S.E. Degradation rate of bio-based agricultural mulch is influenced by mulch composition and biostimulant application. J. Polym. Environ. 2019, 27, 498–509. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.; Hayes, D.; Wadsworth, L.; Anunciado, M.; English, M.; Bandopadhyay, S.; Schaeffer, S.; DeBruyn, J.; Miles, C.; et al. In Situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, L.; Zhang, M.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of P(3HB,4HB)/PLA blends in real soil environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, E.; Briassoulis, D. Comparative biodegradation in soil behavior of two biodegradable polymers based on renewable resources. J. Polym. Environ. 2011, 19, 18–39. [Google Scholar] [CrossRef]
- Rudnik, E.; Briassoulis, D. Degradation behavior of poly(lactic acid) films and fibers in soil under Mediterranean field conditions and laboratory simulations testing. Ind. Cros. Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and hydrolysis rate of aliphatic aromatic polyesters. Polym. Degr. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]
- Müller, R.J.; Kleeberg, I.; Deckwer, W.D. Biodegradation of polyesters containing aromatic constituents. J. Biotech. 2001, 86, 87–95. [Google Scholar] [CrossRef]
- Saadi, Z.; Cesar, G.; Bewa, H. Fungal degradation of poly(butylene adipate-co-terephthalate) in soil, and in compost. J. Polym. Environ. 2013, 21, 893–901. [Google Scholar] [CrossRef]
- Jarerat, A.; Tokiwa, Y.; Tanaka, H. Production of poly(L-lactide)-degrading enzyme by Amycolatopsis orientalis for biological recycling of poly(L-lactide). Appl. Microbiol. Biotechnol. 2006, 72, 726–731. [Google Scholar] [CrossRef]
- Mellon, J.E.; Cotty, P.J.; Dowd, M.K. Aspergillus flavus hydrolases: Their roles in pathogenesis and substrate utilization. Appl. Microbiol. Biotechnol. 2007, 77, 497–504. [Google Scholar] [CrossRef]
- Moore-Kucera, J.; Karich, K.; Inglis, D.A.; Miles, C.; Kinloch, K.; Bailes, G.; Brodhagen, M. Native soil fungi associated with compostable plastics in three contrasting agricultural settings. Appl. Microbiol. Biotechnol. 2014, 98, 6467–6485. [Google Scholar] [CrossRef]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]
- DeVetter, L.W.; Watkinson, S.; Zasada, I.A.; Weiland, J.E.; Hesse, C.; Walters, T.W. Effectiveness of nontarped broadcast fumigation and root removal on root lesion nematode and Fusarium and Pythium species in a red raspberry system. Plant Health Prog. 2018, 19, 168–175. [Google Scholar] [CrossRef]
- Rudolph, R.E.; Zasada, I.A.; Hesse, C.; DeVetter, L.W. Brassicaceous seed meal, root removal, and chemical fumigation vary in their effects on soil quality parameters and Pratylenchus penetrans in a replanted floricane raspberry production system. Appl. Soil Ecol. 2019, 133, 44–51. [Google Scholar] [CrossRef]
| Mulch Product z | Thickness (μm) | Converter | Key Product Ingredient(s) y |
|---|---|---|---|
| BASF 0.5 | 12.7 | Organix Solutions, Bloomington, MN, USA | PLA + PBAT z |
| BASF 0.6 | 15.2 | Organix Solutions, Bloomington, MN, USA | PLA + PBAT |
| Novamont 0.5 | 12.7 | PolyExpert Inc., Laval, QC, Canada | Starch-based, PBAT copolyester |
| Novamont 0.6 | 15.2 | PolyExpert Inc., Laval, QC, Canada | Starch-based, PBAT copolyester |
| PE | 25.4 | FilmTech, LLC., Stanley, WI, USA | Polyethylene |
| Environmental Variables z | April–October 2018 | November 2018–March 2019 | April–October 2019 |
|---|---|---|---|
| Average daily air temperature (°C) | 14.4 | 5.2 | 14.3 |
| Average daily maximum air temperature (°C) | 20.5 | 10.5 | 19.8 |
| Average daily minimum air temperature (°C) | 8.8 | 1.0 | 9.2 |
| Total solar radiation (MJ/m2) | 3278 | 891 | 2889 |
| Relative humidity (%) | 84 | 84 | 77 |
| Total rainfall (mm) | 335 | 351 | 493 |
| Machine Direction | Transverse Direction | |||
|---|---|---|---|---|
| Treatment | Elongation (%) | Breaking Force (N) | Elongation (%) | Breaking Force (N) |
| BASF 0.5 | 9.9 ± 0.2 b z | 4.9 ± 0.6 ab | 9.5 ± 0.6 b | 3.1 ± 0.5 b |
| BASF 0.6 | 12.4 ± 0.9 b | 6.4 ± 0.6 a | 12.5 ± 0.8 b | 4.8 ± 0.4 a |
| Novamont 0.5 | 10.0 ± 1.0 b | 2.5 ± 0.4 b | 8.1 ± 0.3 b | 1.5 ± 0.1 c |
| Novamont 0.6 | 12.4 ± 4.1 b | 2.9 ± 0.5 b | 12.2 ± 0.9 b | 3.0 ± 0.2 b |
| PE | 174.8 ± 21.2 a | 7.7 ± 0.2 a | 261.4 ± 51.2 a | 5.6 ± 0.2 a |
| p-value | <0.0001 | 0.002 | <0.0001 | <0.0001 |
| Mulch area (%) | ||||
|---|---|---|---|---|
| Treatment z | 0 MAB y | 6 MAB | 12 MAB | 18 MAB |
| BASF 0.5 | 100.0 ± 0 x | 95.6 ± 0.8 | 89.3 ± 1.9 | 92.0 ± 1.7 |
| BASF 0.6 | 100.0 ± 0 | 96.7 ± 0.7 | 96.4 ± 1.8 | 93.7 ± 1.1 |
| BASF 0.6NS | 100.0 ± 0 | 96.4± 0.7 | 95.1± 1.3 | 92.0± 2.0 |
| Novamont 0.5 | 100.0 ± 0 | 97.6 ± 0.3 | 82.3 ± 7.0 | 87.1 ± 4.1 |
| Novamont 0.6 | 100.0 ± 0 | 96.0 ± 1.9 | 84.9 ± 5.6 | 88.4 ± 1.7 |
| p-value | 1 | 0.74 | 0.18 | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Flury, M.; Miles, C.; Liu, H.; DeVetter, L. Soil-Biodegradable Plastic Mulches Undergo Minimal in-Soil Degradation in a Perennial Raspberry System after 18 Months. Horticulturae 2020, 6, 47. https://doi.org/10.3390/horticulturae6030047
Zhang H, Flury M, Miles C, Liu H, DeVetter L. Soil-Biodegradable Plastic Mulches Undergo Minimal in-Soil Degradation in a Perennial Raspberry System after 18 Months. Horticulturae. 2020; 6(3):47. https://doi.org/10.3390/horticulturae6030047
Chicago/Turabian StyleZhang, Huan, Markus Flury, Carol Miles, Hang Liu, and Lisa DeVetter. 2020. "Soil-Biodegradable Plastic Mulches Undergo Minimal in-Soil Degradation in a Perennial Raspberry System after 18 Months" Horticulturae 6, no. 3: 47. https://doi.org/10.3390/horticulturae6030047
APA StyleZhang, H., Flury, M., Miles, C., Liu, H., & DeVetter, L. (2020). Soil-Biodegradable Plastic Mulches Undergo Minimal in-Soil Degradation in a Perennial Raspberry System after 18 Months. Horticulturae, 6(3), 47. https://doi.org/10.3390/horticulturae6030047





