1. Introduction
Avocado has become an increasingly popular fruit in recent years, with global consumption experiencing annual growth rates of ~4.6% [
1]. Per capita consumption in Australia, for example, increased by 50% in the past five years from 2.5 to 3.8 kg [
2]. Nonetheless, providing fruit that consistently meet consumers’ quality expectations remains a challenge in the industry. Inconsistent quality was recently identified by more than half of avocado consumers as a barrier to purchase [
3]. In another study, 45% of avocado shoppers felt dissatisfied with quality at least some of the time upon cutting the fruit at home [
4]. Furthermore, around one in five ‘Hass’ avocado fruit sampled from Australian retail stores had internal defects affecting >10% of the flesh [
5]. This level of damage is known to result in consumer dissatisfaction [
6].
Body rots are a leading internal defect of ripening avocado fruit. At the retail stage, they are second only to flesh bruising [
5]. Body rots are principally anthracnose. This disease manifests when quiescent
Colletotrichum infections, established in the orchard during fruit development, are released from latency in concert with postharvest ripening-related changes in the fruit. These changes include the degradation of antifungal compounds and increased tissue pH [
7]. Body rot expression may, however, be exacerbated by the injury of mature green avocado fruit during harvesting and packing. In a recent study on flesh bruising in avocado, a proportion of the green mature fruit dropped from 50 or 100 cm developed body rots at the impact site upon ripening. In contrast, non-impacted fruit had no rot development [
8].
To date, research on impact injury in avocado fruit has focused mostly on flesh bruising. Mature green unripe fruit are hard and tend not to bruise upon impact [
8,
9]. This characteristic has led to a general assumption that gentle handling is not important at harvest and packing. Here, we hypothesised that impact injury during early stages in the supply chain may promote body rot development in the subsequently ripe fruit. Eight experiments were conducted across three seasons, with ‘Hass’ fruit sourced from two commercial orchards in south-east Queensland, Australia. Body rot development in fully ripe fruit in response to impact from drop heights of 0, 15, 30 and 60 cm at harvest or packing was recorded separately for the impact site and elsewhere on the fruit. The influence of postharvest temperature and fungicide treatment on body rot development in impacted and non-impacted fruit were also investigated.
3. Results
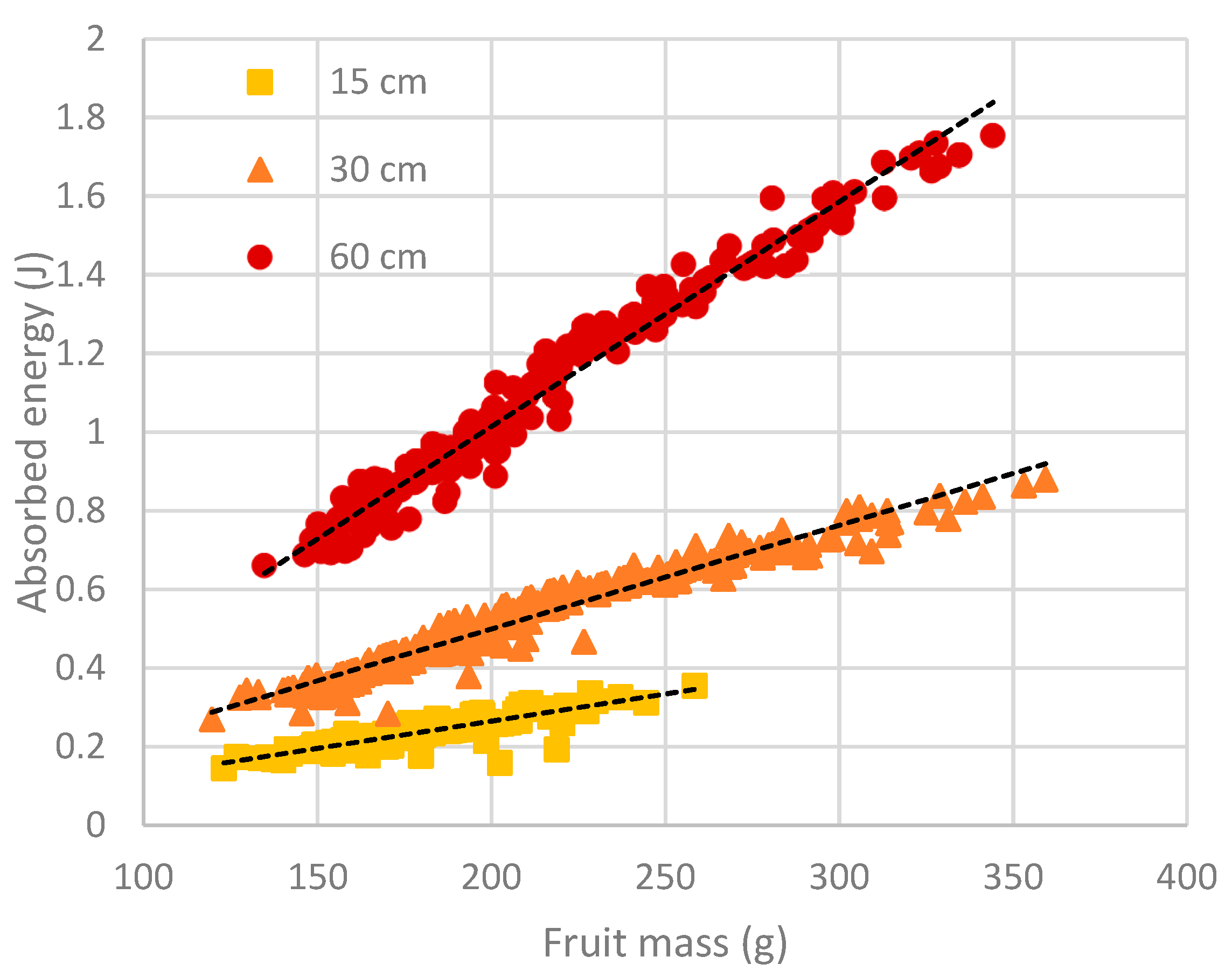
Absorbed energy is a measure of impact severity, and its magnitude is influenced by both drop height and fruit size, as shown by Equation (1). For each drop height, a linear relationship was found to exist between absorbed energy and fruit size (
Figure 2). Between experiments, little difference in fruit size was found between fruit harvested in 2016 from Spring Creek and those harvested in 2017 from Adare (
Table 2). The mean mass of fruit harvested in 2018 from a different block of trees at Adare was at least 32% greater than that of fruit used in the earlier experiments. As a result, fruit from the 2018 season experiments were subjected to greater absorbed energy levels at a particular drop height than fruit from previous seasons’ experiments (
Table 2). The difference was most pronounced for the 60 cm drop height treatment, with the absorbed energy of fruit from the 2018 season being almost twice that of fruit from the 2016 season.
Despite these seasonal differences across years in fruit size and absorbed energy, disease incidence in response to impact was similar between experiments. At soft-ripe stage, disease incidence generally increased with increasing drop height (
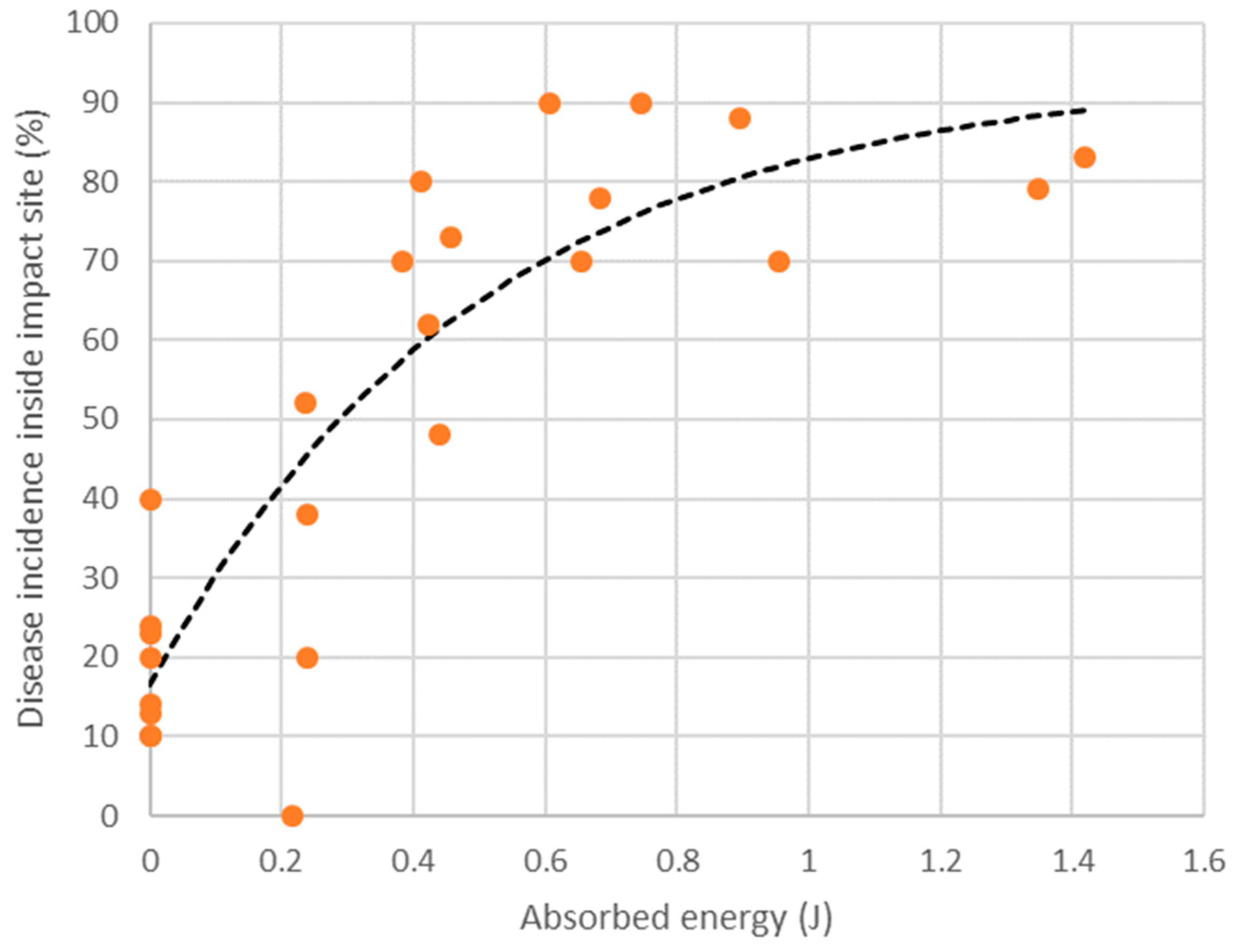
Table 3). Disease incidence did not exceed 40% in fruit subjected to no impact, whereas impact from a drop height of 30 cm caused a two- to eight-fold increase in disease incidence as compared with non-impacted fruit. A nonlinear relationship was observed to exist between disease incidence at the impact site and the energy absorbed by the fruit upon impact (
Figure 3). Modelling of the relationship suggested that an absorbed energy of 0.54 J (roughly equivalent to dropping a 220 g fruit from a height of 30 cm) is sufficient to cause two out of every three fruit to express body rots at soft-ripe stage. Body rots were rarely observed in fruit assessed at firm-ripe stage in Experiment 3. Although flesh bruising was not the focus of this study, it was noted that no flesh bruising was apparent in any of the fruit subjected to impact.
Experiments conducted in 2016 on fruit from the Spring Creek orchard revealed that impact from 30 or 60 cm, but not 15 cm, induced more lesions at the impact site than occurred in a similarly sized and located site arbitrarily marked on non-impacted fruit on the day of harvest (
Table 4 and
Table 5). Lesion area at the impact site was seven to nine times greater in fruit dropped 30 cm than in non-impacted fruit. A 60 cm drop height caused the lesion area at the impact site to be 15 times higher than in non-impacted fruit.
Body rot development elsewhere on the fruit was almost non-existent for all treatments and, therefore, disease expression at a ‘whole fruit’ level was determined solely by the occurrence of body rots at the impact site. Fruit dropped 30 cm had substantially fewer lesions and a smaller lesion area at firm-ripe stage than soft-ripe stage, whereas non-impacted fruit had a similarly low lesion number and area at both ripeness stages (
Table 5).
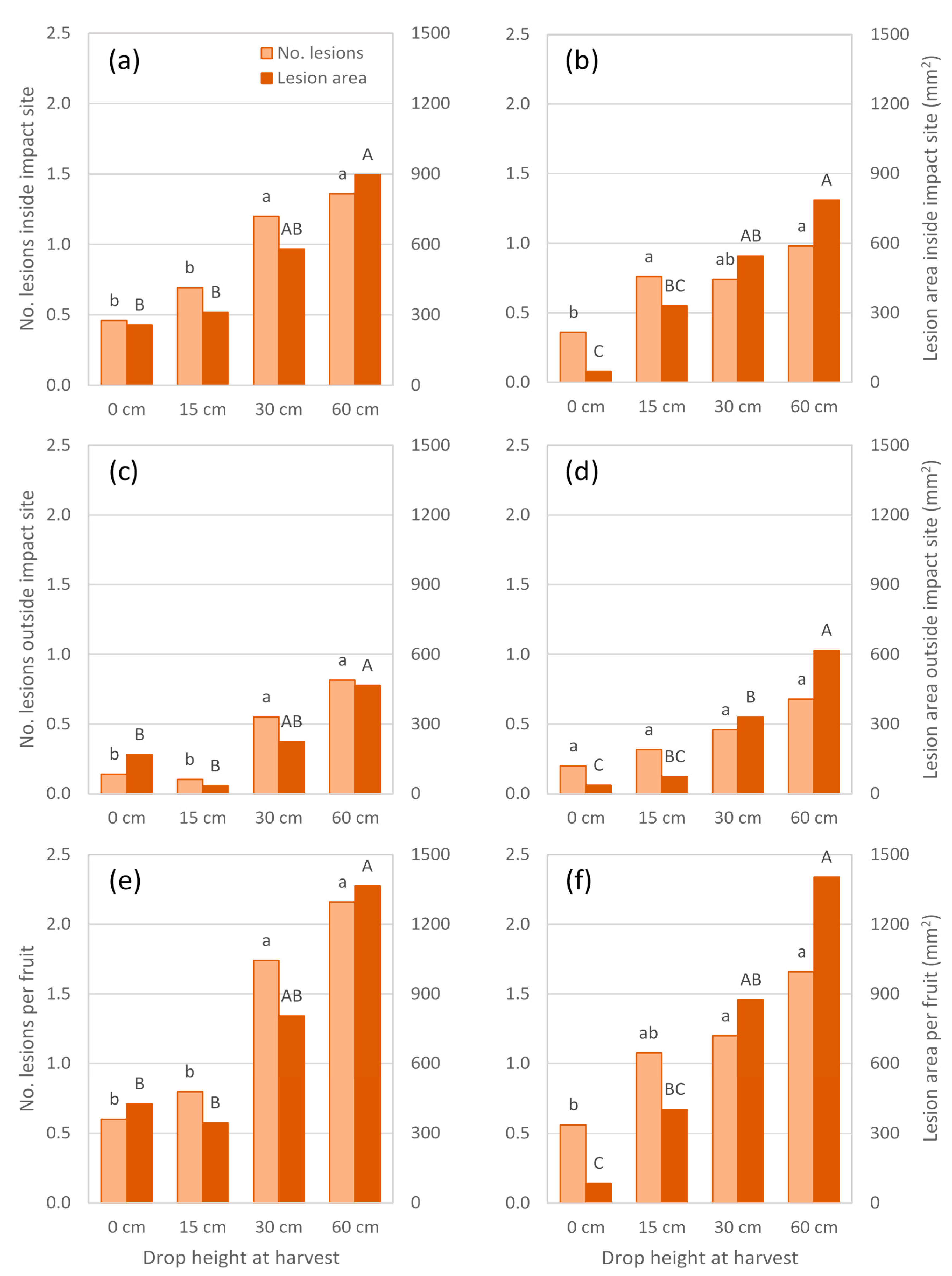
Experiments conducted in 2017 also showed a trend of increasing lesion number and area at the impact site as drop height increased (
Figure 4 and
Figure 5a,b). In Experiment 4, a drop height of 30 cm was sufficient to produce more lesions, and more than twice the lesion area at the impact site than occurred at a similar-sized site marked on non-impacted fruit (
Figure 5a). When the experiment was repeated on fruit harvested from the same trees five weeks later (Experiment 5), fruit dropped 30 cm had a lesion area at the impact site that was eleven times greater than that observed in non-impacted fruit (
Figure 5b). Furthermore, a drop height of 15 cm produced more lesions, but not a larger lesion area, at the impact site than occurred in non-impacted fruit.
In both 2017 season experiments, body rots also developed outside the impact site and generally increased in number and/or area as fruit were dropped from greater heights (
Figure 5c,d). At least one of these responses differed significantly between non-impacted fruit and those dropped 30 or 60 cm, but not 15 cm. Within a treatment, lesions elsewhere on the fruit did not appear to be as numerous or extensive as those observed at the impact site. Hence, treatment differences in body rot expression at a ‘whole fruit’ level (
Figure 5e,f) largely reflected those found at the impact site.
In 2018 season experiments, disease expression in response to impact was generally consistent with previous seasons’ findings (
Figure 6,
Figure 7 and
Figure 8). For Experiment 6, impact at harvest resulted in mean lesion areas at the impact site that were seven to 19 times larger than those observed in non-impacted fruit from the same cooling treatment (
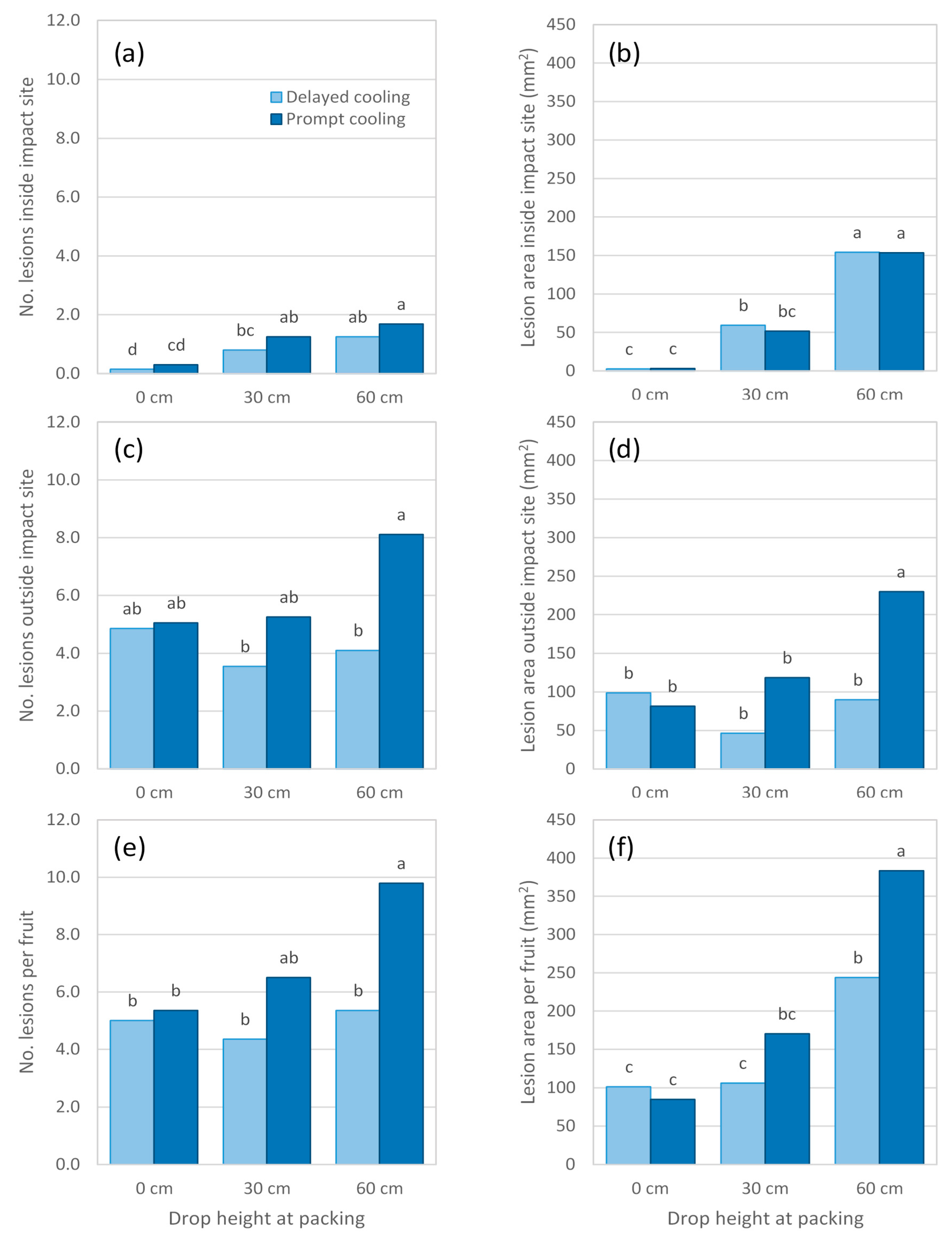
Figure 6b). Irrespective of cooling treatment, lesion area at the impact site almost doubled as drop height increased from 30 to 60 cm, despite no associated increase in lesion number (
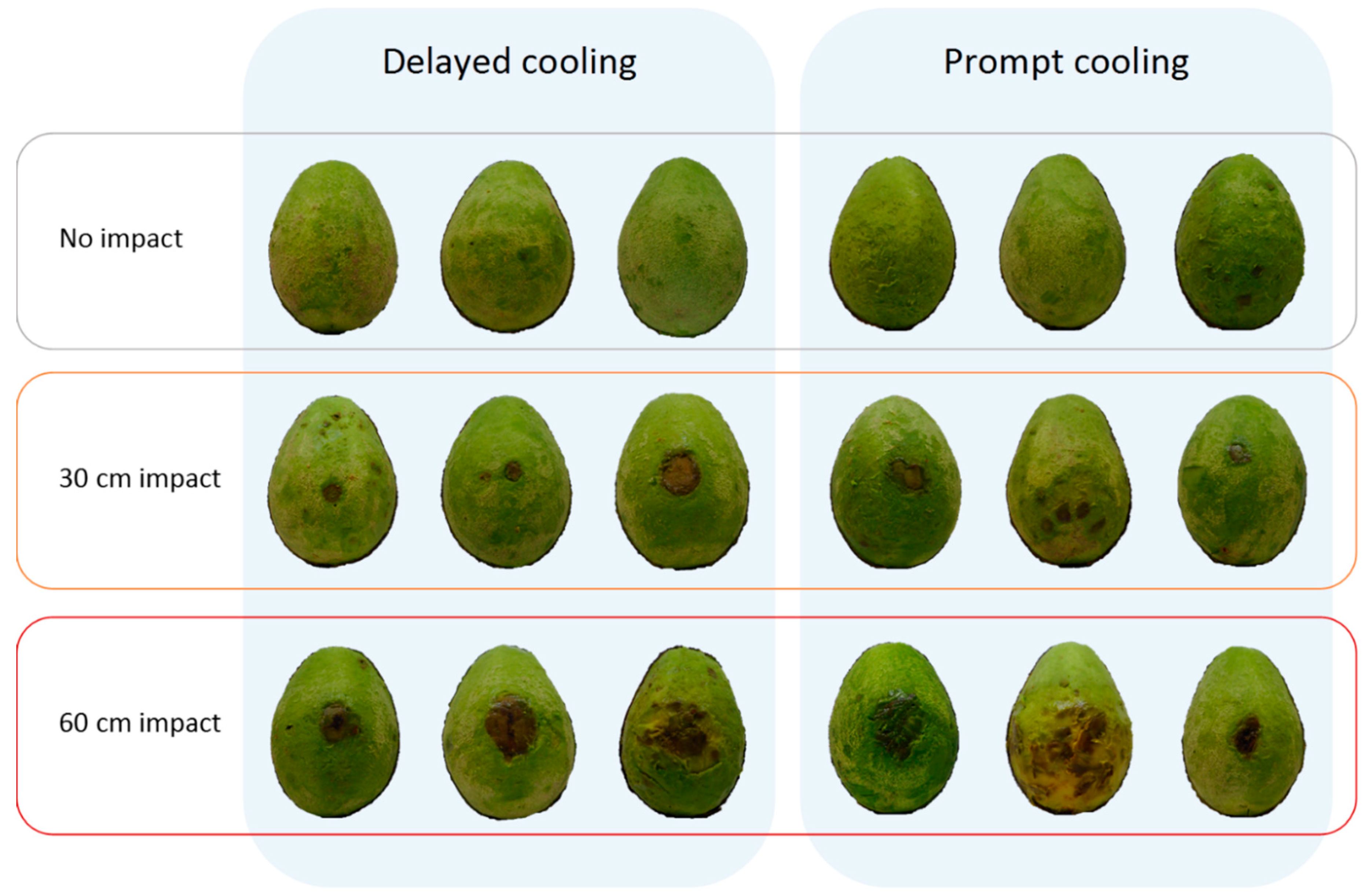
Figure 6a). Fruit subjected to a 60 cm drop height followed by cooling to 5 °C displayed a larger lesion area at the impact site than did fruit from any other treatment. Many fruit in this experiment displayed extensive body rot development, causing lesions to coalesce and to cover the entire fruit surface in some cases. As a result, lesion area at the impact site could only be accurately measured for 108 of the 120 fruit sampled and no data were collected for lesions occurring outside the impact site.
Body rot assessment in Experiment 7 was conducted earlier, at 1 d after reaching firm-ripe stage, than it had been for Experiment 6, at soft-ripe stage. Doing so enabled a more accurate measurement of lesions, as they tended to be smaller and less numerous than in Experiment 6. Postharvest temperature regime in Experiment 7 had no influence on body rot expression in non-impacted fruit or fruit dropped 30 cm (
Figure 7 and
Figure 8). For fruit dropped 60 cm, prompt as opposed to delayed cooling resulted in twice as many lesions occurring outside the impact site (
Figure 8c). The total area of these lesions was 2.5 times higher in fruit that received prompt cooling (
Figure 8d).
Experiment 7 also showed that increasing impact severity caused more lesions and a larger lesion area at the impact site (
Figure 8a,b). These findings were similar to those of previous experiments, despite disease assessment being conducted at a slightly earlier ripeness stage. In all treatments, relatively more body rots occurred outside the impact site than inside it. These rots were randomly distributed across the fruit surface and did not appear to be more concentrated towards the impact site. Fruit subjected to prompt cooling and a 60 cm drop height had more lesions outside the impact site and almost three times the lesion area than found outside the impact site in promptly cooled, non-impacted fruit. This finding suggests that impact can trigger disease expression elsewhere on the fruit, not only at the site of impact. At a ‘whole fruit’ level, the number of lesions was largely driven by body rots outside the impact site (
Figure 8e), whereas body rots occurring inside and outside the impact site both substantially contributed to the overall lesion area (
Figure 8f). Hence, a drop height of 60 cm produced larger lesion areas per fruit than were found in any other impact treatment, and the response was greater for promptly cooled fruit.
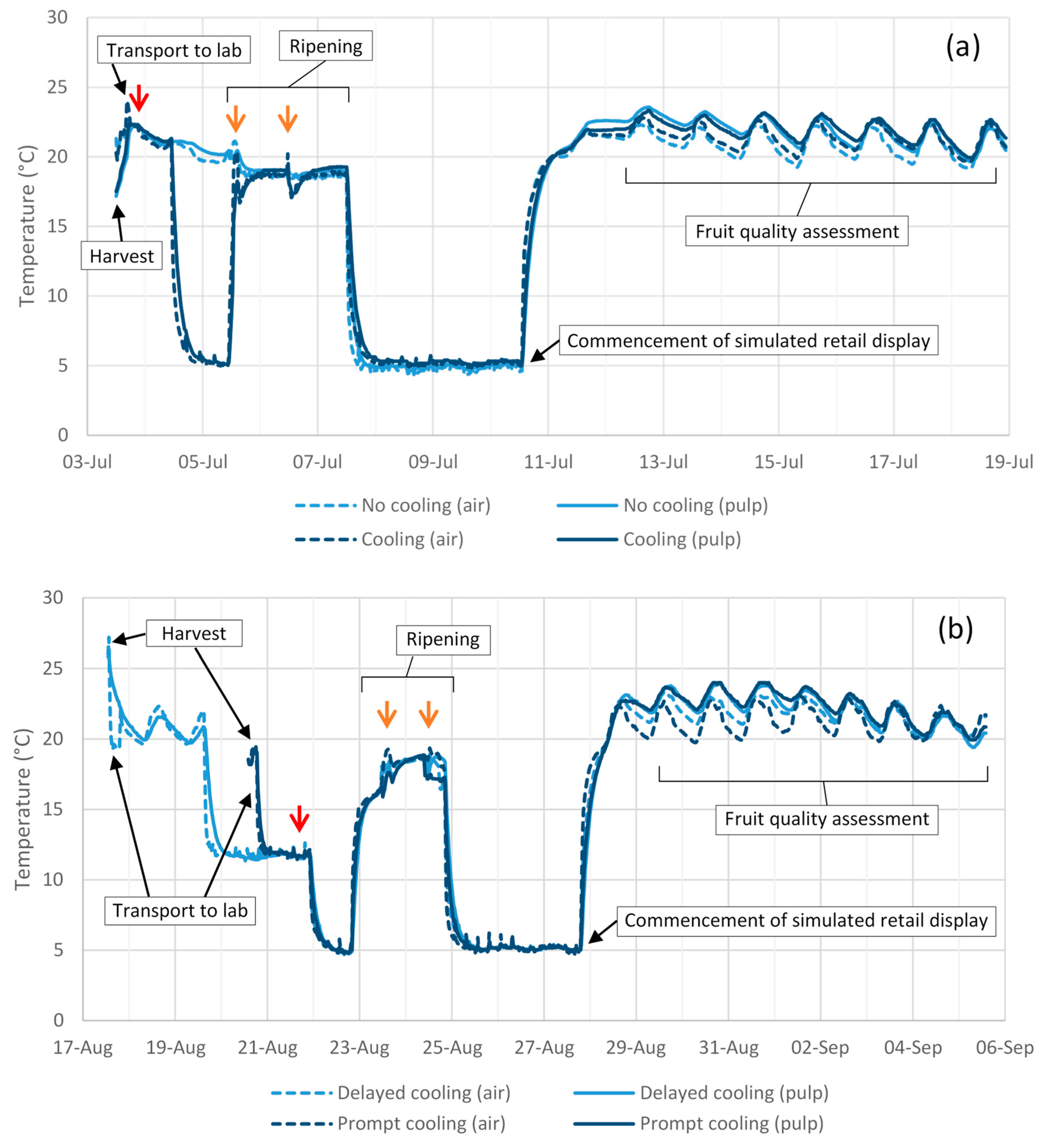
No differences in fruit weight loss were observed between treatments in Experiment 6 or 7 (
Table 6). Ripening time in these experiments was not affected by impact treatment, but was ~1 d longer in fruit that received prompt as opposed to delayed cooling in Experiment 7 and in cooled as opposed to uncooled fruit subjected to a 60 cm drop height in Experiment 6 (
Table 6).
Mineral nutrients in the pulp of 2018 season fruit showed little variation between harvests (
Table 7). Fruit from Experiment 7 had lower iron, zinc and manganese levels than fruit harvested five weeks earlier for Experiment 6, but all other nutrients (and their ratios) did not differ between experiments.
Application of a postharvest fungicide in Experiment 8 did not affect body rot expression at soft-ripe stage (
Table 8). As in previous experiments, more lesions and larger lesion areas were observed at the impact site in fruit dropped 30 cm as compared with non-impacted fruit. Disease expression outside the impact site was extensive and did not significantly differ between treatments. The overall means were 8.8 ± 1.0 (± s.e.) and 290 ± 40 mm
2 (± s.e.) for lesion number and area, respectively.
Fruit from Experiment 8 were harvested on the same day from the same orchard, albeit from a different tree, and subjected to a postharvest temperature regime that closely resembled the prompt cooling treatment of Experiment 7. The exception was a 3 h break in the cold chain to accommodate fungicide dipping and air drying. Despite these similarities between experiments, body rot development was seemingly more pronounced in Experiment 8 than in Experiment 7.
In all experiments, impact-induced body rot symptoms were typical of anthracnose caused by
Colletotrichum spp. Gene sequencing conducted on fungal isolates from diseased fruit in Experiment 6 confirmed this to be the case.
C. siamense was found to be associated with impact-induced lesions in fruit from four of the five trees used in the experiment.
C. alienum was associated with impact-induced lesions in fruit from the fifth tree. Both
C. siamense and
C. alienum have previously been reported as common causal agents of anthracnose (body rot) in ‘Hass’ avocado fruit in eastern Australia [
16].
4. Discussion
All experiments in this study indicated that impact injury to unripe ‘Hass’ avocado fruit increased the frequency and severity of body rots subsequently observed at the eating ripe stage. Furthermore, two
Colletotrichum species from the
C. gloeosporioides species complex [
17] were found to be associated with the impact-induced lesions, suggesting that the rots were more than likely anthracnose. The pathogenicity of
C. gloeosporioides in avocado fruit has been the focus of research [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44] and review [
7,
45,
46,
47]. Consequently, there is relatively good understanding of the infection processes. Quiescent infections of the fruit cuticle that occur in the orchard [
18,
19,
20] are activated postharvest as the fruit ripen [
21]. An increase in fruit peel pH above 5.8 [
24] and a decline in naturally occurring antifungal compounds [
22,
23] during ripening have been shown to promote anthracnose development. Whether impact injury accelerates these changes in avocado is not known. However, an injury-related decline in antifungal compounds is possible. Prusky et al. [
37] showed that the potent antifungal compound persin declined to sub-fungitoxic levels 6 d after applying multiple puncture wounds to freshly harvested ‘Fuerte’ avocado fruit. Persin degradation in avocado fruit has been associated with increased lipoxygenase (LOX) activity [
30]. It is likely that wounding produced a rapid increase in LOX activity, as has been shown in studies on other fruit [
48,
49]. Although puncture and impact are different forms of mechanical injury, the latter does cause apparent mesocarp cell damage in hard green mature ‘Hass’ fruit [
9]. Proton magnetic resonance imaging of fruit dropped 100 cm revealed a region of hyperintensity at the impact site, which Mazhar et al. [
9] interpreted as a leakage of symplastic cellular fluids from damaged cells into apoplastic air space.
Fruit responses to impact injury in the current study did not appear to be limited to the impact site. Experiments 4, 5 and 7 showed that dropping fruit from 30 cm or more caused an increase in the number and/or size of lesions occurring elsewhere on the fruit. Evidence for a ‘whole fruit’ response to impact injury was reported by Ben-Yehoshua et al. [
50]. As compared with uninjured fruit, they found an elevated respiration rate and earlier respiratory climacteric peak in ‘Hass’ fruit subjected to an undefined “shaking” treatment applied 1 d after harvest. They also reported accelerated softening of shaken fruit. However, our results (
Table 6) and those of Arpaia et al. [
51] showed no effect of impact injury on ripening time as measured by fruit firmness. Mechanical wounding by slicing produced an oxidative burst characterised by increased hydrogen peroxide concentration and peroxidase activity in unripe ‘Hass’ avocado fruit, but the response was localised to within 1 cm of the wound site [
52]. Considering these limited and contradictory findings for avocado, it remains unclear as to what impact-related changes might account for increased disease expression across the entire fruit.
Postharvest cooling treatment had no effect on body rot development in non-impacted fruit or fruit dropped from 30 cm. However, cooling as opposed to no cooling (Experiment 6;
Figure 6) and prompt as opposed to delayed cooling (Experiment 7;
Figure 8) resulted in a greater expression of body rots in fruit dropped 60 cm. A strong impact-induced pathogen response, coupled with a longer ripening time for fruit from these cooling treatments, is a possible explanation for this interaction. Studies have shown that avocado fruit that ripen earlier are less prone to body rot development than slow-ripening fruit [
53,
54]. These findings support the view of others [
42,
55] that fruit softening per se is not the key mediator of
C. gloeosporioides growth. In our experiments, fruit dropped 60 cm and subjected to cooled or prompt cooling treatments took ~1 d longer to ripen than their un-cooled or delayed cooling counterparts (
Table 6). Disease development occurs rapidly in the latter stages of avocado ripening. An extra day at 20 °C is capable of yielding a twofold increase in body rot incidence [
54].
A rapid rate of body rot development is one likely reason for the much higher disease expression observed in Experiment 3 for soft-ripe fruit as compared with firm-ripe fruit (
Table 5). Although ripening time was not recorded in this experiment, ‘Hass’ fruit generally require ~2 d at 20 °C to progress from firm- to soft-ripe stage. Moreover, firm-ripe ‘Hass’ fruit may better resist
Colletotrichum invasion if the peel contains higher levels of antifungal compounds and/or has a lower pH than that of soft-ripe fruit. Antifungal compound levels in ‘Hass’ fruit peel have been shown to decrease as fruit ripens [
56]. Although ripening-related changes in peel pH have been established for ‘Fuerte’ fruit [
24,
30], they are cultivar-dependent [
24] and do not appear to have been investigated for ‘Hass’ fruit. Nonetheless, our findings suggest that impact-induced body rots pose little threat for ‘Hass’ fruit consumed at firm-ripe stage. However, the popularity of using avocados as a spread or in guacamole or dips [
3,
4] suggests that most fruit are consumed at the soft-ripe stage. For this reason, it is important that unripe fruit be carefully handled to minimise the appearance of body rots at time of consumption.
Fruit from the 2018 season (Experiments 6 to 8) showed an overall high propensity for body rot development at soft-ripe stage. Compared to values found in the literature, the pulp of these fruit contained low calcium (Ca) concentrations [
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67] and very high N concentrations [
67,
68,
69], with a consequentially high N/Ca ratio of 40. Studies on avocado fruit have revealed that both of these nutrients influence anthracnose susceptibility [
58,
59,
67,
70,
71,
72,
73,
74,
75,
76]. For example, Marques et al. [
67] reported that ‘Hass’ fruit with a pulp N/Ca ratio of 45 developed body rots affecting 20% of the flesh volume, as opposed to just 5% in fruit that had a N/Ca ratio of 33. Ca forms cross-linkages with cell wall pectic substances, physically restricting the access of cell-wall-degrading enzymes produced by fungal pathogens [
77]. Furthermore, Ca signalling in avocado mesocarp cells is believed to initiate defence responses to pathogen invasion [
27]. N, however, supports anthracnose development.
C. gloeosporioides uses N to produce ammonia, thereby raising pH of the surrounding environment and triggering secretion of the tissue-degrading enzyme pectate lyase (PL) [
31]. Increased N supply stimulates greater PL secretion by the pathogen [
28]. Nevertheless, the extent to which nutrient imbalance led to the high anthracnose susceptibility of 2018 season fruit can only be speculated. Other factors, including antifungal compound concentrations, fruit tissue pH and initial pathogen inoculum load, may well have contributed to the response.
Treatment of fruit with fungicide on the day after harvest had no effect on body rot development in either impacted or non-impacted fruit. Everett [
78] reported that delayed postharvest application of the fungicide prochloraz reduced its efficacy. Application within 2 h of harvest was recommended to give the best control of rots in avocado fruit. Despite our use of a different fungicide, it may be similarly possible that the ~24 h delay between harvest and treatment contributed to the lack of efficacy in Experiment 8. In further work, Experiment 8 might be revisited with prompter fungicide application. This would also avoid the 3 h cool chain break that accompanied fungicide treatment in the current study. Other researchers have shown that a 5 h break in the cool chain is sufficient to increase anthracnose incidence in ‘Hass’ avocado fruit upon ripening [
79,
80]. Such a phenomenon may account for the overall relatively higher body rot expression observed for fruit in Experiment 8 as compared with fruit in Experiment 7. The latter were harvested on the same day from the same orchard and subjected to the same postharvest temperature regime, but without a cool chain break.
 ), 30 (
), 30 ( ) or 60 (
) or 60 ( ) cm. Data points represent individual fruit values obtained from eight experiments. Dotted lines represent linear regression equations for 15 cm (absorbed energy = -0.01147 + 0.001382 fruit mass; R2 = 0.75; p < 0.001; n = 140), 30 cm (absorbed energy = -0.03181 + 0.002645 fruit mass; R2 = 0.96; p < 0.001; n = 300) and 60 cm (absorbed energy = -0.1290 + 0.005716 fruit mass; R2 = 0.97; p < 0.001; n = 190) drop heights.
) cm. Data points represent individual fruit values obtained from eight experiments. Dotted lines represent linear regression equations for 15 cm (absorbed energy = -0.01147 + 0.001382 fruit mass; R2 = 0.75; p < 0.001; n = 140), 30 cm (absorbed energy = -0.03181 + 0.002645 fruit mass; R2 = 0.96; p < 0.001; n = 300) and 60 cm (absorbed energy = -0.1290 + 0.005716 fruit mass; R2 = 0.97; p < 0.001; n = 190) drop heights.
 ), 30 (
), 30 ( ) or 60 (
) or 60 ( ) cm. Data points represent individual fruit values obtained from eight experiments. Dotted lines represent linear regression equations for 15 cm (absorbed energy = -0.01147 + 0.001382 fruit mass; R2 = 0.75; p < 0.001; n = 140), 30 cm (absorbed energy = -0.03181 + 0.002645 fruit mass; R2 = 0.96; p < 0.001; n = 300) and 60 cm (absorbed energy = -0.1290 + 0.005716 fruit mass; R2 = 0.97; p < 0.001; n = 190) drop heights.
) cm. Data points represent individual fruit values obtained from eight experiments. Dotted lines represent linear regression equations for 15 cm (absorbed energy = -0.01147 + 0.001382 fruit mass; R2 = 0.75; p < 0.001; n = 140), 30 cm (absorbed energy = -0.03181 + 0.002645 fruit mass; R2 = 0.96; p < 0.001; n = 300) and 60 cm (absorbed energy = -0.1290 + 0.005716 fruit mass; R2 = 0.97; p < 0.001; n = 190) drop heights.