Abstract
Iris yellow spot (IYS), a disease caused by Iris yellow spot virus (IYSV) and spread by onion thrips, is a devastating disease of onion bulb and seed production. The development of onion germplasm resistant to IYS and/or thrips is crucial to onion production, since host plant resistance is unknown for both pests. During the summer of 2010 and 2012, plants with fewer IYS disease symptoms were selected from a screening of plant introduction accessions (PIs) and first-generation selections, respectively. The resulting progeny from these selected plants were evaluated during the summers of 2013 and 2014 for thrips numbers and IYS symptom expression, and compared with their respective original PIs and a susceptible check, ‘Rumba’. The field experiment was designed such that every plant in the field screening had an equal chance of being infected with IYSV. This study shows that variation for thrips and IYS existed among PIs and first- and second-generation selections. Even though not enough progress towards minimizing IYS severity was evident from this study, we did identify several lines with improved tolerance to onion thrips in first- and second-generation selections. The majority of the selected lines exhibited lower thrips and IYS severity compared to ‘Rumba’, which suggests that the progress towards developing insect- and ultimately disease-resistant germplasm can be achieved.
1. Introduction
Iris yellow spot (IYS), the disease caused by Iris yellow spot virus (IYSV; family Bunyaviridae, genus Tospovirus), poses a serious threat to the sustainable production of onion bulbs and seed [1]. Since first reported in Netherlands [2], IYSV has spread to the following countries: Australia [3], Brazil [4], Chile [5], India [6], Iran [7], Israel [8], Italy [9], Japan [10], New Zealand [11], Peru [12], Réunion Island [13], Slovenia [13], Spain [14], and the United States [15]. In the United States, IYSV has been found in all major onion producing states, including Arizona [16], California [17], Colorado [18], Georgia [12], Idaho [15], Nevada [19], New Mexico [20], New York [21], Oregon [22], Texas [23], and Washington [24].
IYS produces variable, typically diamond-shaped, chlorotic lesions on leaves and scapes that later turn necrotic, and results in reduced bulb yield due to a reduction in photosynthetic area [1,18,24,25,26]. No disease symptoms are observed on bulb scale tissue [27].
IYSV is vectored by onion thrips (Thrips tabaci) [1], which was thought to be the only known vector, but recently it has been reported that tobacco thrips (Frankliniella fusca) can also transmit IYSV [28]. Differences in virus titer and thrips number can be observed within a plant due to the preferential feeding of thrips on the inner leaves [1]. Thrips damage can reduce bulb yield by more than 50% [29], and the yield loss can escalate when accompanied by IYS symptoms. Thrips usually acquire IYSV in their larval stage, and spread it by feeding on healthy plants [30,31]. IYSV can be acquired by adult thrips, but it is not transmitted due to lessened multiplication and a lack of movement of IYSV to the salivary glands [32]. IYSV is not seed transmitted [30]; however, weeds, volunteer plants, and infected bulbs can act as sources of IYSV inoculum [1].
In Colorado, IYS incidence has been reported up to 73.2% [33]. du Toit and coworkers reported a significant reduction in the percentage of colossal and jumbo bulbs when the crop was infected with IYSV [24]. In a four hectare seed crop in central Oregon, Crowe and Pappu (2005) observed that half of the field was 100% symptomatic and had 95% lodged plants, and the other half was 30–40% symptomatic and had 15% lodged plants [22]. IYSV has been reported to cause up to 100% damage to bulb and seed crops in Brazil [4].
For the effective control of IYS, there is a need for an integrated approach that includes thrips control, host plant resistance, and cultural practices. Good control of the thrips population can provide an indirect control over IYS [34]. In some cases, chemical control can provide some thrips number reduction, but this control becomes ineffective for temperatures over 30 °C, because thrips populations increase dramatically under such conditions [35]. Onion growers rely on pesticides to control thrips, but this creates other problems, such as environmental pollution [1] and insecticide resistance [36]. Varying cultivar response to thrips injury and differential cultivar performance under similar thrips infestation conditions [37] can be important factors in developing thrips resistant germplasm [38]. Host plant resistance can provide an economic and environmentally friendly solution for controlling thrips and IYSV.
Differential thrips feeding on different cultivars can be attributed to thrips preference for leaf color and amount of wax [39,40,41,42]. It has been observed that leaves with less epicuticular wax (glossy and semi-glossy types) tend to be less preferred by the thrips, as compared to waxy leaves (non-glossy type) [42]. As suggested by some studies, thrips also prefer bluish foliage as compared to greenish foliage [1,43]. Generally, leaves with high amounts of wax appear bluish, and those with less wax appear green in color. In New York, two cultivars, Yankee and Nebula, both with bluish foliage, had a higher number of thrips larvae per plant when compared to other cultivars with different foliage [44]. In a study of four cultivars, ‘Nebuka’ had 48%, 78% and 67% fewer adult thrips than ‘Texas Early Grano 502,’ and ‘951,’ and ‘Creole,’ respectively [40]. In New York, plants of ‘Colorado 6’ and ‘NMSU 03-52-1’ exhibited the fewest number of thrips, and antibiosis and/or antixenosis were suggested as the cause [29]. Long term breeding objectives can be focused on developing semi-glossy onion germplasm with greenish foliage, for sustainable control of onion thrips.
Differences for IYS severity exist among cultivars, however, no cultivar has shown resistance to IYS so far [1,24,26,33,45,46,47]. In New Mexico, NMSU 03-52-1, NMSU 04-41, NMSU 04-44-1, and ‘NuMex Jose Fernandez’ exhibited the fewest IYS symptoms among 18 winter-sown entries screened [47]. Plants of NMSU 05-33-1 showed delayed IYS symptom expression when 13 winter-sown entries were screened [45]. New Mexico State University released eight onion germplasm lines that were selected from plant introduction accessions for reduced IYS symptom expression [48]. In a screening of PI accessions, plants of PI 289689 were less attractive to onion thrips, and plants of PIs 239633 and 546192 exhibited less severe IYS disease symptoms than plants of other accessions [49]. Another follow up study found several first-generation selections performed better than original PIs for both thrips and IYS disease severity [50]. The objective of this study was to evaluate the lines that were selected from accessions in the previous study, to determine if the selected lines performed better than their original PIs in terms of reduced number of thrips and IYS disease incidence and severity.
2. Materials and Methods
2.1. Plant Material
Twenty-seven entries, including five plant introductions (PIs), eight first-generation selections (prefix NMSU-10), 13 second-generation selections (prefix NMSU-12), and one commercial cultivar ‘Rumba,’ were evaluated in 2013 and 2014. Original PIs were selected from the Germplasm Resources Information Network (GRIN) database [51], and were tested in field in 2009 [49]. First-generation breeding lines were developed in 2010 by selecting individual plants from the original PIs for reduced IYS symptoms. Similarly, second-generation lines were selected from first-generation lines in 2012. Pedigree information for NMSU breeding lines is given in Table 1.

Table 1.
Average number of onion thrips per plant, Iris yellow spot (IYS) severity rating, and IYS incidence for entries measured at several time intervals throughout 2013 and 2014 growing seasons at the Leyendecker Plant Science Research Center, Las Cruces, NM.
2.2. Planting and Field Design
Seeds for all entries were sown in 4 inch deep black plastic flats filled with Metro Mix-360 (Sun Gro, Bellevue, WA) in a greenhouse at the Fabian Garcia Science Center. Ridomil (Syngenta, Wilmington, DE) was applied as a soil drench as per manufacturer’s recommendations, to prevent seedling mortality due to fungal infection. Osmocote (18-6-12; N-P-K; Scotts Miracle-Gro Company, Marysville, OH) was applied to enhance seedling growth. Seedlings at the four to five leaf stage were transplanted to the field at the Leyendecker Plant Science Research Center, Las Cruces, NM (32.20° N, −106.74° W, 1187.2 m, climate data in Reference [52], soil type—Glendale loam, pH 7.7).
Two rows of seedlings were transplanted on three meters long raised bed-plots with 10 cm spacing between seedlings. Beds were arranged in a randomized complete block design with three replications. For the plants to have an equal chance of getting infected with IYS, bulbs carrying viruliferous thrips from the previous year were placed around the periphery of the evaluation field in both years. Every third bed in the field was designated as a spreader row, and was sown with the commercial cultivar ‘NuMex Dulce,’ which is susceptible to onion thrips and IYS (Figure 1). One meter spacing between the beds was ensured for irrigation and equipment movement. Plants were grown using standard cultural practices recommended for onion production in southern New Mexico [53]. However, no insecticide was applied to plants, in order to preserve the thrips population for them to spread IYSV throughout the field. Furrow irrigation was done weekly, with fertigation every third time with Uran-32 (32:0:0; N-P-K; Helena Chemical Co., Collierville, TN, USA) at the rate of 48 L ha−1. Manual weed control was done within the entry plots, and chemical weed control was done in between the beds using Goal® 2XL (oxyfluorfen) (Dow AgroSciences, Indianapolis, IN) for broad leaf weeds and Select® (clethodim) (Arysta LifeScience North America, Cary, NC) for grass weed control.
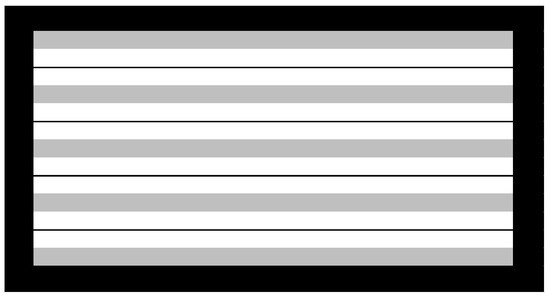
Figure 1.
Field design utilized in this study. Black border represents border rows grown from viruliferous thrips-containing onion bulbs saved from previous year. Gray rows represent spreader rows. White rows represent test entries including plant introductions (PIs), first-, and second-generation selections.
2.3. Data Collection
Data were collected for onion thrips number at 10, 13, and 16 weeks after transplanting (WAT). Ten plants were arbitrarily selected from each plot, and the numbers of thrips (adults and larvae) were counted on each plant. Ten plants per plot were rated for IYS severity at 13, 16, and 19 WAT. Plants scored for onion thrips and IYS severity were arbitrarily selected and might be different. A rating scale from 0 to 4 was used for IYS severity (Figure 2), where 0 = no symptoms; 1 = 1–2 small lesions per leaf; 2 = more than 2 medium-sized lesions; 3 = lesions coalescing on more than 25% of the leaf tissue; 4 = more than 50% leaf is dead [49,54]. Disease incidence data were also computed at 13 WAT as percentage of plants infected with IYS symptoms. Incidence data were not shown for the later weeks, because the incidence had reached 100%. Additionally, data were collected for foliage color and waxiness as previously described [49,54]. Some plots matured or were harvested before the last severity rating, and these plots were considered missing plots and were not considered for the last rating. Any plot with less than five plants, or any entry with less than two replications, was considered missing, and was excluded from the data analysis. Based on foliar disease expression, plants were selected that exhibited fewer IYS symptoms than the other plants within the same plot. Those bulb selections were sacked separately from the other bulbs from the same plot.
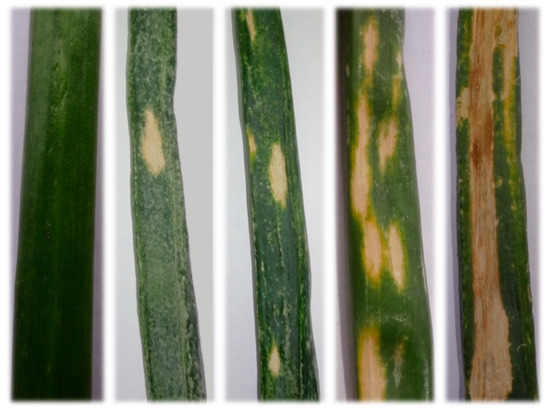
Figure 2.
Typical Iris yellow spot (IYS) severity rating scale. IYS severity rating starting from left, 0 = no symptoms; 1 = 1–2 small lesions per leaf; 2 = more than 2 medium-sized lesions; 3 = lesions coalescing on more than 25% of the leaf tissue; 4 = more than 50% leaf is dead.
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
Leaf tissue samples were collected from border and spreader rows to confirm the presence of IYSV and to measure virus titer using ELISA [55]. Leaf tissue samples about 5–8 cm long were taken arbitrarily from the tips of 10 random plants per plot. Leaves from each plot were put into separate plastic bags that were carefully labeled and temporarily stored in ice in an ice chest in the field. Later, the samples were transferred to a freezer and stored at −4 °C until the end of the season. Each sample was analyzed using the ELISA reagent set SRA 60500 (Agdia Inc., Elkhart, IN, USA), following the manufacturer’s instructions. A small portion, about 0.5 cm, was used from all of the ten leaves for each sample plot. The leaf tissues were placed in 2 ml Eppendorf microcentrifuge tubes (Eppendorf North America, Hauppauge, NY, USA). Tubes were dipped in liquid N to quick freeze, to allow easy and fast grinding. After grinding, 1 ml of prepared general extract buffer (ELISA reagent set SRA 60500) was added to each tube. After shaking, each tube was centrifuged at 10,000 rpm for 4 min using VWR Galaxy 16DH Centrifuge (VWR, Randor, PA, USA). Immuno 96 microwell solid plates (Nalge Nunc International, Rochester, NY, USA) were used for the ELISA. Positive and negative controls for IYSV were developed by running ELISA on various tissue samples with the highest and lowest IYS severity ratings, respectively. Samples were considered positive when their ELISA OD values were at least three times than that of negative control, plus four times the standard deviation of respective negative control.
2.5. Data Analysis
Means for all traits were calculated over three replications in SAS (SAS 9.2, SAS Institute Inc., Cary, NC, USA). Analysis of variance (ANOVA) was performed separately for both years, to compare lines with each other. Single degree contrasts were performed between the original PIs and selections. Dunnett’s test was performed to compare all the selected lines with control ‘Rumba’.
3. Results and Discussion
3.1. Virus Detection and IYS Spread
Border rows were grown using the bulbs carrying virus infected thrips from the preceding year’s screening study. Every third row in the field was planted with a susceptible cultivar, to serve as the spreader row to spread virus (Figure 1). The idea was that once the temperatures were conducive, the thrips would move from border to spreader rows, and ultimately to test entries and infect them with virus. IYS symptoms were not observed on the plants until mid-May in both years. By the end of May, small diamond- to irregular-shaped, chlorotic lesions were visible on some plants. As time progressed, chlorotic lesions increased in size, started to coalesce, and became necrotic. To confirm the presence of virus in these plants, ELISA was performed (data not shown). Random leaf samples from symptomatic tissue exhibited positive ELISA reactions with polyclonal anti-sera against IYSV, confirming the presence of virus. ELISA confirmed the presence of IYSV in leaf tissue.
3.2. Leaf Color and Waxiness
For both years, first- and second-generation selections did not show any significant differences in foliage color and waxiness ratings among themselves or compared to their original PIs. Since the selections were performed based on their IYS severity rating, and not on color and waxiness, no defined pattern was expected. Previous studies have correlated the leaf color and waxiness with thrips feeding [6,7].
3.3. Thrips and IYS Severity
For both years, data for thrips count were recorded at three separate times, however, IYS severity was recorded twice in 2013 and thrice in 2014. Plants matured earlier in 2013, and were harvested earlier before the third IYS rating in 2013. Expected trends for onion thrips and IYS severity were observed in both years. Thrips count was lowest at the initial date and highest at the second recording. Thrips counts tended to be lower later in the season, because plants started to mature and lose green leaf tissue. Contrastingly, IYS severity tended to increase with every severity rating. Plant senescence can also be confounded with the IYSV severity, which can result in higher severity late in the season. Incidence was recorded 10, 13, and 16 WAT, however, only recording at 13 WAT was informative, as incidence was very scarce at earlier observations, and no variation existed in the last observation.
Overall, higher thrips numbers and IYS severity were observed in 2013 than 2014 (Figure 3). This difference possibly arose due to difference in planting dates and temperature variation in both years. In 2013, seeds were sown in a greenhouse on Jan 7 and transplanted into the field from Mar 6–13, whereas in 2014, the sowing and transplanting was delayed by more than one week. Seeds were sown in a greenhouse on Jan 15 and transplanted into the field from Mar 17–20. Consequently, the data recording dates were also different. Levene’s test for homogeneity of variance between both years revealed significant difference for all dates (p ≤ 0.001), except IYS severity 10 WAT. Due to this variation, data for both years were analyzed separately.
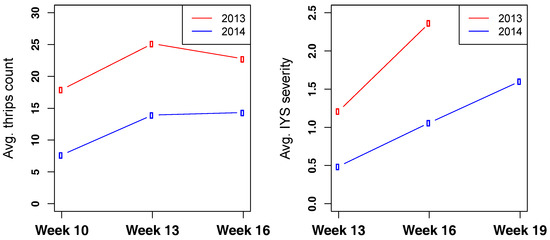
Figure 3.
(Left) Average number of thrips per counting date for 2013 and 2014. (Right) Average IYS severity rating per rating date for 2013 and 2014.
In 2013, significant differences in thrips counts were observed starting 10 WAT. Almost all the original PIs, and first- and second-generation selections had significantly lower thrips counts than the susceptible cultivar ‘Rumba’ (Table 1). First-generation selections did not show significantly lower counts from their original PIs. However, several second-generation selections showed significant difference from their original PIs. Moreover, NMSU 12-279, 12-255, 12-298, and 12-289 had lower thrips counts compared to their first-generation selections.
A similar pattern was observed for the thrips counts at 13 WAT (Table 1). ‘Rumba’ had the highest thrips count, and all other lines had significantly lower thrips counts compared to ‘Rumba’. Except for NMSU 10-579-1, all first- and second-generation selections from PI 172703 had significantly lower thrips counts than the original PI. There was no significant difference among first- and second-generation selections. At 16 WAT, a similar pattern was observed, with a few differences. ‘Rumba’ had a higher thrips count than most of the lines, except PI 258956 and its selections. No significant differences were observed among original PIs and their first- and second-generation selections. However, selections from PI 172703 and 289689 and the PIs themselves had significantly lower thrips count compared to ‘Rumba’.
Significant differences in IYS severity were observed at 13 WAT in 2013. At this initial rating, ‘Rumba’ exhibited a low disease severity. Only one PI and a second-generation selection from a different PI showed significantly lower severity than ‘Rumba’. However, by the second severity rating at 16 WAT, significant differences were more evident. One original PI 239633, two first-generation lines (NMSU 10-700 and NMSU 10-589-1), and two second-generation lines (NMSU 12-298 and NMSU 12-289) had significantly lower severity than ‘Rumba’. Two second-generation lines had lower severity than their parental first-generation lines. IYS incidence only showed significant differences at 13 WAT. All lines had 100% incidence at 16 WAT.
In 2014, ‘Rumba’ had the highest thrips count at 10 WAT. Several PI, first-, and second-generation selection lines showed significantly lower thrips counts than ‘Rumba’. First- and second-generation lines showed significant differences from their original PI. Several second-generation lines, especially in the PI 172703 group, showed progress from their first-generation selections. A similar trend was observed at 13 WAT, where ‘Rumba’ had the highest thrips count. Except for PI 172703, PI 546140, and NMSU 12-299, all other lines had significantly lower thrips count than ‘Rumba’. One second-generation selection, NMSU 12-258, had a significantly lower thrips count compared to its first-generation parent. The same trend was followed through the last thrips count observation. An original PI 258956 had a higher thrips count than ‘Rumba’; however, its first- and second-generation selections had a significantly lower thrips count. Again, except for this one PI and NMSU 12-283, all lines had a lower thrips count than ‘Rumba’.
For IYS severity, no line, except for one second-generation, NMSU 12-285, showed lower IYS severity compared to ‘Rumba’ at 13 WAT. No first- or second-generation selections showed any progress as compared to their original PIs. At 16 WAT, NMSU 10-579-1 had a lower IYS severity than its original PI. One second-generation line, NMSU 12-298, showed progress compared to its first-generation parent. By the last severity observation, PI 258956 and its first- and second-generation selections exhibited a lower severity than ‘Rumba’. Few selection lines had a lower IYS severity compared to their original PIs. NMSU 12-299 had a lower severity compared to its first-generation parent. Only one second-generation line, NMSU 12-285, had significantly lower incidence compared to ‘Rumba’ at 13 WAT. All lines had a 100% incidence at later dates.
3.4. Selection Progress for Thrips and IYS
Tolerance to onion thrips and IYS are quantitative and complex traits. As indicated by our data, not much progress for reduced IYS severity was achieved after two rounds of selection (Figure 4). This might be due to the segregating nature of the original PIs, and also to the amount of selection pressure exerted in the first selection cycle. It is also possible that the selected bulbs were just escapes, and were not truly tolerant to IYSV. The inability to detect differences statistically, due to the yearly differences in the experimental evaluation, could be another possible explanation. However, even with these challenges, several first- and second-generation selections showed progress for reduced thrips numbers (Figure 4 and Table 1). In both years, first- and second-generation selections performed better than susceptible cultivar check ‘Rumba’ (Figure 4). Larger standard error around ‘Rumba’ is the result of the low number of replications for this susceptible check. Additional selection cycles are needed to make more progress towards developing IYS and thrips tolerant germplasm.
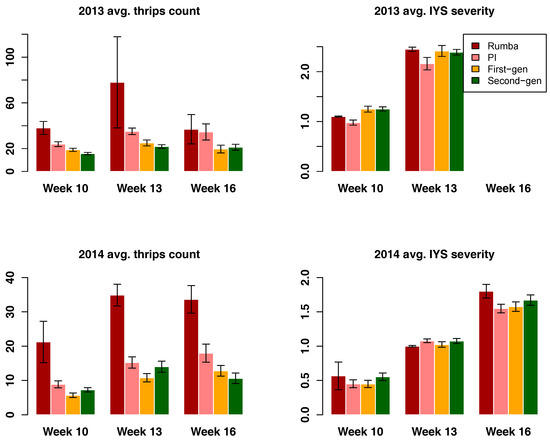
Figure 4.
Barplots showing group-wise average (top left) thrip counts for each counting date for 2013, (top right) IYS severity for each rating date for 2013, (bottom left) thrip counts for each counting date for 2014, and (bottom right) IYS severity for each rating date for 2014. IYS severity rating was not performed for the third recording in 2013. Standard error is shown on top of each bar. Grouping legend is provided in the top right plot.
3.5. Future Direction towards Developing Better Germplasm
Selection efforts that maximize selection efficiency and genetic gain per cycle are challenging with a complex plant disease such as Iris yellow spot. Field screening for onion thrips and IYS rely on the insect vector and its management, the disease causal agent, and environmental conditions conducive to vector and disease development. These factors make it difficult to achieve uniform disease evaluation conditions within and across years. These factors also contribute to a large amount of environmental and non-plant genetic variation associated with disease development. To overcome these shortcomings, efficient mechanical inoculation methods are required for reliable and efficient IYSV transmission and disease development in the field. Artificial or mechanical virus inoculation has been attempted in maize [56] and wheat [57] by physical wounding, and Chinese cabbage [58] and potato [59] by physical contact. The use of abrasives, such as carborundum and celite, has also been tested for transmission of tobacco mosaic virus [60,61]. However, these methods have low efficiency and are low throughput, which makes them unsuitable for field studies. New innovations in this area have the potential to revolutionize virus screening. Environmental variation could be reduced through the use of multiple locations within a year, smaller field sizes, more uniform soil type distribution throughout a field, a soil type more conducive for plant stress, selection based upon families rather than individual plants, and multiple year evaluations for selection rather than a single year. While these changes could improve the genetic gain made per selection cycle, they may negatively impact physical and financial resources, and reduce the number of populations and number of individuals within those populations that can be evaluated. Given that no known resistance to onion thrips, IYSV, and IYS currently exists, numerous, diverse populations are being evaluated for any level of resistance to these plant problems. With the identification of putative germplasm for resistance, breeding efforts can focus on this genetic material for an evaluation of a larger number of individuals.
In addition to environmental factors, several plant factors can influence the evaluation process. Plant maturity appears to influence disease expression [49]. As a plant nears maturity, plant leaves begin to senesce, and disease symptom expression increases relative to those plants not nearing maturity. The evaluation of genetically diverse populations expressing varying bulb maturity times within and between populations can be challenging to conduct collectively within a single year study. In addition, the expression of leaf senescence can be confounded with IYS symptoms [47]. Onions exhibit a high degree of genetic–environmental interaction with respect to plant growth, bulb initiation, bulb maturity, and bulb dormancy. Local adaptation or the lack thereof can impact the disease evaluation process. In addition, a plant’s ability to tolerate abiotic and biotic stresses can influence IYS symptom expression. These plant and environmental factors offer opportunities to improve the selection process; however, the development of an onion cultivar resistant to thrips, IYSV, and/or IYS will be a challenge for years to come.
Author Contributions
N.S. performed the analysis and wrote the manuscript; C.S.C. collected data and wrote the manuscript.
Funding
This research was funded in part by the USDA-NIFA Specialty Crop Research Initiative grant number 2008-51180-04875, a germplasm evaluation grant from the National Plant Germplasm System, USDA-ARS, the New Mexico Agricultural Experiment Station, and the New Mexico Dry Onion Commission.
Acknowledgments
The authors would like to thank Ray Muhyi for his assistance in managing plants in the greenhouse and the field.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Gent, D.H.; du Toit, L.J.; Fichtner, S.F.; Mohan, S.K.; Pappu, H.R.; Schwartz, H.F. Iris yellow spot virus: An emerging threat to onion bulb and seed production. Plant Dis. 2006, 90, 1468–1480. [Google Scholar] [CrossRef]
- Cortes, I.; Livieratos, I.C.; Derks, A.; Peters, D.; Kormelink, R. Molecular and serological characterization of iris yellow spot virus, a new and distinct tospovirus species. Phytopathology 1998, 88, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Coutts, B.A.; McMichael, L.A.; Tesoriero, L.; Rodoni, B.C.; Wilson, C.R.; Wilson, A.J.; Persley, D.M.; Jones, R.A.C. Iris yellow spot virus found infecting onions in three Australian states. Australas. Plant Pathol. 2003, 32, 555–557. [Google Scholar] [CrossRef]
- Pozzer, L.; Bezerra, I.C.; Kormelink, R.; Prins, M.; Peters, D.; Resende, R.D.; de Avila, A.C. Characterization of a tospovirus isolate of iris yellow spot virus associated with a disease in onion fields in Brazil. Plant Dis. 1999, 83, 345–350. [Google Scholar] [CrossRef]
- Rosales, M.; Pappu, H.; Lopez, L.; Mora, R.; Aljaro, A. 1457801. Iris yellow spot virus in onion in Chile. Plant Dis. 2005, 89, 1245. [Google Scholar] [CrossRef]
- Ravi, K.; Kitkaru, A.; Winter, S. Iris yellow spot virus in onions: A new tospovirus record from India. Plant Pathol. 2006, 55, 288. [Google Scholar] [CrossRef]
- Ghotbi, T.; Shahraeen, N.; Winter, S. Occurrence of tospoviruses in ornamental and weed species in Markazi and Tehran provinces in Iran. Plant Dis. 2005, 89, 425–429. [Google Scholar] [CrossRef]
- Gera, A.; Cohen, J.; Salomon, R.; Raccah, B. Iris yellow spot tospovirus detected in onion (Allium cepa) in Israel. Plant Dis. 1998, 82, 127. [Google Scholar] [CrossRef]
- Tomassoli, L.; Tiberini, A.; Masenga, V.; Vicchi, V.; Turina, M. Characterization of Iris yellow spot virus isolates from onion crops in northern Italy. J. Plant Pathol. 2009, 91, 733–739. [Google Scholar]
- Murai, T. Current status of the onion thrips, Thrips tabaci, as a pest thrips in Japan. Agrochem. Jpn. 2004, 84, 7–10. [Google Scholar]
- Ward, L.I.; Perez-Egusquiza, Z.; Fletcher, J.D.; Corona, F.M.O.; Tang, J.Z.; Liefting, L.W.; Martin, E.J.; Quinn, B.D.; Pappu, H.R.; Clover, G.R.G. First report of Iris yellow spot virus on Allium cepa in New Zealand. Plant Pathol. 2009, 58, 406. [Google Scholar] [CrossRef]
- Nischwitz, C.; Pappu, H.R.; Mullis, S.W.; Sparks, A.N.; Langston, D.R.; Csinos, A.S.; Gitaitis, R.D. Phylogenetic analysis of Iris yellow spot virus isolates from onion (Allium cepa) in Georgia (USA) and Peru. J. Phytopathol. 2007, 155, 531–535. [Google Scholar] [CrossRef]
- Robène-Soustrade, I.; Hostachy, B.; Roux-Cuvelier, M.; Minatchy, J.; Hédont, M.; Pallas, R.; Couteau, A.; Cassam, N.; Wuster, G. First report of Iris yellow spot virus in onion bulb- and seed-production fields in Réunion Island. Plant Pathol. 2006, 55, 288. [Google Scholar] [CrossRef]
- Córdoba-Sellés, C.; Martínez-Priego, L.; Muńoz-Gómez, R.; Jordá-Gutiérrez, C. Iris yellow spot virus: A new onion disease in Spain. Plant Dis. 2005, 89, 1243. [Google Scholar] [CrossRef]
- Hall, J.; Mohan, K.; Knott, E.; Moyer, J. Tospoviruses associated with scape blight of onion (Allium cepa) seed crops in Idaho. Plant Dis. 1993, 77, 952. [Google Scholar] [CrossRef]
- Pappu, H.; Matheron, M. Characterization of Iris yellow spot virus from onion in Arizona. Plant Health Prog. 2008, 9, 44. [Google Scholar] [CrossRef]
- Poole, G.J.; Pappu, H.R.; Davis, R.M.; Turini, T.A. Increasing outbreaks and impact of Iris yellow spot virus in bulb and seed onion crops in the Imperial and Antelope Valleys of California. Plant Health Prog. 2007, 8, 50. [Google Scholar] [CrossRef]
- Schwartz, H.; Brown Jr, W.; Blunt, T.; Gent, D. Iris yellow spot virus on onion in Colorado. Plant Dis. 2002, 86, 560. [Google Scholar] [CrossRef]
- Bag, S.; Singh, J.; Davis, R.; Chounet, W.; Pappu, H. Iris yellow spot virus in onion in Nevada and northern California. Plant Dis. 2009, 93, 674. [Google Scholar] [CrossRef]
- Creamer, R.; Sanogo, S.; Moya, A.; Romero, J.; Molina-Bravo, R.; Cramer, C. Iris yellow spot virus on onion in New Mexico. Plant Dis. 2004, 88, 1049. [Google Scholar] [CrossRef]
- Hoepting, C.; Schwartz, H.; Pappu, H. First report of Iris yellow spot virus on onion in New York. Plant Dis. 2007, 91, 327. [Google Scholar] [CrossRef]
- Crowe, F.; Pappu, H. Outbreak of Iris yellow spot virus in onion seed crops in central Oregon. Plant Dis. 2005, 89, 105. [Google Scholar] [CrossRef]
- Miller, M.; Saldana, R.; Black, M.; Pappu, H. First report of Iris yellow spot virus on onion (Allium cepa) in Texas. Plant Dis. 2006, 90, 1359. [Google Scholar] [CrossRef]
- du Toit, L.; Pappu, H.; Druffel, K.; Pelter, G. Iris yellow spot virus in onion bulb and seed crops in Washington. Plant Dis. 2004, 88, 222. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): A global pest of increasing concern in onion. J. Econ. Entomol. 2011, 104, 1–13. [Google Scholar] [CrossRef]
- Shock, C.C.; Feibert, E.; Jensen, L.; Mohan, S.K.; Saunders, L.D. Onion variety response to Iris yellow spot virus. HortTechnology 2008, 18, 539–544. [Google Scholar] [CrossRef]
- Kritzman, A.; Beckelman, H.; Alexandrov, S.; Cohen, J.; Lampel, M.; Zeidan, M.; Raccah, B.; Gera, A. Lisianthus leaf necrosis: A new disease of lisianthus caused by Iris yellow spot virus. Plant Dis. 2000, 84, 1185–1189. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sundaraj, S.; Pappu, H.R.; Diffie, S.; Riley, D.G.; Gitaitis, R.D. Transmission of Iris yellow spot virus by Frankliniella fusca and Thrips tabaci (Thysanoptera: Thripidae). J. Econ. Entomol. 2012, 105, 40–47. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Shelton, A.M. Evaluation of onion cultivars for resistance to onion thrips (Thysanoptera: Thripidae) and Iris yellow spot virus. J. Econ. Entomol. 2010, 103, 925–937. [Google Scholar] [CrossRef]
- Kritzman, A.; Lampel, M.; Raccah, B.; Gera, A. Distribution and transmission of Iris yellow spot virus. Plant Dis. 2001, 85, 838–842. [Google Scholar] [CrossRef]
- Nagata, T.; Almeida, A.C.L.; Resende, R.O.; de Ávila, A.C. The identification of the vector species of Iris yellow spot tospovirus occurring on onion in Brazil. Plant Dis. 1999, 83, 399. [Google Scholar] [CrossRef]
- Riley, D.G.; Joseph, S.V.; Srinivasan, R.; Diffie, S. Thrips vectors of tospoviruses. J. Integr. Pest Manag. 2011, 2, I1–I10. [Google Scholar] [CrossRef]
- Gent, D.H.; Schwartz, H.F.; Khosla, R. Distribution and incidence of Iris yellow spot virus in Colorado and its relation to onion plant population and yield. Plant Dis. 2004, 88, 446–452. [Google Scholar] [CrossRef]
- Schwartz, H.; Gent, D. High Plains IPM Guide, a Cooperative Effort of the University of Wyoming; University of Nebraska, Colorado State University and Montana State University Montana State University, 2004. Available online: https://wiki.bugwood.org/index.php?title=HPIPM:Onion_Iris_Yellow_Spot&oldid=56075 (accessed on 15 January 2019).
- Mau, R.F.L.; Kessing, J.L.M. Thrips tabaci (Linderman). Crop Knowl. Master 2019. Available online: http://www.extento.hawaii.edu/kbase/crop/Type/t_tabaci.htm (accessed on 5 February 2019).
- Cranshaw, W.S.; Schweissing, F. Control of organophosphate resistant onion thrips, 1986. Insectic. Acaric. Tests 1989, 14, 128. [Google Scholar]
- Mahaffey, L.A. Diversity, Seasonal Biology, and IPM of Onion-Infesting Thrips in Colorado; Colorado State University: Fort Collins, CO, USA, 2006. [Google Scholar]
- Aldosari, S.A. Development of an IPM System for Onion Thrips (Thrips Tabaci Lindeman) as a Pest of Bulb Onions. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 1997. [Google Scholar]
- Jones, H.; Bailey, S.; Emsweller, S. Thrips resistance in the onion. Hilgardia 1934, 8, 213–232. [Google Scholar] [CrossRef]
- Coudriet, D.L.; Kishaba, A.N.; McCreight, J.D.; Bohn, G.W. Varietial resistance in onions to thrips. J. Econ. Entomol. 1979, 72, 614–615. [Google Scholar] [CrossRef]
- Molenaar, N.D. Genetics, thrips (Thrips tabaci L.) resistance and epicuticular wax characteristics of nonglossy and glossy onions (Allium cepa L.). Diss. Abstr. Int. B Sci. Eng. 1984, 45, 224. [Google Scholar]
- Damon, S.J.; Groves, R.L.; Havey, M.J. Variation for Epicuticular Waxes on Onion Foliage and Impacts on Numbers of Onion Thrips. J. Am. Soc. Hortic. Sci. 2014, 139, 495–501. [Google Scholar] [CrossRef]
- KIRK, W.D. Ecologically seIective coIoured traps. Ecol. Entomol. 1984, 9, 35–41. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fail, J.; Deutschlander, M.; Nault, B.A.; Shelton, A.M. Characterization of resistance, evaluation of the attractiveness of plant odors, and effect of leaf color on different onion cultivars to onion thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2012, 105, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Mohseni-Moghadam, M.; Cramer, C.S.; Steiner, R.L.; Creamer, R. Evaluating winter-sown onion entries for Iris yellow spot virus susceptibility. HortScience 2011, 46, 1224–1229. [Google Scholar] [CrossRef]
- Boateng, C.O.; Schwartz, H.F.; Havey, M.J.; Otto, K. Evaluation of Onion Germplasm for Resistance to Iris Yellow Spot (Iris Yellow Spot Virus) and Onion Thrips, Thrips tabaci. Southwest. Entomol. 2014, 39, 237–260. [Google Scholar] [CrossRef]
- Multani, P.S.; Cramer, C.S.; Steiner, R.L.; Creamer, R. Screening winter-sown onion entries for Iris yellow spot virus tolerance. HortScience 2009, 44, 627–632. [Google Scholar] [CrossRef]
- Cramer, C.S. Onion germplasm lines selected for reduced Iris yellow spot symptom expression. N.M. Agr. Expt. Stn. Germ. Rel. Not. 2013, 5. [Google Scholar]
- Cramer, C.S.; Singh, N.; Kamal, N.; Pappu, H.R. Screening Onion Plant Introduction Accessions for Tolerance to Onion Thrips and Iris Yellow Spot. Hortscience 2014, 49, 1253–1261. [Google Scholar] [CrossRef]
- Kamal, N.; Cramer, C.S. Selection Progress for Resistance to Iris Yellow Spot in Onions. HortScience 2018, 53, 1088–1094. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; National Genetic Resources Program. Germplasm Resources Information Network–(GRIN); National Germplasm Resources Laboratory: Beltsville, MD, USA, 2015. [Google Scholar]
- NM Climate Center. Leyendecker II PSRC. Available online: https://weather.nmsu.edu/ziamet/station/nmcc-da-5/ (accessed on 5 February 2019).
- Walker, S.J. Bulb Onion Culture and Management for Southern New Mexico; New Mexico State University: Las Cruces, New Mexico, 2009; pp. 1–16. [Google Scholar]
- Cramer, C.S.; Kamal, N.; Singh, N. Evaluating Iris Yellow Spot Disease Incidence and Severity in Onion Germplasm of Varying Leaf Characteristics. HortScience 2017, 52, 527–532. [Google Scholar] [CrossRef]
- Copeland, R. Assaying levels of plant virus by ELISA. In Plant Virology Protocols; Springer: Berlin/Heidelberg, Germany, 1998; pp. 455–460. [Google Scholar]
- Louie, R. Vascular puncture of maize kernels for the mechanical transmission of maize white line mosaic virus and other viruses of maize. Phytopathology 1995, 85, 139–143. [Google Scholar] [CrossRef]
- Zhang, L.; Zitter, T.; Lulkin, E. Artificial inoculation of maize white line mosaic virus into corn and wheat. Phytopathology 1991, 81, 397–400. [Google Scholar] [CrossRef]
- Fraser, L.; Matthews, R. Efficient mechanical inoculation of turnip yellow mosaic virus using small volumes of inoculum. J. Gen. Virol. 1979, 44, 565–568. [Google Scholar] [CrossRef]
- Salazar, L.; Jayasinghe, U. Fundamentals of Purification of Plant Viruses; International Potato Centre: Lima, Peru, 1999; pp. 1–10. [Google Scholar]
- Kalmus, H. The use of abrasives in the transmission of plant viruses. Ann. Appl. Biol. 1945, 32, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Beraha, L.; Varzandeh, M.; Thornberry, H. Mechanism of the action of abrasives on infection by tobacco mosaic virus. Virology 1955, 1, 141–151. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).