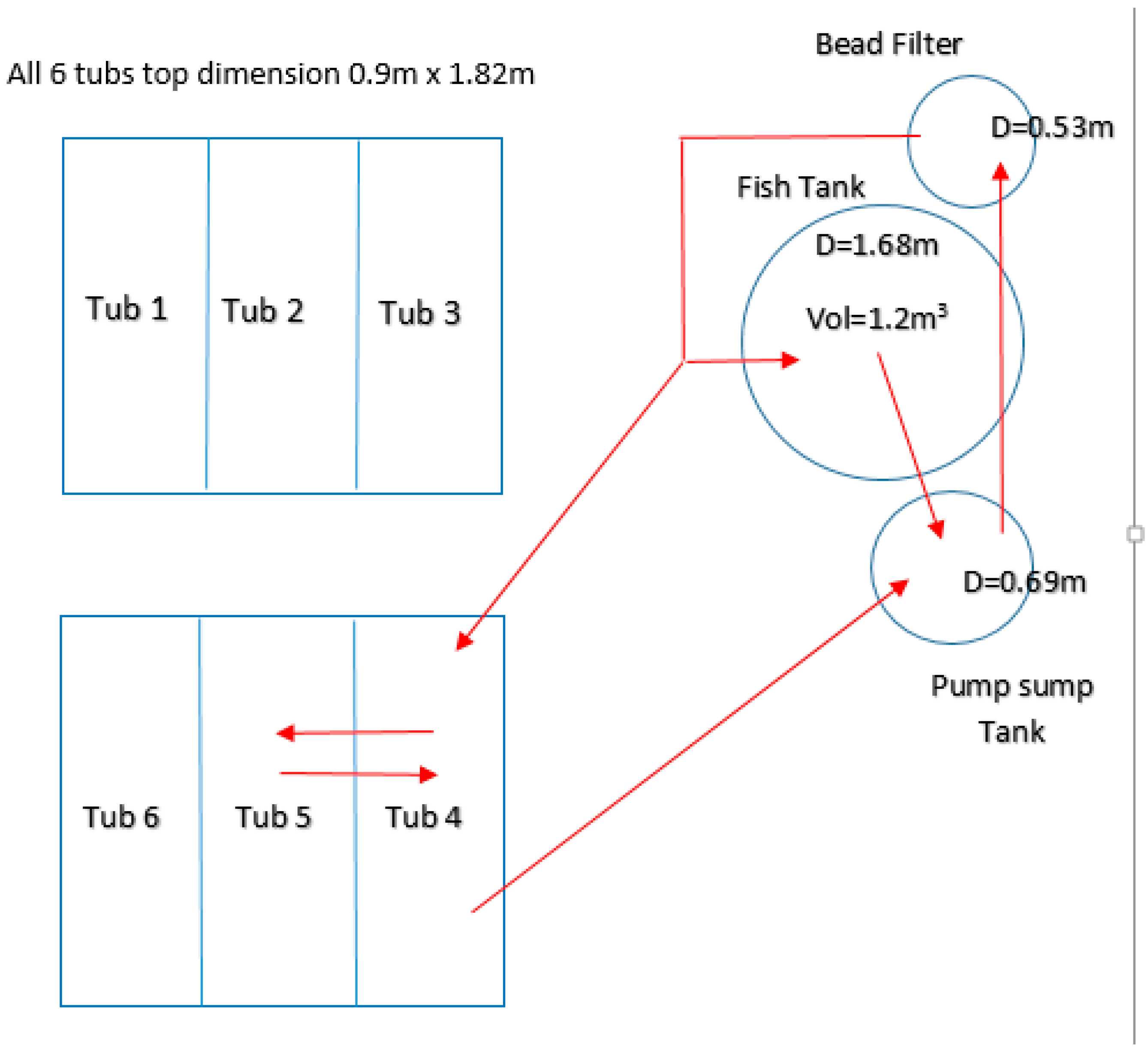
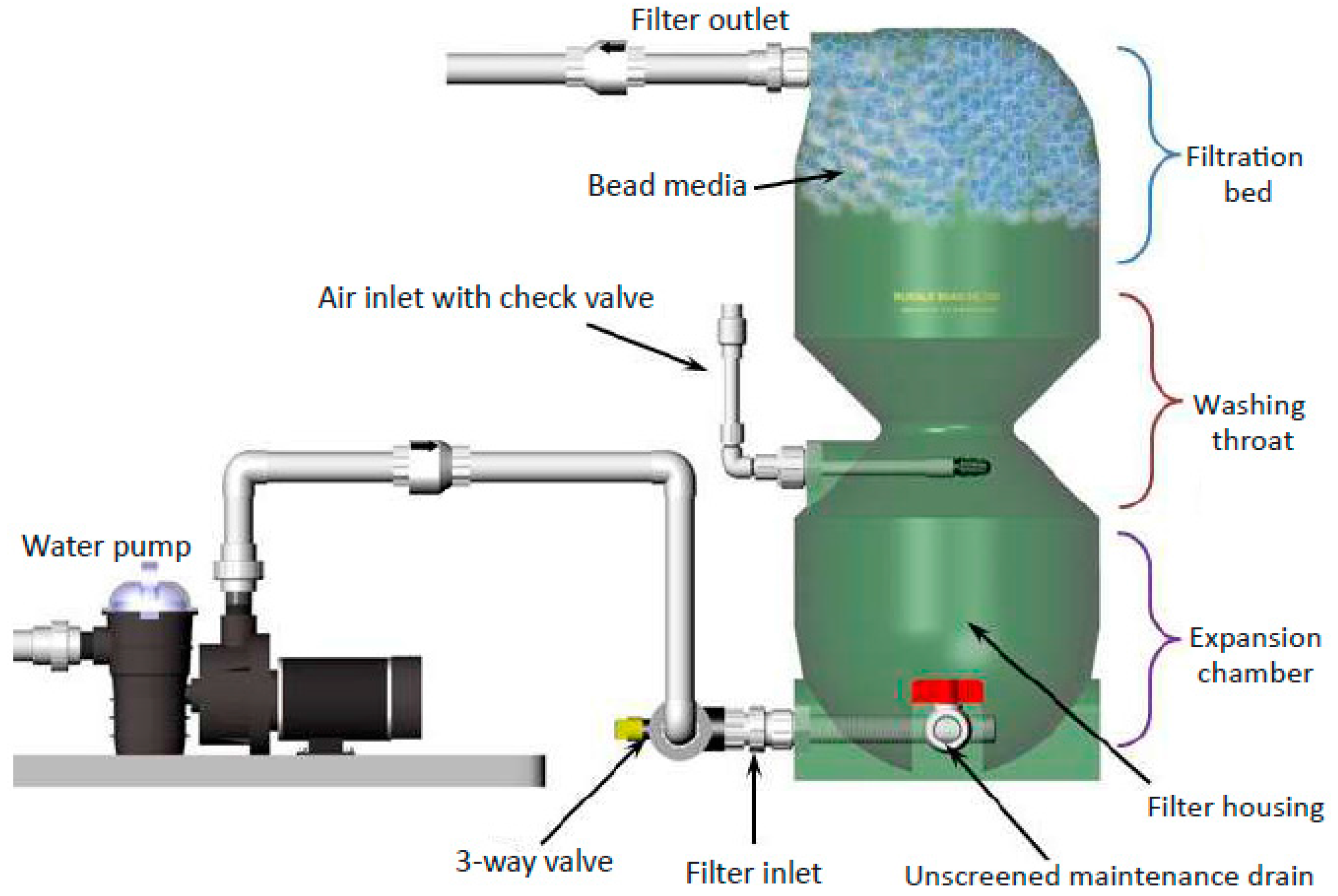
4.2. Tissue Elements
The differences found in microelements between the treatments were generally proportional to the differences in the nutrient solutions. A proportional uptake of micronutrients has been reported by others, e.g., data from Liedl et al. [
21] showing a proportional response of Mo tissue content to nutrient solution concentrations.
The tissue elemental comparisons generally did not differ, which is a significant positive result for the aquaponics treatment (A7). We hypothesized that the aquaponic plants would suffer biomass and tissue elemental effects from deviations from an ‘ideal’ nutrient solution formulated specifically for lettuce. For example, the elemental concentrations of B, Ca, Cu, P, Mn, Mo, and Sr were all much higher in the hydroponic pH 5.8 treatment (H5) at the end of each trial than in the aquaponic treatment at pH 7.0 (A7).
Most of the differences in elemental tissue concentrations were unlikely to have been causal or meaningful to biomass response. Very significant and large differences such as those found with Zn, Mo, Cu, and Mn were all within the normal bounds of lettuce tissue content, and may be explained by significantly elevated or diminished concentrations or ratios in the nutrient solutions.
Among the macroelements included in the Sonneveld nutrient solution (K, Ca, N, P, Mg, S), a tissue difference in Ca between the hydroponic pH 5.8 treatment (H5) and the aquaponic pH 7.0 treatment (A7) was the only difference, and this difference was proportional to the difference in Ca levels in the nutrient solutions. The A7 Ca tissue content was ~11% lower than H5, and there was no difference between the two hydroponic treatments (H5 and H7) or H7 and A7. The Ca nutrient solution in both H7 and A7 was below the nutrient solution targets, A7 much more so. However, the pH alone did not appear to influence the availability of Ca to the lettuce, since the H7 value was not different from H5, where the only difference was pH. It is also possible that lettuce has a critical level for Ca, above which there are no deficiencies observed in plant tissue.
The mean nitrogen (N) tissue content was 5.82%, 5.68%, and 5.84% for hydroponic pH 5.8 (H5), hydroponic pH 7.0 (H7), and aquaponic pH 7.0 (A7), respectively; there were no significant differences among these pairwise comparisons. Our previous experiment found N contents of 5.42% and 4.95% for H5 and H7, respectively [
14]. Our values from both experiments were slightly higher than the 4.5% N reported by Resh [
1] and Seawright et al. [
22], and much higher than the 2.9% reported by Pantanella et al. [
23]. These comparisons indicate that a system’s design, its management, and the N bioavailability of the nutrient solution may affect N assimilation by the plant, and the values reported from one study have to be carefully evaluated before assuming applicability to another growing and management system.
The mean copper (Cu) tissue concentrations were different for hydroponic pH 5.8 (H5) compared to both hydroponic pH 7.0 (H7) and aquaponic pH 7.0 (A7); H7 and A7 were not different. The Cu nutrient solution concentrations were close to equivalent or slightly smaller in H7 and A7 than in H5. Despite the less than or equivalent Cu in solution, the tissue Cu concentrations in H7 and A7 suggest that pH may have some effect on the uptake of Cu in hydroponic solutions. However, others have shown that lower pH values should increase lettuce tissue Cu content [
24], which suggests that our measurements of higher Cu in tissues at higher pH may have resulted from other complex molecular substances containing Cu, such as slightly more DTPA chelator transport of Cu at pH 7.
The mean sodium (Na) tissue concentrations were 720, 717, and 2027mg/kg for hydroponic pH 5.8 (H5), hydroponic pH 7.0 (H7), and aquaponic pH 7.0 (A7), respectively. The difference in A7 can be attributed to the 10-times higher concentration of Na in the nutrient solution for A7 than for either H5 or H7. Na is not included in the Sonneveld nutrient solution. The lack of difference between H5 and H7 in Na nutrient solution and tissue concentrations suggest no pH effect. The increased Na concentration in the A7 nutrient solution correlated with the increased A7 tissue concentration. Caution may be needed in RAS to guard against elevated tissue Na, since Na in the nutrient solution can become elevated due to the constant recycling of water, concentration due to evaporation, and the difficulty of eliminating Na from the fish feed source. Minimizing Na in the fish diet may need to be considered in aquaponics, especially where the grower is attempting to minimize Na tissue levels in the lettuce product.
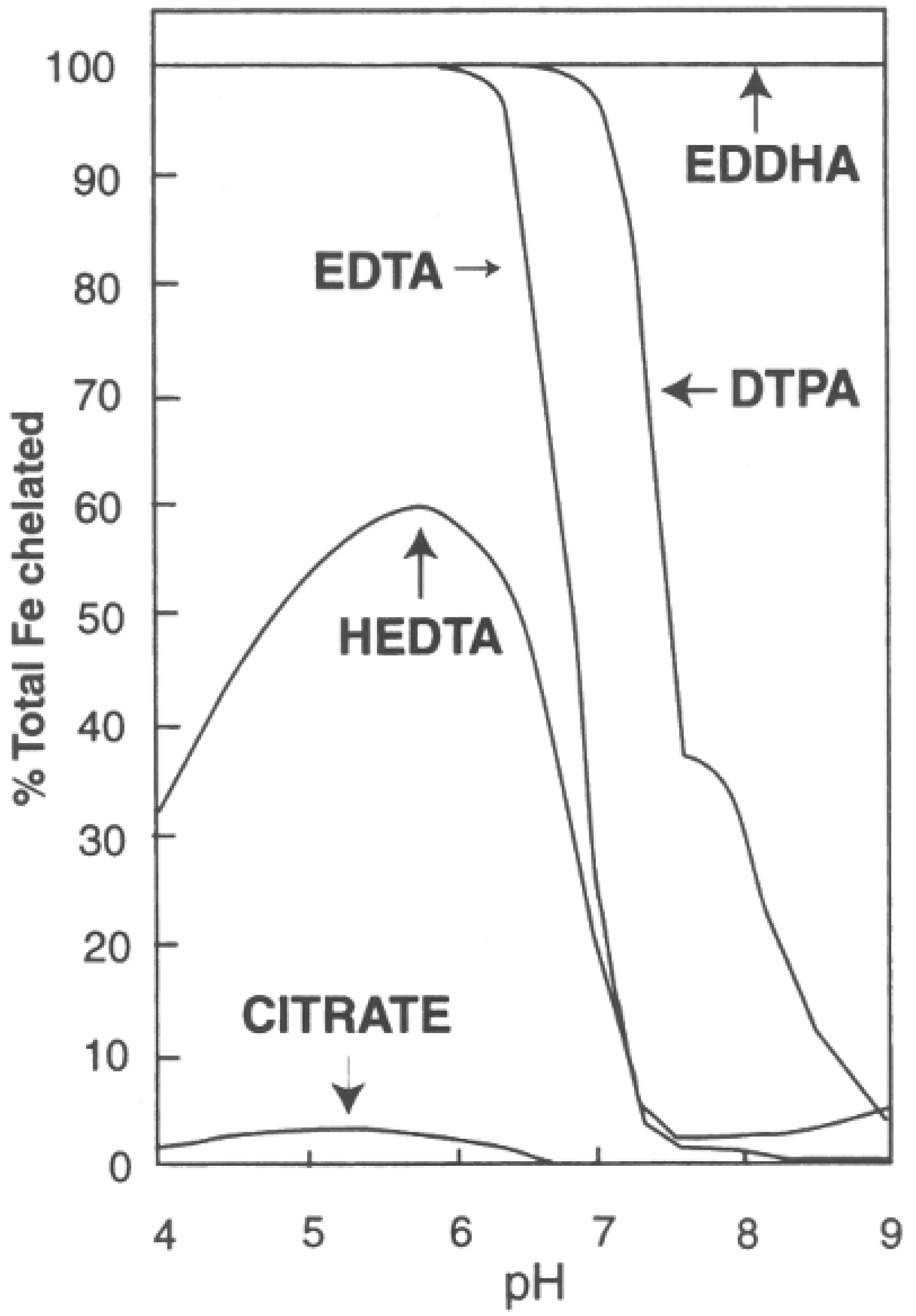
The Fe tissue concentrations were very consistent among all treatments. The percentage of bound Fe for common chelators between pH 4 and 9 is shown in
Figure 4. Within the typical pH range used in conventional hydroponics, the DTPA chelator, as used in this experiment for all treatments, is at or very nearly 100% selective for Fe. As the solution approaches pH 7.0, as is the case for aquaponic pH 7.0 (A7) or hydroponic pH 7.0 (H7), the DTPA chelator is still very effective at ≥97% selective for Fe. However, given that a chelator may bind to other elements, the ~3% of chelator not bound to iron may aid in the transport of other elements into the plant for the two higher pH treatments. For example, Blaylock et al. [
25] has reported that EDTA additions increased the transport into plants of five metals including Pb. Thus, while elevated pH values generally result in less nutrient availability, the free chelator may counterbalance a portion of this negative pH effect.
The mean boron (B) tissue concentrations were not different among treatments, even though the aquaponic treatment (A7) nutrient solution concentrations of B were an order of magnitude smaller than either of the hydroponic treatments (H5 and H7). If B is passively acquired, as suggested by Brown et al. [
27], then our hypothesis is that B usage is low enough and B is sufficient in the nutrient solutions such that equilibrium is reached between the plants and solution. This would explain the order of magnitude lower nutrient solution concentrations resulting in the same plant tissue concentrations.
The mean silicon (Si) tissue concentrations were not different among the treatments. Even though Si was not in our Sonneveld nutrient solution, Si was present in the three treatment solutions. The starting Si nutrient solution concentrations were 0.05, 0.10, and 1.27mg/L for hydroponic pH 5.8 (H5), hydroponic pH 7.0 (H7), and aquaponic pH 7.0 (A7), respectively, while the ending nutrient solution concentrations were 0.12, 0.30, and 1.55mg/L for H5, H7, and A7 respectively, showing an accumulation over the growing cycle in all three treatments. The very low concentrations in the nutrient solution appear to still accumulate significant quantities in the shoots, yet they had no measured effect on the biomass shoot yield between H5 and A7. One explanation may be that Si was acquired from the rockwool cube fibers, which are silicate-based. A larger discussion on a rockwool cube elemental analysis can be found in our previous paper [
14], where we included a hot plate acid digestion and ICP-AES analysis of the rockwool cube stock used in our experiments.
The order of magnitude larger nutrient solution concentration in Si for the aquaponic pH 7.0 treatment (A7) did not increase the tissue concentration between treatments. This is potentially important, since a grower could choose to add Si to the nutrient solution as a preventative measure against reduced production to combat potential stress that the crop may experience. There are several reports of silicon reducing stress in hydroponically grown plants [
28,
29], including the alleviation of abiotic and biotic stresses [
30,
31].
4.3. Precipitation from System
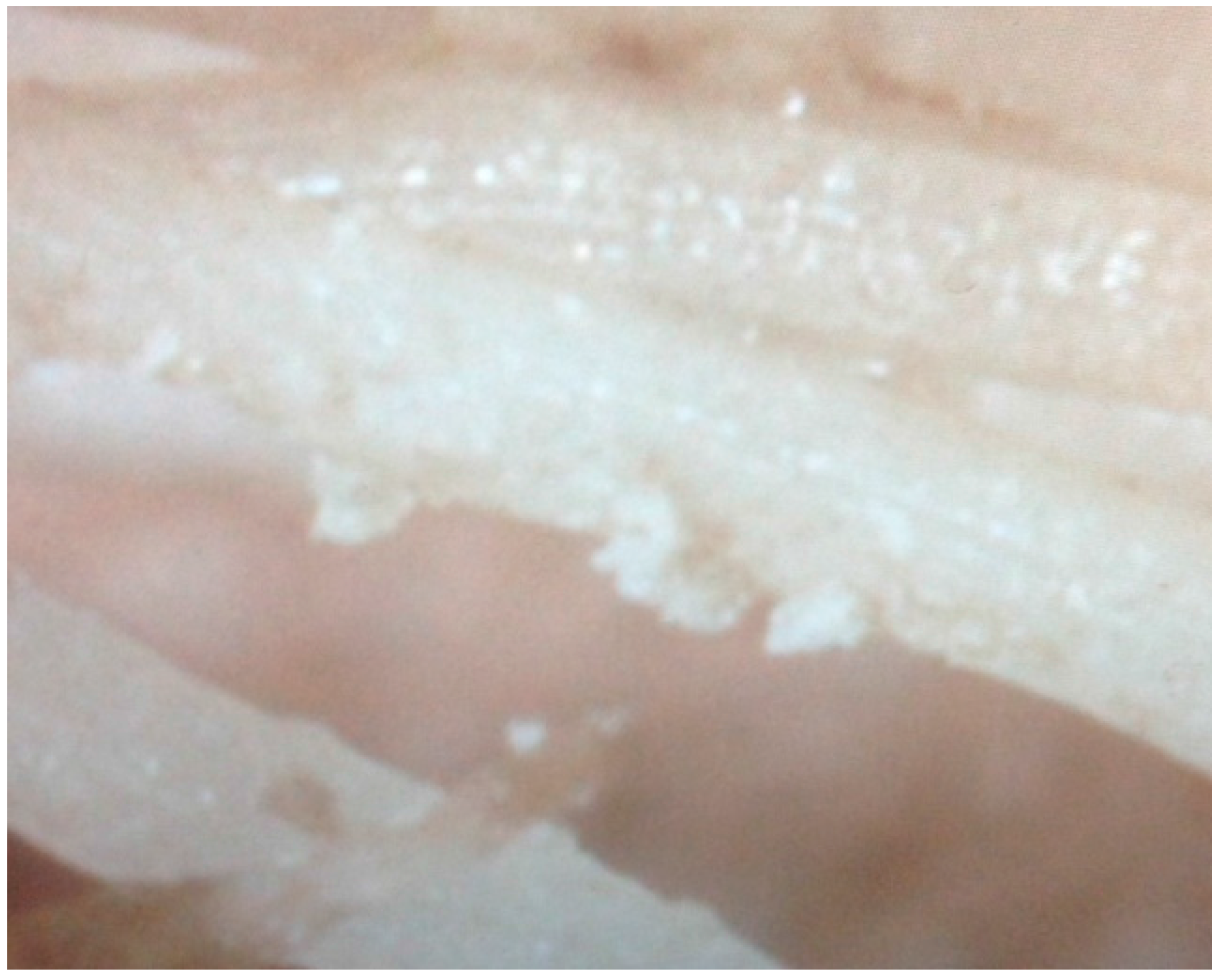
There was significant precipitate as granular “sand” in the hydroponic pH 7.0 (H7) HDPE tubs, and the formation of a precipitate “skin” on the tub sides. White precipitate was also observed on the roots of the H7 treatment (
Figure 5). Despite the slow addition of acid and base with vigorous mixing, localized white precipitate plumes did occasionally appear in the H7 treatment. The H7 treatment required daily pH adjustment, including during the first week, when the plants were proportionally having very little influence on the nutrient solution. The hydroponic pH 5.8 treatment (H5) required little to no adjustments the first week, and typically much smaller adjustments compared to H7 as each trial proceeded. Seawright et al. [
22] also reported on the continual precipitation of calcium phosphate from solution in an integrated aquaponic system with the fish systems maintained at pH 7.5.
The precipitate’s digestion results ranged between 97–98% as Ca and P (as PO43−), and the average Ca to P molar ratio of the precipitate was 1.39:1 (standard deviation (SD) = 0.06). Since the precipitation was unique to the hydroponic pH 7.0 treatment (H7) and correlated to a daily requirement to raise the pH with KOH, this indicates that the form(s) of precipitating calcium phosphate resulted in donated hydrogen ion(s) to the solution.
The precipitation of P in the hydroponic pH 7.0 treatment (H7) and the resulting decreased nutrient solution concentration caused no differences in P tissue contents among treatments. This lack of difference in P was surprising considering the 3-times higher concentration of P in the nutrient solution of the hydroponic pH 5.8 treatment (H5) compared to either H7 or the aquaponic pH 7.0 treatment (A7). H7 started at the target concentration, but dropped in availability to a third of the starting concentration, while A7 remained fairly constant at a third of the target nutrient solution concentration.
4.4. Summary
This experiment demonstrated that aquaponics can produce high quality lettuce in both size and elemental nutrient content while not suffering any significant decrease in biomass in comparison to a best practices conventional inorganic hydroponics at pH 5.8. We hope this research expands the discussion on using targeted inorganic nutrient solutions that provide a repeatable process versus nutrient solutions provided primarily from organic sources, e.g., aquaponics. The next question to consider is whether the byproducts of the aquaponic system, the direct secretions and waste from the fish, and the decomposition and mineralization of complex nutrients, hormones, enzymes, and compounds are a causal factor in the results, or whether some direct or short lived aspects of the flora, fauna, and their byproducts are causal to these results.
Prior to this research, we believed aquaponic systems to be more complicated and more risky because two complex systems were being joined (hydroponics plus RAS). However, the aquaponics system proved to be surprisingly simple to manage in daily operations. Our data suggested that the aquaponics system (A7), which was operated at a higher pH 7.0, was able to offset any negative biomass and elemental effects that occurred in the inorganic hydroponic pH 7.0 condition (H7) from its increased pH and less optimized nutrient solution elemental concentrations. This study shows that caution should be taken when raising the standard hydroponic solution pH from 5.8 to pH 7.0, which can slow plant growth, negatively affecting product yields.