New Trends in the Fertigation Management of Irrigated Vegetable Crops
Abstract
:1. Introduction
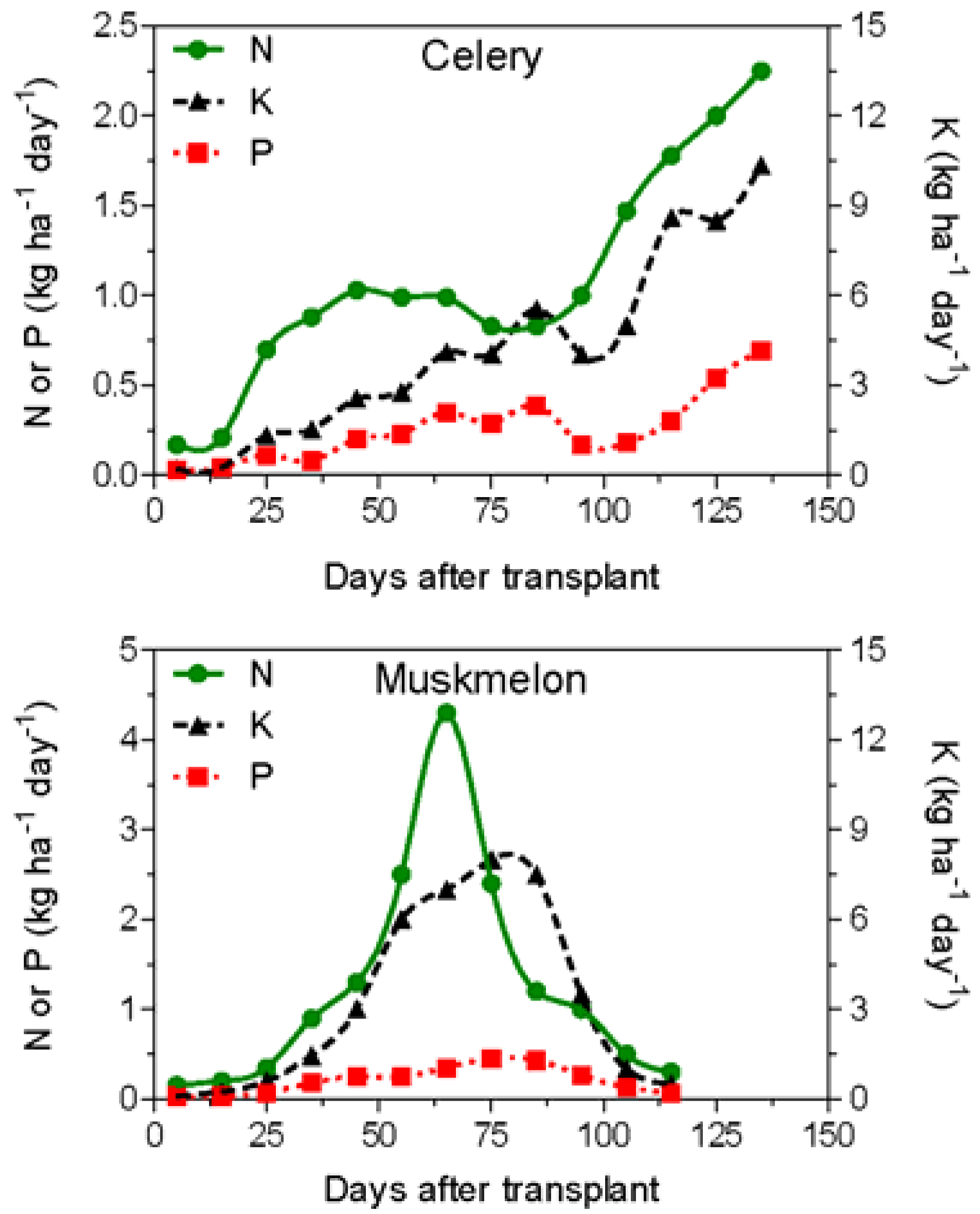
2. Nutrients Applied by Fertigation
3. Optimal Fertigation Management
4. Techniques for Prescriptive Fertigation Management
4.1. Simulation Models
4.2. Decision Support Systems Based on Simulators
5. Techniques for Corrective Fertigation Management
5.1. Plant Monitoring
5.2. Root Zone Monitoring
5.3. Decision Support Systems Based on Crop Monitoring
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Goldberg, D.; Gornat, B.; Rimon, D. Drip Irrigation Principles, Design and Agricultural Practices; Drip Irrigation Scientific Publications: Kfar Shmariahu, Israel, 1976. [Google Scholar]
- Dasberg, S.; Bresler, E. Drip Irrigation Manual; Publ. No. 9; International Irrigation Information Center: Bet Dagan, Israel, 1985. [Google Scholar]
- Bar-Yosef, B. Advances in fertigation. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: London, UK, 1999; Volume 65, pp. 1–77. [Google Scholar]
- Reinders, F.B. Micro-irrigation: World overview on technology and utilization. In Proceedings of the 7th International Micro-Irrigation Congress, Kuala Lumpur, Malaysia, 13–15 September 2007. [Google Scholar]
- International Commission on Irrigation and Drainage (ICID). Agricultural Water Management for Sustainable Rural Development: Annual Report 2015–2016; International Commission on Irrigation and Drainage: New Delhi, India, 2016; pp. 79–80. [Google Scholar]
- Howell, T.A. Irrigation Efficiency; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Pardossi, A.; Incrocci, L. Traditional and new approaches to irrigation scheduling in vegetable crops. HortTechnology 2011, 21, 309–313. [Google Scholar]
- Hagin, J.; Lowengart, A. Fertigation for minimizing environmental pollution by fertilizers. Fert. Res. 1995, 43, 5–7. [Google Scholar] [CrossRef]
- Bryan, B.B.; Thomas, E.L. Distribution of Fertilizer Materials Applied through Sprinkler Irrigation Systems; Arkansas System Division of Agriculture Experiment Station: Fayetteville, AR, USA, 1958; p. 12. [Google Scholar]
- Sagiv, B.; Kafkafi, U. Fertilization and manuring of pepper plants in sandy soils. Hassadeh 1976, 56, 1726–1730. [Google Scholar]
- Agostini, F.; Tei, F.; Silgram, M.; Farneselli, M.; Benincasa, P.; Aller, M. Decreasing nitrate leaching in vegetable crops with better N management. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Springer: Berlin, Germany, 2010; pp. 147–200. [Google Scholar]
- Solaimalai, A.; Baskar, M.; Sadasakthi, A.; Subburamu, K. Fertigation in high value crops—A review. Agric. Rev. 2005, 1, 1–13. [Google Scholar]
- Thompson, R.B.; Martínez-Gaitán, C.; Gallardo, M.; Giménez, C.; Fernández, M.D. Identification of irrigation and N management practices that contribute to nitrate leaching loss from an intensive vegetable production system by use of a comprehensive survey. Agric. Water Manag. 2007, 89, 261–274. [Google Scholar] [CrossRef]
- Harter, T.; Lund, J.R. Addressing Nitrate in California’s Drinking Water; Report for the State Water Resources Control Board; Center for Watershed Sciences, University of California: Davis, CA, USA, 2012. [Google Scholar]
- Alvino, A.; Marino, S. Remote and proximal sensing for precision agriculture and irrigation management. Horticulturae 2017, in press. [Google Scholar]
- Cahn, M. New approaches to irrigation scheduling of vegetables. Horticulturae 2017, 3, 28. [Google Scholar] [CrossRef]
- George, R. Sensor sense in irrigation management. Horticulturae 2017. submitted. [Google Scholar]
- Bar-Yosef, B. Advances in fertigation. Adv. Agron. 1999, 65, 1–77. [Google Scholar]
- Hanson, B.R.; Šimůnek, J.; Hopmans, J.W. Evaluation of urea-ammonium-nitrate fertigation with drip irrigation using numerical modeling. Agric. Water Manag. 2006, 86, 102–113. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Rao, M. Wetting patterns and nitrogen distributions as affected by fertigation strategies from a surface point source. Agric. Water Manag. 2004, 67, 89–104. [Google Scholar] [CrossRef]
- Haynes, R.J. Movement and transformations of fertigated nitrogen below trickle emitters and their effects on pH in the wetted soil volume. Fert. Res. 1990, 23, 105–112. [Google Scholar] [CrossRef]
- Abalos, D.; Sanchez-Martin, L.; Garcia-Torres, L.; van Groenigen, J.W.; Vallejo, A. Management of irrigation frequency and nitrogen fertilization to mitigate GHG and NO emissions from drip-fertigated crops. Sci. Total Environ. 2014, 490, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Shedeed, S.I.; Zaghloul, S.M.; Yassen, A. Effect of method and rate of fertilizer application under drip irrigation on yield and nutrient uptake by tomato. Ozean J. Appl. Sci. 2009, 2, 139–147. [Google Scholar]
- Farneselli, M.; Benincasa, P.; Tosti, G.; Simonne, E.; Guiducci, M.; Tei, F. High fertigation frequency improves nitrogen uptake and crop performance in processing tomato grown with high nitrogen and water supply. Agric. Water Manag. 2015, 154, 52–58. [Google Scholar] [CrossRef]
- Badr, M.; El-Yazied, A. Effect of fertigation frequency from subsurface drip irrigation on tomato yield grown on sandy soil. Aust. J. Basic Appl. Sci. 2007, 1, 279–285. [Google Scholar]
- Simonne, E.; Studstill, D.; Hochmuth, R.C. Understanding water movement in mulched beds on sandy soils: An approach to ecologically sound fertigation in vegetable production. Acta Hortic. 2006, 700, 173–178. [Google Scholar] [CrossRef]
- Hartz, T.; Hochmuth, G.J. Fertility management of drip-irrigated vegetables. HortTechnology 1996, 6, 168–172. [Google Scholar]
- Everaarts, A.P.; de Moel, C.P. The effect of nitrogen and the method of application on yield and quality of white cabbage. Eur. J. Agric. 1998, 9, 203–211. [Google Scholar] [CrossRef]
- Hartz, T.K.; Bendixen, W.E.; Wierdsma, L. The value of presidedress soil nitrate testing as a nitrogen management tool in irrigated vegetable production. HortScience 2000, 35, 651–656. [Google Scholar]
- Heckman, J.R.; Morris, T.; Sims, J.T.; Sieczka, J.B.; Krogmann, U.; Nitzsche, P.; Ashley, R. Pre-sidedress soil nitrate test is effective for fall cabbage. HortScience 2002, 37, 113–117. [Google Scholar]
- Bottoms, T.G.; Smith, R.F.; Cahn, M.D.; Hartz, T.K. Nitrogen requirements and N status determination of lettuce. HortScience 2012, 47, 1768–1774. [Google Scholar]
- Tei, F.; Benincasa, P.; Guiducci, M. Nitrogen fertilisation on lettuce, processing tomato and sweet pepper: Yield, nitrogen uptake and the risk of nitrate leaching. Acta Hortic. 1999, 506, 61–67. [Google Scholar] [CrossRef]
- Lord, E.; Johnson, P.; Archer, J. Nitrate Sensitive Areas: A study of large scale control of nitrate loss in England. Soil Use Manag. 1999, 15, 201–207. [Google Scholar] [CrossRef]
- Bar-Yosef, B.; Kramer, S.; Ben Basat, S. Trickle Irrigation and Fertilization in the Northern Arava Valley in Israel: Fresh Tomatoes (cv. 675); Annual Report; Agricultural Research Organization: Bet-Dagan, Israel, 1982. [Google Scholar]
- Dafne, O. Nitrogen Effect on Dry Matter Production, Mineral Absorption, Yield and Quality of Processing Tomatoes; University of Jerusalem: Jerusalem, Israel, 1984. [Google Scholar]
- Bar-Yosef, B.; Kramer, S.; Ben Basat, S. Trickle Irrigation and Fertilization in the Northern Arava Valley in Israel: Egg plant; Annual Report; Agricultural Research Organization: Bet-Dagan, Israel, 1981. [Google Scholar]
- Feigin, A.; Sagiv, B. Broccoli Response to Manure and N Fertilization in a Loessial Soil in Southern Israel; Agricultural Research Organization: Bet Dagan, Israel, 1971. [Google Scholar]
- Bar-Yosef, B.; Sagiv, B. Trickle Irrigation and Fertilization of Iceberg Lettuce. In Proceedings of the 9th International Plant Nutrition Colloquium, Coventry, England, 22–27 August 1982; pp. 33–38. [Google Scholar]
- Feigin, A.; Sagiv, B.; Aviram, H.; Zipilevich, Y. Celery Response to Manure and N Fertilization in a Loessial Soil in Southern Israel; Report on Project 301–046; Agricultural Research Organization: Bet Dagan, Israel, 1976. [Google Scholar]
- Sagiv, B.; Bar-Yosef, B.; Eliah, E. Drip Irrigation and Fertilization of Spring Muskmelon at Besor; Pub. No. 17; Division of Scientific Publications, The Volcani Center: Bet Dagan, Israel, 1980.
- Giller, K.E.; Chalk, P.; Dobermann, A.; Hammond, L.; Heffer, P.; Ladha, J.K.; Nyamudeza, P.; Maene, L.; Ssali, H.; Freney, J. Emerging technologies to increase the efficiency of use of fertilizer nitrogen. In Agriculture and the Nitrogen Cycle; Mosier, A., Syers, J.K., Freney, J.R., Eds.; Island Press: New York, NY, USA, 2004; p. 344. [Google Scholar]
- Thornley, J.H.M.; Johnson, I.R. Plant and Crop Modelling: A Mathematical Approach to Plant and Crop Physiology; Clarendon Press: Oxford, UK, 1990; p. 670. [Google Scholar]
- Massa, D.; Mattson, N.S.; Lieth, H.J. Effects of saline root environment (NaCl) on nitrate and potassium uptake kinetics for rose plants: A Michaelis-Menten modelling approach. Plant Soil 2009, 318, 101–115. [Google Scholar] [CrossRef]
- Louison, L.; Omrane, A.; Ozier-Lafontaine, H.; Picart, D. Modeling plant nutrient uptake: Mathematical analysis and optimal control. Lect. Notes Pure Appl. 2015, 4, 193–203. [Google Scholar] [CrossRef]
- Karlberg, L.; Ben-Gal, A.; Jansson, P.E.; Shani, U. Modelling transpiration and growth in salinity-stressed tomato under different climatic conditions. Ecol. Model. 2006, 190, 15–40. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Bibbiani, C.; Carmassi, G.; Malorgio, F.; Pardossi, A. Simulation of crop water and mineral relations in greenhouse soilless culture. Environ. Model. Softw. 2011, 26, 711–722. [Google Scholar] [CrossRef]
- Thompson, R.B.; Gallardo, M.; Rodríguez, J.S.; Sánchez, J.A.; Magán, J.J. Effect of N uptake concentration on nitrate leaching from tomato grown in free-draining soilless culture under Mediterranean conditions. Sci. Hortic. 2013, 150, 387–398. [Google Scholar] [CrossRef]
- Pardossi, A.; Incrocci, L.; Malorgio, F.; Campiotti, C.A. The relationship between mineral nutrition and fruit yield components in melon grown in recirculating nutrient solution culture. Agric. Mediterr. 2004, 134, 8–14. [Google Scholar]
- Voogt, W.; Kipp, J.A.; de Graaf, R.; Spaans, L. A fertigation model for glasshouse crops grown in soil. Acta Hortic. 2000, 537, 495–502. [Google Scholar] [CrossRef]
- Voogt, W.; Steinbuch, F.; van Winkel, A. Evaluation of the ‘fertigation model’, a decision support system for water and nutrient supply for soil grown greenhouse crops. Acta Hortic. 2006, 718, 531–538. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Carmassi, G.; Campiotti, C.A.; Pardossi, A. Strategies to decrease water drainage and nitrate emission from soilless cultures of greenhouse tomato. Agric. Water Manag. 2010, 97, 971–980. [Google Scholar] [CrossRef]
- Incrocci, L. Gestire la nutrizione in serra con due software gratuiti. L’Inf. Agrar. 2012, 68, 50–51. [Google Scholar]
- Seginer, I. A dynamic model for nitrogen-stressed lettuce. Ann. Bot. 2003, 91, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Battilani, A. Fertirrigere V2.11: A multi-target DSS to manage water and nutrient supply at macrozone level. Acta Hortic. 2006, 724, 111–118. [Google Scholar] [CrossRef]
- Battilani, A.; Bussières, P.; Dumas, Y. Fertirrigere: A simple tool-model for managing water and nutrient supply in drip-irrigated processing tomatoes. Acta Hortic. 2003, 613, 155–158. [Google Scholar] [CrossRef]
- Gallardo, M.; Giménez, C.; Martínez-Gaitán, C.; Stöckle, C.O.; Thompson, R.B.; Granados, M.R. Evaluation of the VegSyst model with muskmelon to simulate crop growth, nitrogen uptake and evapotranspiration. Agric. Water Manag. 2011, 101, 107–117. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Goudriaan, J. Modelling biomass production and yield of horticultural crops: A review. Sci. Hortic. 1998, 74, 83–111. [Google Scholar] [CrossRef]
- Causton, D.R.; Dale, P.M. The monomolecular and rectangular hyperbola as empirical models of the response of photosynthetic rate to photon flux density, with applications to three Veronica species. Ann. Bot. 1990, 65, 389–394. [Google Scholar] [CrossRef]
- Wang, Y.P. A refinement to the two-leaf model for calculating canopy photosynthesis. Agr. For. Meteorol. 2000, 101, 143–150. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Thornley, J.H.M. Temperature and CO2 responses of leaf and canopy photosynthesis: A clarification using the non-rectangular hyperbola model of photosynthesis. Ann. Bot. 1998, 82, 883–892. [Google Scholar] [CrossRef]
- Lieth, J.H.; Pasian, C.C. A model for net photosynthesis of rose leaves as a function of photosynthetically active radiation, leaf temperature, and leaf age. J. Am. Soc. Hortic. Sci. 1990, 115, 486–491. [Google Scholar]
- Thornley, J.H.M. Dynamic model of leaf photosynthesis with acclimation to light and nitrogen. Ann. Bot. 1998, 81, 421–430. [Google Scholar] [CrossRef]
- Hikosaka, K. Optimality of nitrogen distribution among leaves in plant canopies. J. Plant Res. 2016, 129, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.W.; Dayan, E.; Allen, L.H.; van Keulen, H.; Challa, H. A dynamic tomato growth and yield model (TOMGRO). Am. Soc. Agric. Eng. 1991, 34, 663–672. [Google Scholar] [CrossRef]
- Silberbush, M.; Lieth, J.H. Nitrate and potassium uptake by greenhouse roses (Rosa hybrida) along successive flower-cut cycles: A model and its calibration. Sci. Hortic. 2004, 101, 127–141. [Google Scholar] [CrossRef]
- Mattson, N.; Lieth, H. ‘Kardinal’ rose exhibits growth plasticity and enhanced nutrient absorption kinetics following nitrate, phosphate, and potassium deprivation. J. Am. Soc. Hort. Sci. 2008, 133, 341–350. [Google Scholar]
- Van Diepen, C.A.; Wolf, J.; van Keulen, H.; Rappoldt, C. WOFOST: A simulation model of crop production. Soil Use Manag. 1989, 5, 16–24. [Google Scholar] [CrossRef]
- Hutson, J.L.; Wagenet, R.J. Leaching Estimation and Chemistry Model: A Process-based Model of Water and Solute Movement, Transformation, Plant Uptake and Chemical Reaction in the Unsaturated Zone; The Department of Soil, Crop and Atmospheric Sciences, Cornell University: New York, NY, USA, 1991; p. 121. [Google Scholar]
- Mary, B.; Guérif, J. Intérêts et limites des modèles de prévision de l'évolution des matières organiques et de l‘azote dans le sol. Cah. Agric. 1994, 3, 247–257. [Google Scholar]
- Webb, T.H.; Lilburne, L.R.; Francis, G.S. Validation of the GLEAMS simulation model for estimating net nitrogen mineralisation and nitrate leaching under cropping in Canterbury, New Zealand. Aust. J. Soil Res. 2001, 39, 1015–1025. [Google Scholar] [CrossRef]
- Hansen, S.; Abrahamsen, P.; Petersen, C.T.; Styczen, M. Daisy: Model use, calibration, and validation. Trans. ASABE 2012, 55, 1315–1333. [Google Scholar] [CrossRef]
- Heinen, M. FUSSIM2: Brief description of the simulation model and application to fertigation scenarios. Agronomie 2001, 21, 285–296. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Modeling Subsurface Water Flow and Solute Transport with HYDRUS and Related Numerical Software Packages; Garcia-Navarro, P., Ed.; Numerical Modelling of Hydrodynamics for Water Resources; Taylor & Francis Group: London, UK, 2007. [Google Scholar]
- Stöckle, C.O.; Donatelli, M.; Nelson, R. CropSyst, a cropping systems simulation model. Eur. J. Agric. 2003, 18, 289–307. [Google Scholar] [CrossRef]
- Martínez-Gaitán, C.; Gallardo, M.; Thompson, R.B.; Stöckle, C.O.; Granados, M.R.; Fernández, M.D.; Giménez, C. Use of CropSyst to simulate growth, ETc and N uptake for the development of irrigation and N fertiliser programs in intensive vegetable crop production. Acta Hortic. 2008, 802, 337–342. [Google Scholar] [CrossRef]
- Giménez, C.; Gallardo, M.; Martínez-Gaitán, C.; Stöckle, C.O.; Thompson, R.B.; Granados, M.R. VegSyst, a simulation model of daily crop growth, nitrogen uptake and evapotranspiration for pepper crops for use in an on-farm decision support system. Irrig. Sci. 2013, 31, 465–477. [Google Scholar] [CrossRef]
- Gallardo, M.; Thompson, R.B.; Giménez, C.; Padilla, F.M.; Stöckle, C.O. Prototype decision support system based on the VegSyst simulation model to calculate crop N and water requirements for tomato under plastic cover. Irrig. Sci. 2014, 32, 237–253. [Google Scholar] [CrossRef]
- Rahn, C.R.; Zhang, K.; Lillywhite, R.; Ramos, C.; Doltra, J.; de Paz, J.M.; Riley, H.; Fink, M.; Nendel, C.; Thorup-Kristensen, K.; et al. Eu-Rotate_N—A decision support system—To predict environmental and economic consequences of the management of nitrogen fertiliser in crop rotations. Eur. J. Hortic. Sci. 2010, 75, 20–32. [Google Scholar]
- Nendel, C.; Venezia, A.; Piro, F.; Ren, T.; Lillywhite, R.D.; Rahn, C.R. The performance of the EU-Rotate-N model in predicting the growth and nitrogen uptake of rotations of field vegetable crops in a Mediterranean environment. J. Agric. Sci. 2013, 151, 538–555. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, K.; Fan, Z.; Wei, Y.; Lin, S.; Wang, J. Simulating the fate of nitrogen and optimizing water and nitrogen management of greenhouse tomato in North China using the EU-Rotate_N model. Agric. Water Manag. 2013, 128, 72–84. [Google Scholar] [CrossRef]
- Suárez-Rey, E.M.; Romero-Gámez, M.; Giménez, C.; Thompson, R.B.; Gallardo, M. Use of EU-Rotate_N and CropSyst models to predict yield, growth and water and N dynamics of fertigated leafy vegetables in a Mediterranean climate and to determine N fertilizer requirements. Agric. Syst. 2016, 149, 150–164. [Google Scholar] [CrossRef]
- Zhang, K.; Greenwood, D.J.; Spracklen, W.P.; Rahn, C.R.; Hammond, J.P.; White, P.J.; Burns, I.G. A universal agro-hydrological model for water and nitrogen cycles in the soil-crop system SMCR_N: Critical update and further validation. Agric. Water Manag. 2010, 97, 1411–1422. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, D.; Greenwood, D.J.; Rahn, C.R.; Thorup-Kristensen, K. Development and critical evaluation of a generic 2-D agro-hydrological model (SMCR_N) for the responses of crop yield and nitrogen composition to nitrogen fertilizer. Agric. Ecosyst. Environ. 2009, 132, 160–172. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Pardossi, A.; Paoli, P.D.; Battilani, A. Application of a decision support system for increasing economic and environmental sustainability of processing tomato cultivated in Mediterranean climate. Acta Hortic. 2013, 971, 51–58. [Google Scholar] [CrossRef]
- Elia, A.; Conversa, G. A decision support system (GesCoN) for managing fertigation in open field vegetable crops. Part I-methodological approach and description of the software. Front. Plant Sci. 2015, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Conversa, G.; Bonasia, A.; Di Gioia, F.; Elia, A. A decision support system (GesCoN) for managing fertigation in vegetable crops. Part II-model calibration and validation under different environmental growing conditions on field grown tomato. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cahn, M.; Smith, R.; Hartz, T. Improving Irrigation and Nitrogen Management in California Leafy Greens Production. In Proceedings of the NUTRIHORT: Nutrient Management, Innovative Techniques and Nutrient Legislation in Intensive Horticulture for an Improved Water Quality, Ghent, Belgium, 16–18 September 2013; pp. 65–68. [Google Scholar]
- Stockle, C.O.; Martin, S.A.; Campbell, G.S. CropSyst, a cropping systems simulation model: Water/nitrogen budgets and crop yield. Agric. Syst. 1994, 46, 335–359. [Google Scholar] [CrossRef]
- Soto, F.; Gallardo, M.; Giménez, C.; Peña-Fleitas, T.; Thompson, R.B. Simulation of tomato growth, water and N dynamics using the EU-Rotate_N model in Mediterranean greenhouses with drip irrigation and fertigation. Agric. Water Manag. 2014, 132, 46–59. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, K.; Zhang, K.; Jiang, L.; Xu, Y. Simulation of nitrogen fate for greenhouse cucumber grown under different water and fertilizer management using the EU-Rotate_N model. Agric. Water Manag. 2012, 112, 21–32. [Google Scholar] [CrossRef]
- Gallardo, M.; Fernández, M.D.; Giménez, C.; Padilla, F.M.; Thompson, R.B. Revised VegSyst model to calculate dry matter production, critical N uptake and ETc of several vegetable species grown in Mediterranean greenhouses. Agric. Syst. 2016, 146, 30–43. [Google Scholar] [CrossRef]
- Cahn, M.; Hartz, T.; Smith, R.; Noel, B.; Johnson, L.; Melton, F. CropManage: An online decision support tool for irrigation and nutrient management. In Proceedings of the Western Nutrient Management Conference, Reno, NV, USA, 5–6 March 2015; pp. 9–13. [Google Scholar]
- Lorenz, H.P.; Schlaghecken, J.; Engl, G.; Maync, A.; Ziegler, J.; Kohl, M.; Strohmeyer, K. Ordnungsgemasse Stìckstoff-Versorgung im Freiland-Gemusebau nach dem “Kulturbegleìtenden Nmin-Sollwerte (KNS)-System”; Mìnìsterium fur Landwìrtschaft, Weinbau und Forsten: Rheìnland Pfalz, Maìnz, 1989; p. 85. [Google Scholar]
- Ziegler, J.; Strohmeier, K.; Brand, T. Nitrogen supply of vegetables based on the “KNS-system“. Acta Hortic. 1996, 428, 223–233. [Google Scholar] [CrossRef]
- Fink, M.; Sharpf, H.C. N-Expert—A decision support system for vegetable fertilization in the field. Acta Hortic. 1993, 339, 67–74. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Li, X.; Christie, P.; Horlacher, D.; Liebig, H.P. Use of a modified N-expert system for vegetable production in the Beijing region. J. Plant Nutr. 2005, 28, 475–487. [Google Scholar] [CrossRef]
- Goffart, J.P.; Olivier, M.; Frankinet, M. Crop nitrogen status assessment tools in a decision support system for nitrogen fertilization management of potato crops. Horttechnology 2011, 21, 282–286. [Google Scholar]
- Goffart, J.P.; Olivier, M.; Frankinet, M. Potato Crop Nitrogen Status Assessment to Improve N Fertilization Management and Efficiency: Past-Present-Future. Potato Res. 2008, 51, 355–383. [Google Scholar] [CrossRef]
- Nishina, H. Development of speaking plant approach technique for intelligent greenhouse. Agric. Agric. Sci. Proc. 2015, 3, 9–13. [Google Scholar] [CrossRef]
- Thompson, R.B.; Incrocci, L.; Voogt, W.; Pardossi, A.; Magán, J.J. Sustainable irrigation and nitrogen management of fertigated vegetable crops. Acta Hortic. 2017, 1150, 363–378. [Google Scholar] [CrossRef]
- Farneselli, M.; Tei, F.; Simonne, E. Reliability of petiole sap test for n nutritional status assessing in processing tomato. J. Plant Nutr. 2014, 37, 270–278. [Google Scholar] [CrossRef]
- Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B.; Farneselli, M.; Padilla, F.M. Assessing crop N status of fertigated vegetable crops using plant and soil monitoring techniques. Ann. Appl. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, G.J. Efficiency ranges for nitrate-nitrogen and potassium for vegetable petiole sap quick tests. HortTechnology 1994, 4, 218–222. [Google Scholar]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Loh, F.C.W.; Grabosky, J.C.; Bassuk, N.L. Using the SPAD 502 meter to assess chlorophyll and nitrogen content of benjamin fig and cottonwood leaves. Horttechnology 2002, 12, 682–686. [Google Scholar]
- Swiader, J.M.; Moore, A. Spad-chlorophyll response to nitrogen fertilization and evaluation of nitrogen status in dryland and irrigated pumpkins. J. Plant Nutr. 2002, 25, 1089–1100. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Threshold values of canopy reflectance indices and chlorophyll meter readings for optimal nitrogen nutrition of tomato. Ann. Appl. Biol. 2015, 166, 271–285. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, N.; Bélec, C.; Jenni, S.; Fortier, E.; Mellgren, R. The dualex—A new tool to determine nitrogen sufficiency in broccoli. Acta Hortic. 2009, 824, 121–132. [Google Scholar] [CrossRef]
- Wu, J.D.; Wang, D.; Rosen, C.J.; Bauer, M.E. Comparison of petiole nitrate concentrations, SPAD chlorophyll readings, and QuickBird satellite imagery in detecting nitrogen status of potato canopies. Field Crops Res. 2007, 101, 96–103. [Google Scholar] [CrossRef]
- Gianquinto, G.; Orsini, F.; Fecondini, M.; Mezzetti, M.; Sambo, P.; Bona, S. A methodological approach for defining spectral indices for assessing tomato nitrogen status and yield. Eur. J. Agric. 2011, 35, 135–143. [Google Scholar] [CrossRef]
- Padilla, F.M.; Teresa Peña-Fleitas, M.; Gallardo, M.; Thompson, R.B. Evaluation of optical sensor measurements of canopy reflectance and of leaf flavonols and chlorophyll contents to assess crop nitrogen status of muskmelon. Eur. J. Agric. 2014, 58, 39–52. [Google Scholar] [CrossRef]
- Baret, F.; Houlès, V.; Guérif, M. Quantification of plant stress using remote sensing observations and crop models: The case of nitrogen management. J. Exp. Bot. 2007, 58, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Usha, K.; Singh, B. Potential applications of remote sensing in horticulture—A review. Sci. Hortic. 2013, 153, 71–83. [Google Scholar] [CrossRef]
- Fox, R.H.; Walthall, C.L. Crop monitoring technologies to assess nitrogen status. In Nitrogen in Agricultural Systems; American Society of Agronomy: Madison, WI, USA, 2008; pp. 647–674. [Google Scholar]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to make use of plant sensor-based diagnostic information for nitrogen recommendations. Agron. J. 2009, 101, 800–816. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: New York, NY, USA, 2009; p. 350. [Google Scholar]
- Granados, M.R.; Thompson, R.B.; Fernández, M.D.; Martínez-Gaitán, C.; Gallardo, M. Prescriptive-corrective nitrogen and irrigation management of fertigated and drip-irrigated vegetable crops using modeling and monitoring approaches. Agric. Water Manag. 2013, 119, 121–134. [Google Scholar] [CrossRef]
- Van den Bos, A.L.; de Kreij, C.; Voogt, W. Bemestingsadviesbasis Grond; Proefstation voor Bloemisterij en Glasgroente: Naaldwijk, 1999; p. 54. [Google Scholar]
- Hartz, T.K. The assessment of soil and crop nutrient status in the development of efficient fertilizer recommendations. Acta Hortic. 2003, 627, 231–240. [Google Scholar] [CrossRef]
- Thompson, R.B.; Gallardo, M.; Joya, M.; Segovia, C.; Martínez-Gaitán, C.; Granados, M.R. Evaluation of rapid analysis systems for on-farm nitrate analysis in vegetable cropping. Span. J. Agric. Res. 2009, 7, 200–211. [Google Scholar] [CrossRef]
- Bamsey, M.; Graham, T.; Thompson, C.; Berinstain, A.; Scott, A.; Dixon, M. Ion-specific nutrient management in closed systems: The necessity for ion-selective sensors in terrestrial and space-based agriculture and water management systems. Sensors 2012, 12, 13349–13392. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.E.; Irving, D.E.; Milham, P.J. A critical evaluation of on-farm rapid tests for measuring nitrate in leafy vegetables. Sci. Hortic. 2012, 134, 1–6. [Google Scholar] [CrossRef]
- Bogrekci, I.; Lee, W.S. Spectral measurement of common soil phosphates. Trans. ASAE 2005, 48, 2371–2378. [Google Scholar] [CrossRef]
- Yokota, M.; Okada, T.; Yamaguchi, I. An optical sensor for analysis of soil nutrients by using LED light sources. Meas. Sci. Technol. 2007, 18, 2197–2201. [Google Scholar] [CrossRef]
- Bansod, S.J.; Thakare, S.S. Near Infrared spectroscopy based a portable soil nitrogen detector design. Int. J. Comput. Sci. Inf. Technol. 2014, 5, 3953–3956. [Google Scholar]
- Feller, C.; Fink, M. NMIN target values for field vegetables. Acta Hortic. 2002, 571, 195–201. [Google Scholar] [CrossRef]
- De Kreij, C.; Kavvadias, V.; Assimakopoulou, A.; Paraskevopoulos, A. Development of Fertigation for Trickle Irrigated Vegetables under Mediterranean Conditions. Int. J. Veg. Sci. 2007, 13, 81–99. [Google Scholar] [CrossRef]


| Advantages | Drawbacks |
|---|---|
| Nutrients are mainly applied in the wet root zone, where they can be easily taken up by plants | Higher investment costs of fertigation system |
| More flexibility in nutrient supply and better synchronization with the crop uptake | Risk of insufficient nutrient supply during rainy seasons |
| Automation of fertilizer supply (potentially labor saving) | Risk of hypoxia due to frequent irrigation, especially in clay soils |
| Improved yield and quality | Necessity of specialized labor |
| Reduction of environmental pollution, mainly related to N run-off | Risk of clogging of emitters due to precipitation of insoluble salts |
| Application of specific fertilizers for treating mineral deficiencies | |
| Improvement of medium-high saline water management, with low yield reduction |
| Days after Planting | Tomato Greenhouse (kg ha−1 day−1) | Processing Tomato (kg ha−1 day−1) | Eggplant (kg ha−1 day−1) | Broccoli (kg ha−1 day−1) | Lettuce (kg ha−1 day−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | N | P | K | N | P | K | N | P | K | N | P | K | |
| 1–10 | 1.00 | 0.10 | 2.00 | 0.10 | 0.02 | 0.1 | 0.05 | 0.01 | 0.00 | 0.02 | 0.00 | 0.01 | 0.15 | 0.01 | 0.20 |
| 11–20 | 1.00 | 0.10 | 4.00 | 0.50 | 0.05 | 0.30 | 0.10 | 0.01 | 0.00 | 0.07 | 0.01 | 0.02 | 0.45 | 0.10 | 0.50 |
| 21–30 | 1.00 | 0.10 | 3.50 | 1.00 | 0.16 | 2.00 | 0.20 | 0.01 | 0.30 | 1.08 | 0.12 | 0.74 | 3.40 | 0.50 | 7.80 |
| 31–40 | 2.00 | 0.20 | 3.50 | 2.80 | 0.19 | 2.30 | 0.25 | 0.01 | 0.80 | 1.22 | 0.13 | 0.91 | 2.20 | 0.60 | 8.20 |
| 41–50 | 2.50 | 0.40 | 5.50 | 4.50 | 0.75 | 8.00 | 3.20 | 0.02 | 4.90 | 1.75 | 0.20 | 1.35 | 1.80 | 0.55 | 3.20 |
| 51–60 | 2.50 | 0.60 | 6.00 | 6.50 | 0.80 | 8.50 | 2.90 | 0.08 | 7.20 | 1.04 | 0.13 | 3.04 | - | - | - |
| 61–70 | 2.50 | 0.30 | 4.00 | 7.50 | 1.80 | 9.00 | 0.25 | 0.09 | 1.30 | 3.02 | 0.36 | 4.34 | - | - | - |
| 71–80 | 2.50 | 0.30 | 6.00 | 3.50 | 0.50 | 4.50 | 0.25 | 0.05 | 0.50 | 3.41 | 0.46 | 3.95 | - | - | - |
| 81–90 | 1.50 | 0.30 | 0.10 | 5.00 | 0.50 | 9.20 | 0.25 | 0.05 | 0.50 | 2.79 | 0.38 | 4.09 | - | - | - |
| 91–100 | 1.50 | 0.10 | 0.10 | 8.00 | 0.89 | 9.00 | 0.25 | 0.05 | 0.50 | 2.09 | 0.32 | 3.13 | - | - | - |
| 101–110 | 1.00 | 0.10 | 0.10 | - | - | - | 0.25 | 0.09 | 2.00 | 0.93 | 0.18 | 2.74 | - | - | - |
| 111–120 | 1.00 | 0.10 | 1.00 | - | - | - | 1.20 | 0.15 | 3.00 | 0.20 | 0.09 | 0.96 | - | - | - |
| 121–130 | 1.50 | 0.20 | 1.00 | - | - | - | 2.40 | 0.27 | 3.00 | 0.18 | 0.09 | 0.48 | - | - | - |
| 131–150 | 1.50 | 0.35 | 1.30 | - | - | - | 2.60 | 0.31 | 3.00 | 0.15 | 0.04 | 0.20 | - | - | - |
| 151–180 | 4.00 | 0.50 | 3.80 | - | - | - | 2.30 | 0.38 | 1.60 | - | - | - | - | - | - |
| 181–210 | 2.00 | 0.30 | 3.00 | - | - | - | 1.90 | 0.35 | 160 | - | - | - | - | - | - |
| TOTAL (kg ha−1) | 450 | 65 | 710 | 393 | 59 | 520 | 290 | 33 | 380 | 202 | 26 | 165 | 110 | 22 | 250 |
| Variety | Daniela | VFM82-1-2 | Black Oval | Woltam | Iceberg | ||||||||||
| Planting Date | 25th September | 27th March | 10th September | 30th August | 5th November | ||||||||||
| Harvest | Selective | 18th July | Selective | 17th January | 25th January | ||||||||||
| Plants/ha | 23,000 | 50,000 | 12,500 | 33,000 | 100,000 | ||||||||||
| Soil type | Sandy | Clayey | Sandy | Loam | Sandy | ||||||||||
| Yield (t/ha) | 195 | 160 | 51 | 13 | 45 | ||||||||||
| Source: | Bar-Yosef et al. [34] | Dafne [35] | Bar-Yosef et al. [36] | Feigin and Sagiv [37] | Bar-Yosef and Sagiv [38] | ||||||||||
| DSS | Main Characteristics | Experimental Trials | Species | Comparative Trials | Main Results |
|---|---|---|---|---|---|
| Cropsyst (Stockle et al. [88]) | Prescriptive. Simulation of plant growth, ETc and N uptake | Martínez-Gaitán [81] | Sweet pepper | No | Accurate estimation of the evolution of LAI, ETc, dry matter production, and crop N uptake |
| Suárez-Rey [75] | Lettuce, Escarole | No | Acceptable simulation of dry matter and N uptake | ||
| EU-Rotate_N (Rahn et al. [78]) | Prescriptive. Simulation of N and water balance | Suárez-Rey [75] | Lettuce, Escarole | Yes | −57% N supply and leaching versus grower’s practice |
| Soto et al. [89] | Tomato | No | Simulation scenarios with different level of N fertilization; model validation on fertigated tomato | ||
| Sun et al. [80,90] | Cucumber, Tomato | No | Optimized N management in greenhouse vegetables | ||
| Fertirrigere (Battilani et al. [54,55]) | Prescriptive. N, P, K, Ca, Mg balance | Massa et al. [84] | Processing tomato | Yes | About 2-fold higher N use efficiency and 27% decrease in water footprint in comparison with standard growers’ practice |
| VegSyst (Gallardo et al. [56]) | Prescriptive. Crop biomass, N uptake and crop ETc simulation | Gallardo et al. [91] Gallardo et al. [77] Giménez et al. [76] | Many vegetable species | No | Accurate estimation of crop biomass production, N uptake and crop ETc |
| CropManage (Cahn et al. [87]) | Prescriptive/Corrective. N and water balance for leafy vegetables | Cahn et al. [87,92] | Lettuce | Yes | 30% reduction of N supplied |
| GesCoN (Elia et al. [85]) | Prescriptive. Simulation of growth, N uptake, and yield | Conversa et al. [86] | Tomato | No | Good agreement between simulations and measurements from Italy and Florida (USA) |
| KNS (Lorenz [93]) | Prescriptive/Corrective. Calculation of N balance | Ziegler et al. [94] | Many vegetables | Yes | −57% N on the average of 21 vegetable crops in comparison with growers’ practice |
| N-Expert (Fink and Scharps [95]) | Prescriptive/Corrective. Calculation of N balance | Chen et al. [96] | Spinach, Cauliflower | Yes | −70% N on average |
| CRA-W (Goffart et al. [97,98]) | Prescriptive/Corrective. Nitrogen balance and crop measurements | Goffart et al. [97] | Potato | Yes | 95% of advice met actual crop nutrient requirements |
| Crop | Crop Developmental Stage | Fresh Petiole Sap Concentration (ppm) | Concentration in the Whole Leaf Tissue (%) | ||
|---|---|---|---|---|---|
| N-NO3 | K | N | K | ||
| Broccoli and cabbage | Six-leaf stage | 800–1000 | NA * | 3.5–5.0 | 3.5–4.5 |
| 1 week before first harvest | 500–800 | 3.0–4.5 | 1.5–4.0 | ||
| First harvest | 300–500 | 3.0–4.0 | 1.5–4.0 | ||
| Cucumber | Until first open flower | 800–1000 | NA | 4.0–5.0 | 2.0–3.0 |
| Fruits three-inches long | 600–800 | 2.5–5.0 | 20–30 | ||
| First harvest | 400–600 | 2.5–3.5 | 1.5–2.5 | ||
| Eggplant | Until first fruit are 5 cm | 1200–1600 | 4500–5000 | 4.5–5.5 | 4.5–6.0 |
| First harvest | 1000–1200 | 4000–5000 | 4.5–5.0 | 3.5–5.0 | |
| Mid harvest | 800–1000 | 3500–4000 | 3.5–4.5 | 3.0–4.0 | |
| Muskmelon | Until first open flower | 1100–1200 | NA | 4.5–5.0 | 5.0–6.0 |
| Fruit (length = 5 cm) | 800–1000 | 4.0–5.0 | 4.5–5.0 | ||
| First harvest | 700–800 | 3.5–4.5 | 2.0–4.0 | ||
| Pepper | Until first open flower | 1400–1600 | 3000–3200 | 4.0–4.5 | 4.5–5.0 |
| Fruits half-grown | 1200–1400 | 3000–3200 | 4.0–4.5 | 4.0–5.0 | |
| First harvest | 800–1000 | 2400–3000 | 3.5–4.0 | 3.5–4.5 | |
| Second harvest | 500–800 | 2000–2400 | 2.5–3.0 | 3.0–4.0 | |
| Potato | Until first open flower | 1000–1400 | 4500–5000 | 3.0–4.0 | 3.0–5.0 |
| 50% flowers open | 1000–1200 | 4000–4500 | 3.0–4.0 | 3.0–4.0 | |
| 100% flowers open | 900–1200 | 3500–4000 | 2.5–4.0 | 2.5–4.0 | |
| Tops falling over | 600–900 | 2500–3000 | 2.0–3.0 | 1.5–3.0 | |
| Squash | Until first open flower | 900–1000 | NA | 3.0–5.0 | 3.0–5.0 |
| First harvest | 800–900 | 3.0–5.0 | 2.0–3.0 | ||
| Strawberry | November–December | 800–900 | 3000–3500 | 2.8–3.5 | 1.5–3.0 |
| January–February | 500–800 | 2500–3000 | 3.0–4.0 | 1.5–3.0 | |
| March–April | 200–500 | 1800–2500 | 3.0–3.5 | 1.5–2.5 | |
| Tomato (Field) | First open flowers | 600–800 | 3500–4000 | 3.5–4.0 | 3.5–4.0 |
| Until first fruit are 5 cm | 400–600 | 3000–3500 | 3.0–4.0 | 3.0–4.0 | |
| First harvest | 300–400 | 2500–3000 | 2.5–3.5 | 2.5–3.5 | |
| Second harvest | 200–400 | 2000–2500 | 2.0–3.5 | 2.0–3.0 | |
| Tomato (Greenhouse) | Until to second truss | 1000–1200 | 4500–5000 | 4.0–6.0 | 4.0–5.0 |
| 2th -5th trusses | 800–1000 | 4000–5000 | 4.0–5.0 | 3.5–4.0 | |
| Harvest season | 700–900 | 3500–4000 | 3.5–4.0 | 2.5–3.5 | |
| Watermelon | Until fruit are 5 cm long | 1000–1200 | 4000–5000 | 4.0–5.0 | 3.5–4.0 |
| Half fruit time | 800–1000 | 3500–4000 | 3.5–4.0 | 2.5–3.5 | |
| At first harvest | 600–800 | 3000–3500 | 3.0–4.0 | 2.0–3.0 | |
| Crop | Guide Values (1:2 Volume Extract) for Base Dressing | Standard Nutrient Solution for Top Fertigation | Guide Values (1:2 Volume Extract) for Top Dressing | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | K+ | Ca2+ | Mg2+ | NO3− | SO42− | H2PO4− | NH4+ | K+ | Ca2+ | Mg2+ | NO3− | SO42− | EC | K+ | Ca2+ | Mg2+ | NO3− | SO42− | H2PO4− | |
| Asparagus, haricot bean, strawberry, sweet corn | 1.50 | 1.0 | 1.5 | 1.0 | 2.0 | 2.0 | 0.1 | 0.2 | 2.7 | 1.3 | 0.7 | 5.5 | 0.7 | 0.80 | 1.0 | 1.5 | 1.0 | 2.0 | 2.0 | 0.1 |
| Beet, broccoli, fennel, leeks, purslane, mustard | 1.50 | 1.5 | 1.5 | 1.2 | 3.0 | 2.0 | 0.1 | 0.4 | 3.0 | 1.4 | 0.7 | 6.0 | 0.8 | 0.90 | 1.5 | 1.5 | 1.2 | 3.0 | 2.0 | 0.1 |
| Carrot, onion | 1.50 | 2.0 | 1.3 | 1.2 | 2.0 | 2.5 | 0.1 | 0.3 | 2.2 | 1.0 | 0.6 | 4.5 | 0.6 | 0.80 | 2.0 | 1.2 | 1.2 | 2.0 | 2.5 | 0.1 |
| Cauliflower, broccoli, Chinese cabbage, kohlrabi, endive, red pepper, celery, celery root, zucchini | 1.50 | 1.5 | 1.5 | 1.2 | 3.0 | 2.0 | 0.1 | 0.4 | 3.0 | 1.4 | 0.7 | 6.0 | 0.8 | 0.90 | 1.5 | 1.5 | 1.2 | 3.0 | 2.0 | 0.1 |
| Gherkin, cucumber | 2.20 | 1.8 | 2.2 | 1.2 | 4.0 | 1.5 | 0.1 | 0.9 | 3.5 | 2.0 | 1.0 | 8.4 | 1.0 | 1.00 | 1.8 | 2.2 | 1.2 | 4.0 | 1.5 | 0.1 |
| Chicory | 1.50 | 1.2 | 1.2 | 0.8 | 1.5 | 0.8 | 0.1 | 0.9 | 3.5 | 2.0 | 1.0 | 8.4 | 1.0 | 0.80 | 1.2 | 1.2 | 0.8 | 1.5 | 0.8 | 0.1 |
| Lettuce (summer) | 1.50 | 2.5 | 3.3 | 1.0 | 4.0 | 3.5 | 0.1 | 0.4 | 3.4 | 1.6 | 0.9 | 7.0 | 0.9 | 0.80 | 2.5 | 3.2 | 1.0 | 4.0 | 3.5 | 0.1 |
| Lettuce (winter) | 1.50 | 3.0 | 3.3 | 1.0 | 5.0 | 3.6 | 0.1 | 0.9 | 3.5 | 2.0 | 1.0 | 8.4 | 1.0 | 1.20 | 3.0 | 3.2 | 1.0 | 5.0 | 3.6 | 0.1 |
| Endivie, escarole | 1.50 | 2.5 | 2.0 | 1.3 | 3.0 | 3.0 | 0.1 | 0.9 | 3.5 | 2.0 | 1.0 | 8.4 | 1.0 | 0.80 | 2.5 | 2.0 | 1.2 | 3.0 | 3.0 | 0.1 |
| Eggplant | 1.80 | 1.8 | 2.0 | 1.5 | 4.5 | 2.0 | 0.1 | 0.9 | 3.5 | 2.0 | 1.0 | 8.4 | 1.0 | 1.20 | 1.8 | 2.0 | 1.5 | 4.5 | 2.0 | 0.1 |
| Musk-melon | 1.80 | 1.0 | 1.5 | 1.0 | 2.0 | 2.0 | 0.1 | 0.4 | 4.0 | 2.0 | 1.0 | 8.4 | 1.0 | 1.20 | 1.5 | 1.5 | 1.0 | 3.0 | 2.0 | 0.1 |
| Potato | 1.50 | 1.8 | 1.5 | 1.0 | 3.0 | 1.9 | 0.1 | 0.9 | 3.1 | 1.8 | 1.0 | 7.6 | 1.0 | 0.80 | 1.8 | 1.5 | 1.0 | 3.0 | 1.9 | 0.1 |
| Sweet Pepper | 2.00 | 2.0 | 2.5 | 1.2 | 4.5 | 2.0 | 0.1 | 0.4 | 4.0 | 2.0 | 1.0 | 8.4 | 10 | 1.10 | 2.0 | 2.5 | 1.2 | 4.5 | 2.0 | 0.1 |
| Tomato | 2.30 | 3.5 | 3.5 | 2.7 | 7.5 | 3.5 | 0.1 | 0.4 | 5.0 | 2.0 | 1.5 | 9.4 | 1.5 | 1.40 | 2.2 | 2.5 | 1.7 | 5.0 | 2.5 | 0.1 |
| Radish (autumn–winter) | 2.00 | 3.0 | 3.0 | 1.0 | 3.0 | 3.5 | 0.1 | 0.7 | 6.0 | 2.4 | 1.2 | 10.8 | 16 | 1.20 | 3.0 | 3.0 | 1.0 | 3.0 | 3.5 | 0.1 |
| Radish (spring–summer) | 1.50 | 2.0 | 1.5 | 0.8 | 2.0 | 2.2 | 0.1 | 0.7 | 6.0 | 2.4 | 1.2 | 10.8 | 1.6 | 0.80 | 2.0 | 1.5 | 0.7 | 2.0 | 2.2 | 0.1 |
| Spinach | 2.20 | 1.5 | 1.5 | 1.25 | 3.0 | 2.0 | 0.1 | 0.4 | 3.0 | 1.4 | 0.7 | 6.0 | 0.8 | 0.90 | 1.5 | 1.5 | 1.25 | 3.0 | 2.0 | 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Incrocci, L.; Massa, D.; Pardossi, A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae 2017, 3, 37. https://doi.org/10.3390/horticulturae3020037
Incrocci L, Massa D, Pardossi A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae. 2017; 3(2):37. https://doi.org/10.3390/horticulturae3020037
Chicago/Turabian StyleIncrocci, Luca, Daniele Massa, and Alberto Pardossi. 2017. "New Trends in the Fertigation Management of Irrigated Vegetable Crops" Horticulturae 3, no. 2: 37. https://doi.org/10.3390/horticulturae3020037
APA StyleIncrocci, L., Massa, D., & Pardossi, A. (2017). New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae, 3(2), 37. https://doi.org/10.3390/horticulturae3020037








