Effect of Methyl Jasmonate on Physical and Chemical Properties of Mango Fruit cv. Nam Dok Mai
Abstract
:1. Introduction
2. Experimental Section
2.1. Mango Fruit Preparation
2.2. Disease Incidence of Anthracnose and Stem End Rot
2.3. Colour Changes
2.4. Extraction, Saponification and HPLC Analysis of β-Carotene
2.5. Ethylene Production
2.6. Statistical Analysis
3. Results and Discussion
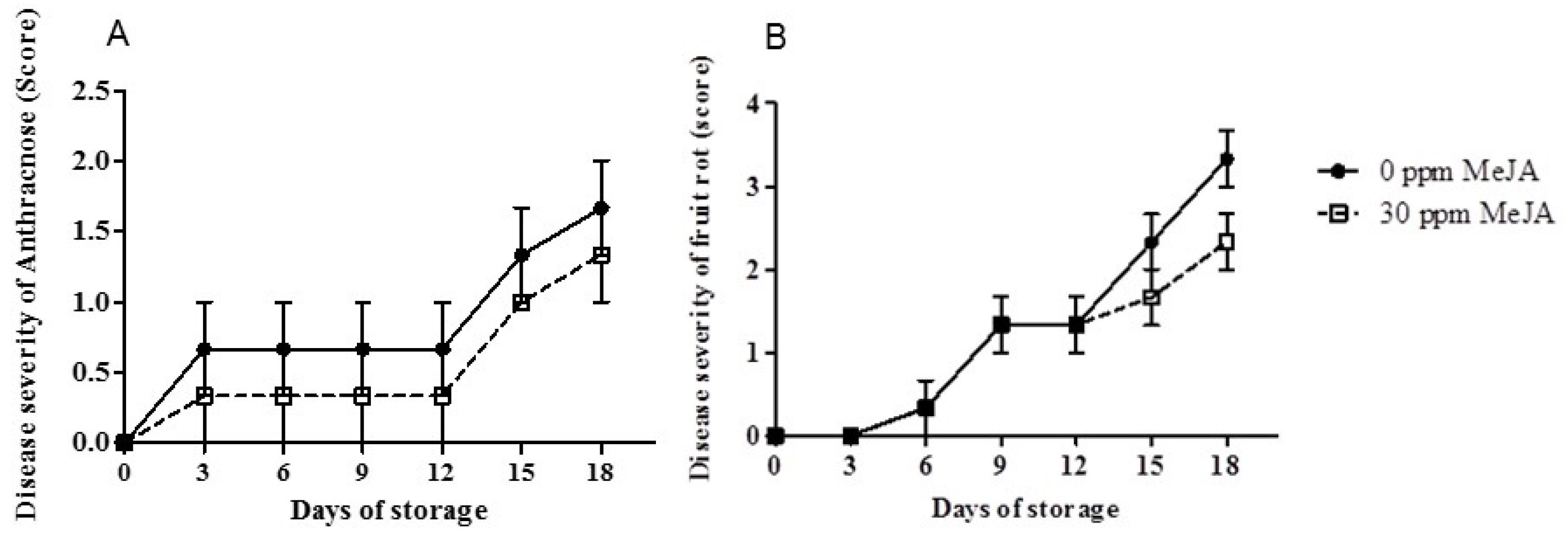
3.1. Disease Severity
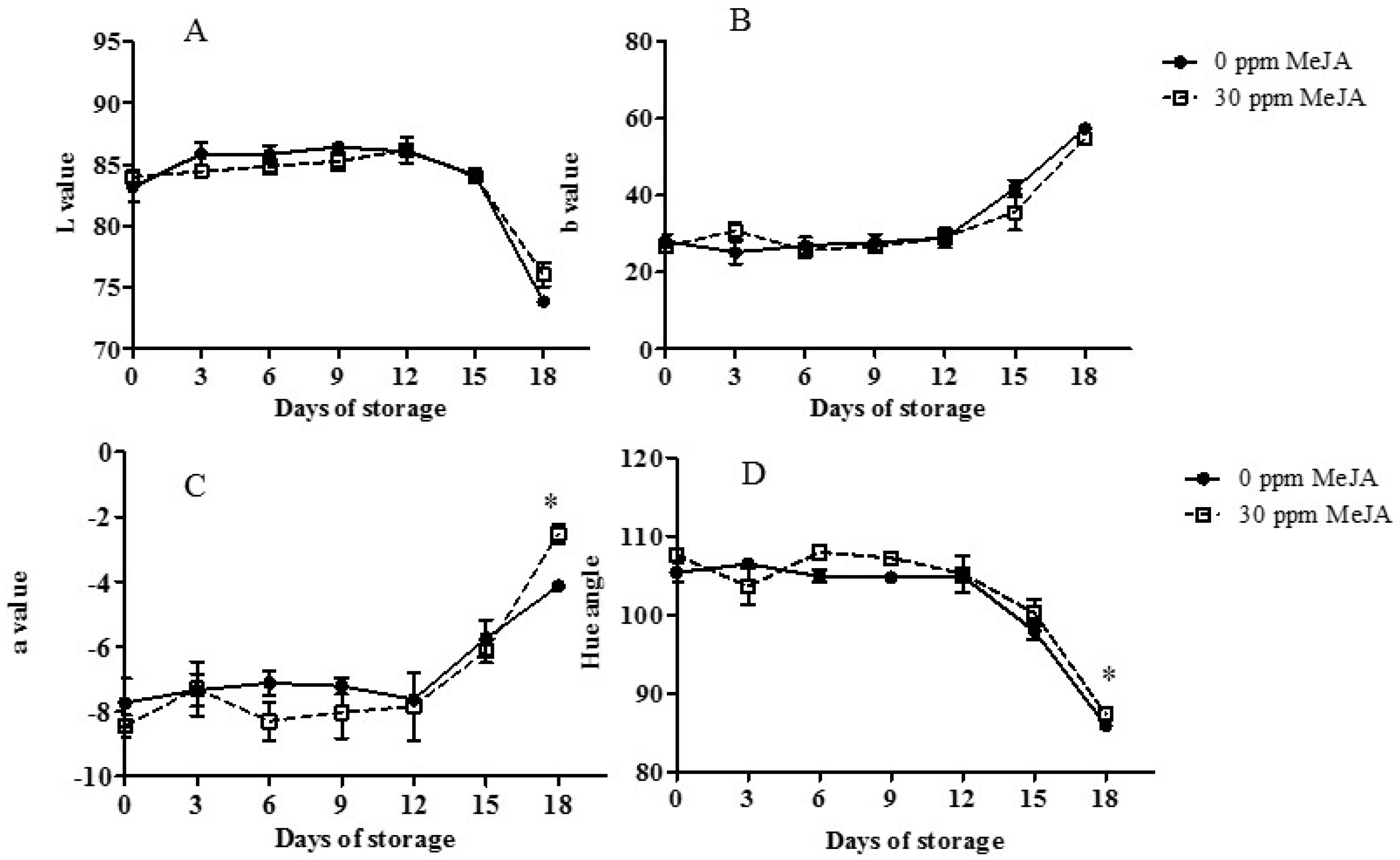
3.2. Internal Pulp Color
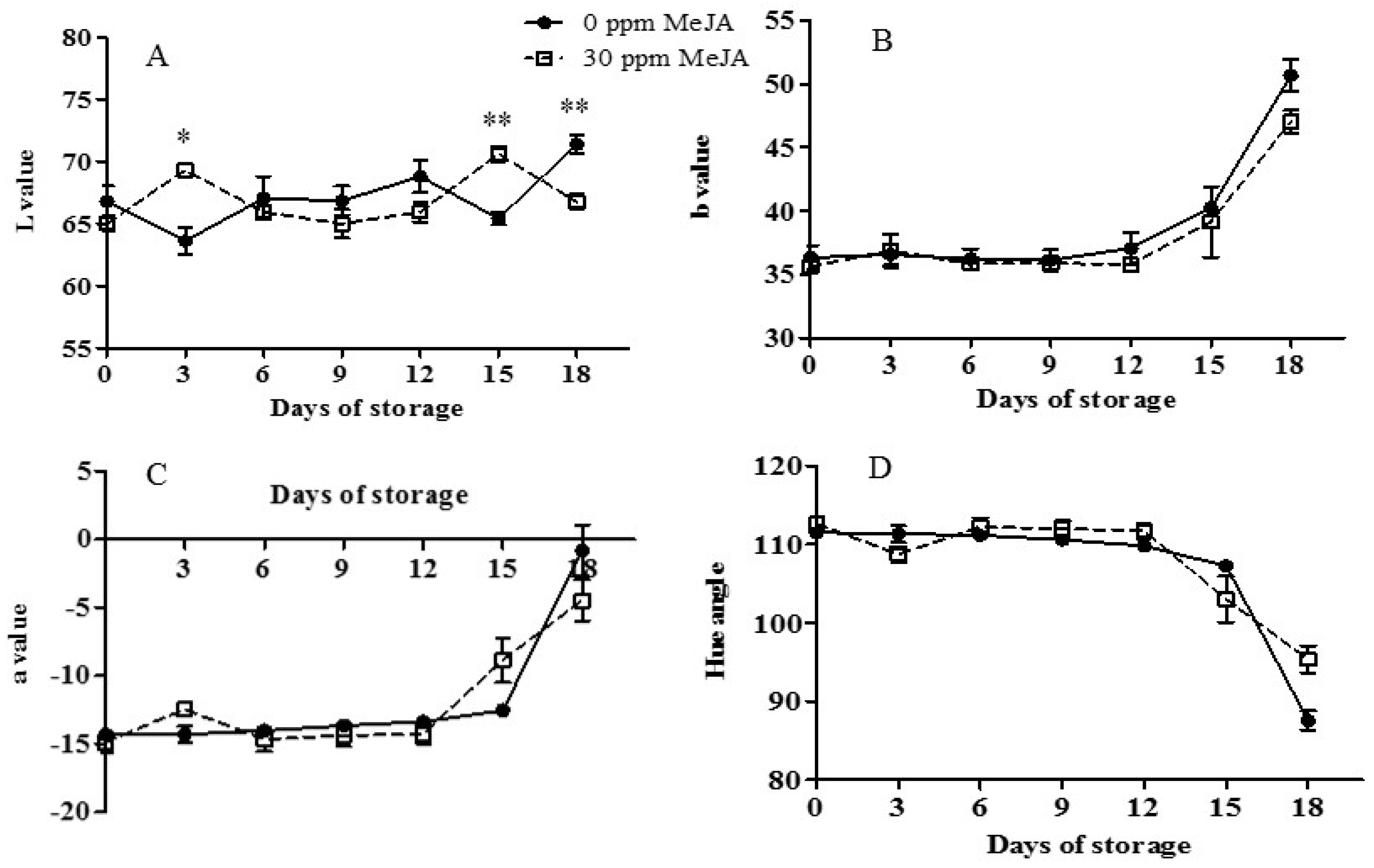
3.3. Peel Color
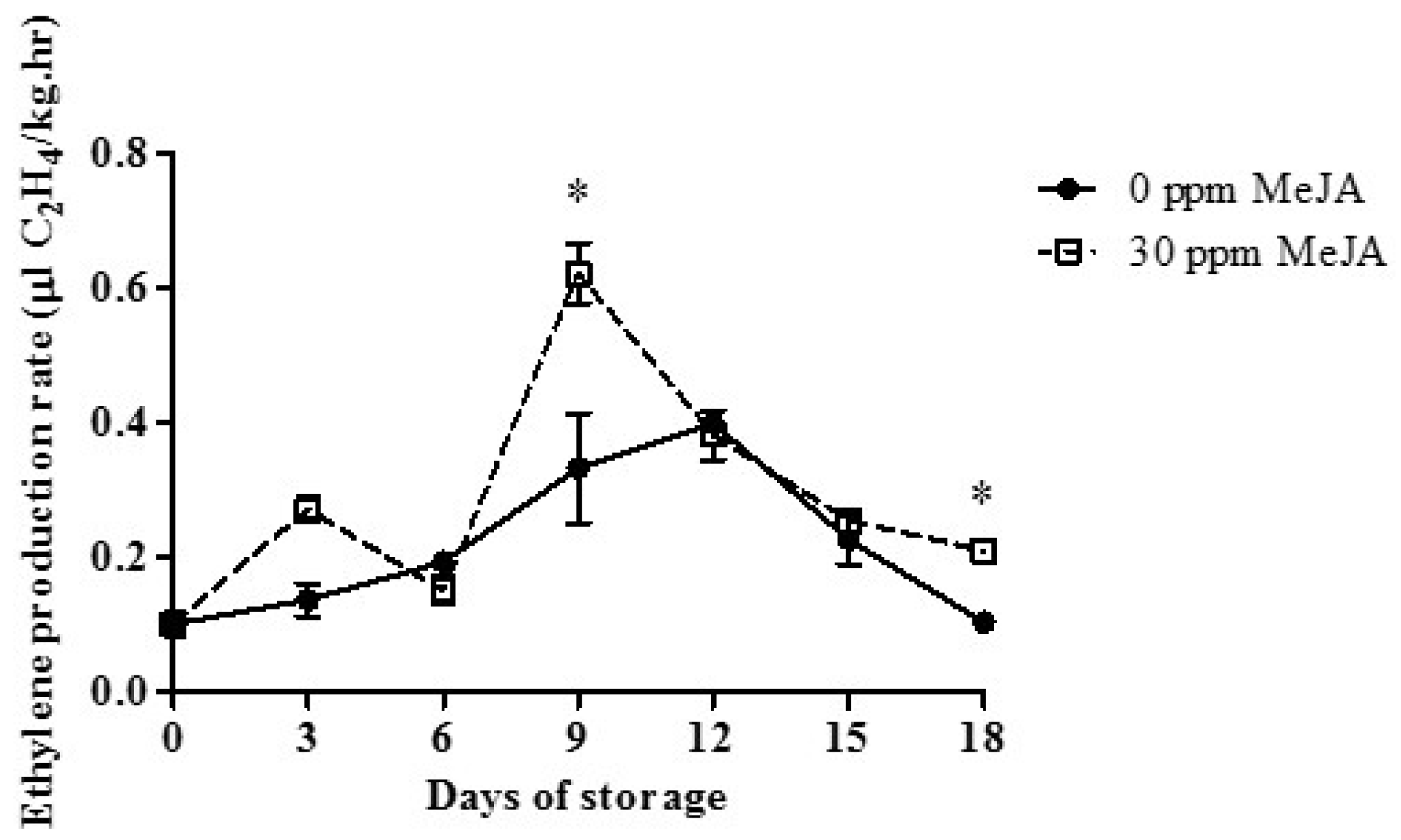
3.4. Ethylene Production
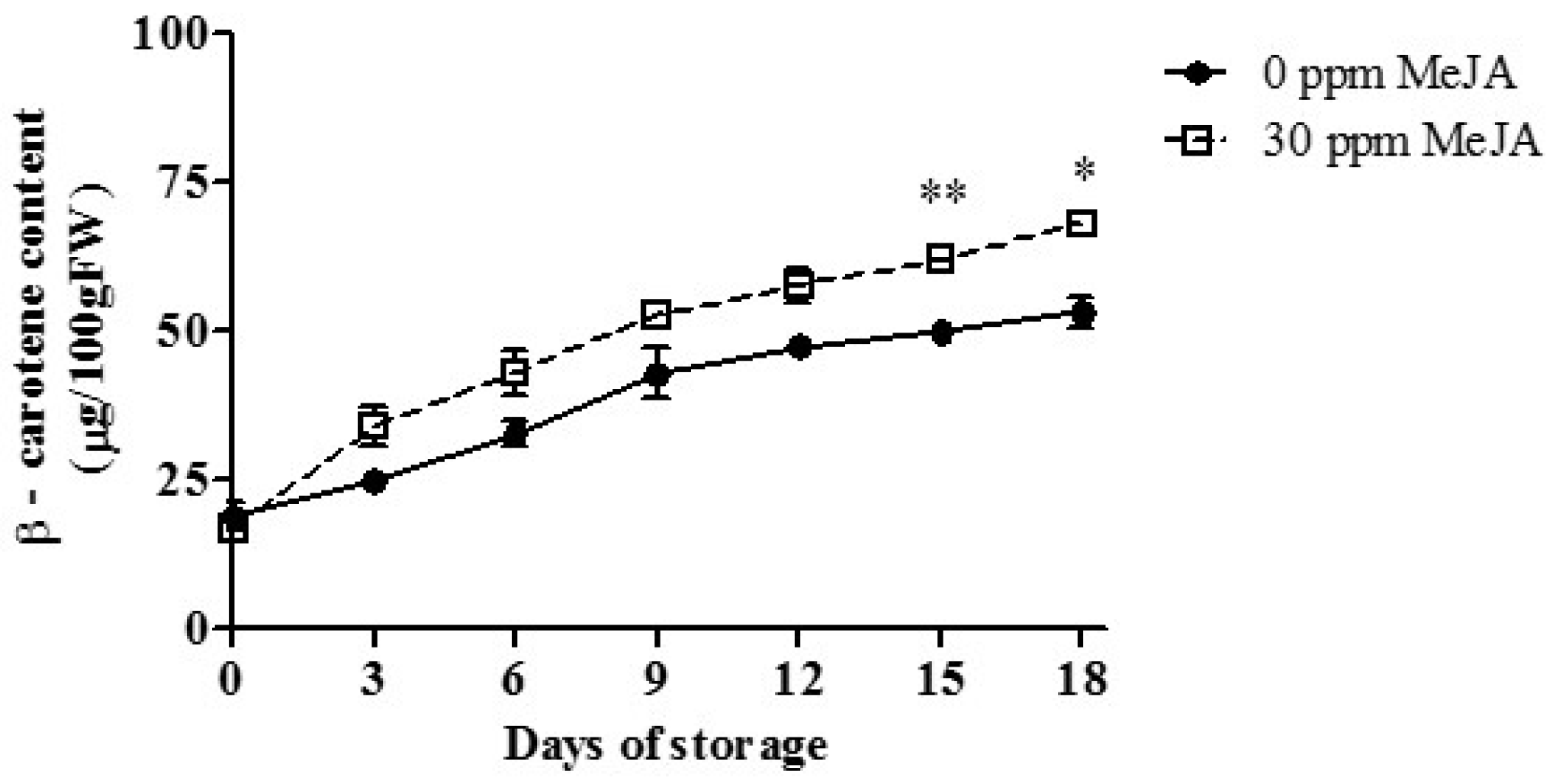
3.5. β-Carotene Content
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raskin, I. Salicylic acid, a new plant hormone. Plant Physiol. 1992, 99, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonate in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Ananieva, E.A.; Christov, K.N.; Popova, L.P. Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plant sexpose to paraquat. J. Plant Physiol. 2004, 161, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, S.; Kong, W.; Li, S.; Archbold, D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock protein of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Fellman, J.K.; Patterson, M.E. Effect of methyl jasmonate on ethylene and volatile production by Summered apples depends on fruit developmental stage. J. Agric. Food Chem. 1997, 45, 208–211. [Google Scholar] [CrossRef]
- Cheong, J.J.; Choi, Y.D. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef]
- Burhan, O.; Ebubekir, A.; Kenan, Y.; Yakup, O.; Onur, S. Effect of methyl jasmonate treatments on the bioactive compounds and physicochemical quality of “Fuji” apples: Ciencia e InvestigaciÓn Agraria. Crop Prod. 2013, 40, 201–211. [Google Scholar]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates: A review. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Heridia, J.B.; Zevallos, C. The effects of exogenous ethylene and methyl jasmonate on the accumulation of phenolic antioxidants in selected whole and wounded fresh produce. Food Chem. 2009, 115, 1500–1508. [Google Scholar] [CrossRef]
- Saniewski, M.; Czapski, J. The effect of methyl jasmonate on lycopene and β-carotene accumulation in ripening red tomatoes. Experientia 1983, 39, 1373–1374. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Fortiz, J.; Cruz, R.; Baez, R.; Wang, C.Y. Methyl jasmonate reduces chilling injury and maintains postharvest quality of mango fruit. J. Agric. Food Chem. 2000, 48, 515–519. [Google Scholar] [CrossRef] [PubMed]
- González-Aguilar, G.A.; Buta, J.G.; Wang, C.Y. Methyl jasmonate and modified atmosphere packaging (MAP) reduce decay and maintain postharvest quality of papaya “Sunrise”. Postharvest Biol. Technol. 2003, 28, 361–370. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Tiznado-Hernández, M.E.; Zavaleta-Gatica, R.; Martínez-Téllez, M.A. Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem. Biophs. Res. Commun. 2004, 313, 694–701. [Google Scholar] [CrossRef]
- Meng, X.; Han, J.; Wang, Q.; Tian, S. Changes in physiology and quality of peach fruits treated by methyl jasmonate under low temperature stress. Food Chem. 2009, 114, 1028–1035. [Google Scholar] [CrossRef]
- Chantrasri, P. Induction of Resistance to Anthracnose Disease of Postharvest Mango Fruit by Antagonist Yeasts. Master’s thesis, Chiangmai University, Chiang Mai, Thailand, 2006. [Google Scholar]
- Buta, J.G.; Moline, H.E. Methyl jasmonate shelf life and reduces microbial contamination of fresh cut celery and peppers. J. Agric. Food. Chem. 1998, 46, 1253–1256. [Google Scholar] [CrossRef]
- Droby, S.; Porat, R.; Cohen, L.; Weiss, B.; Shapira, B.; Philosoph-Hasas, S.; Meir, S. Suppressing green mold decay in grapefruit with postharvest jasmonate application. J. Am. Soc. Hortic. Sci. 1999, 124, 184–188. [Google Scholar]
- Wang, K.; Dickinson, R.E.; Liang, S. Clear sky visibility has decreased over land globally from 1973 to 2007. Science 2009, 323, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Aguilar, G.A.; Buta, J.G.; Wang, C.Y. Methyl jasmonate reduces chilling injury symptoms and enhance color development of “Kent” mangoes. J. Sci. Food Agric. 2001, 81, 1244–1249. [Google Scholar] [CrossRef]
- Perez, A.G.; Sanz, C.; Richardson, D.G.; Olias, M. Methyl jasmonate promotes β-carotene synthesis and chlorophyll degradation in Golden Delicious apple peel. Plant Growth Regul. 1993, 12, 163–167. [Google Scholar] [CrossRef]
- Saniewski, M.; Czapski, J. Stimulatory effect of methyl jasmonate on ethylene production in tomato fruits. Experientia 1985, 41, 256–257. [Google Scholar] [CrossRef]
- Saniewski, M.; Nowacki, J.; Lange, E.; Czapski, J. The effect of methyl jasmonate on anthocyanin accumulation, ethylene production and ethylene forming enzyme activities in apple. Fruit Sci. Rep. 1988, 15, 97–102. [Google Scholar]
- Olias, J.M.; Rios, J.J.; Valle, M.; Zamora, R.; Sanz, L.C.; Axelroad, B.A. Fatty acid hydroperoxide lyase in germinating soybean seedlings. J. Agric. Food Chem. 1990, 38, 624–630. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonyaritthongchai, P.; Chimvaree, C.; Buanong, M.; Uthairatanakij, A.; Jitareerat, P. Effect of Methyl Jasmonate on Physical and Chemical Properties of Mango Fruit cv. Nam Dok Mai. Horticulturae 2017, 3, 18. https://doi.org/10.3390/horticulturae3010018
Boonyaritthongchai P, Chimvaree C, Buanong M, Uthairatanakij A, Jitareerat P. Effect of Methyl Jasmonate on Physical and Chemical Properties of Mango Fruit cv. Nam Dok Mai. Horticulturae. 2017; 3(1):18. https://doi.org/10.3390/horticulturae3010018
Chicago/Turabian StyleBoonyaritthongchai, Panida, Chalida Chimvaree, Mantana Buanong, Apiradee Uthairatanakij, and Pongphen Jitareerat. 2017. "Effect of Methyl Jasmonate on Physical and Chemical Properties of Mango Fruit cv. Nam Dok Mai" Horticulturae 3, no. 1: 18. https://doi.org/10.3390/horticulturae3010018
APA StyleBoonyaritthongchai, P., Chimvaree, C., Buanong, M., Uthairatanakij, A., & Jitareerat, P. (2017). Effect of Methyl Jasmonate on Physical and Chemical Properties of Mango Fruit cv. Nam Dok Mai. Horticulturae, 3(1), 18. https://doi.org/10.3390/horticulturae3010018






