Four Petal-Specific TPS Drive Nocturnal Terpene Scent in Jasminum sambac
Abstract
1. Introduction
2. Materials and Methods
2.1. Nomenclature and Taxonomy
2.2. Plant Materials and Growth Conditions
2.3. Headspace and Petal Extract Volatile Compound Collection
2.4. RNA Extraction and Reverse Transcription
2.5. Transcriptome Data Mining, Full-Length cDNA Cloning, Plasmid Construction, and Sequence Analysis
2.6. Quantitative Real-Time PCR Analysis
2.7. Subcellular Localization of JsTPS Proteins in Protoplasts and N. benthamiana
2.8. Transient Expression in N. benthamiana and Collection of Emitted Volatiles
2.9. Heterologous Expression in Yeast
2.10. Recombinant Proteins and In Vitro Enzyme Assay of Four JsTPSs
2.11. Yeast Microsome Isolation and In Vitro Enzyme Assay of JsTPS01 and JsTPS03
2.12. Gas Chromatography–Mass Spectrometry Analysis
2.13. Statistical Data Analysis
3. Results
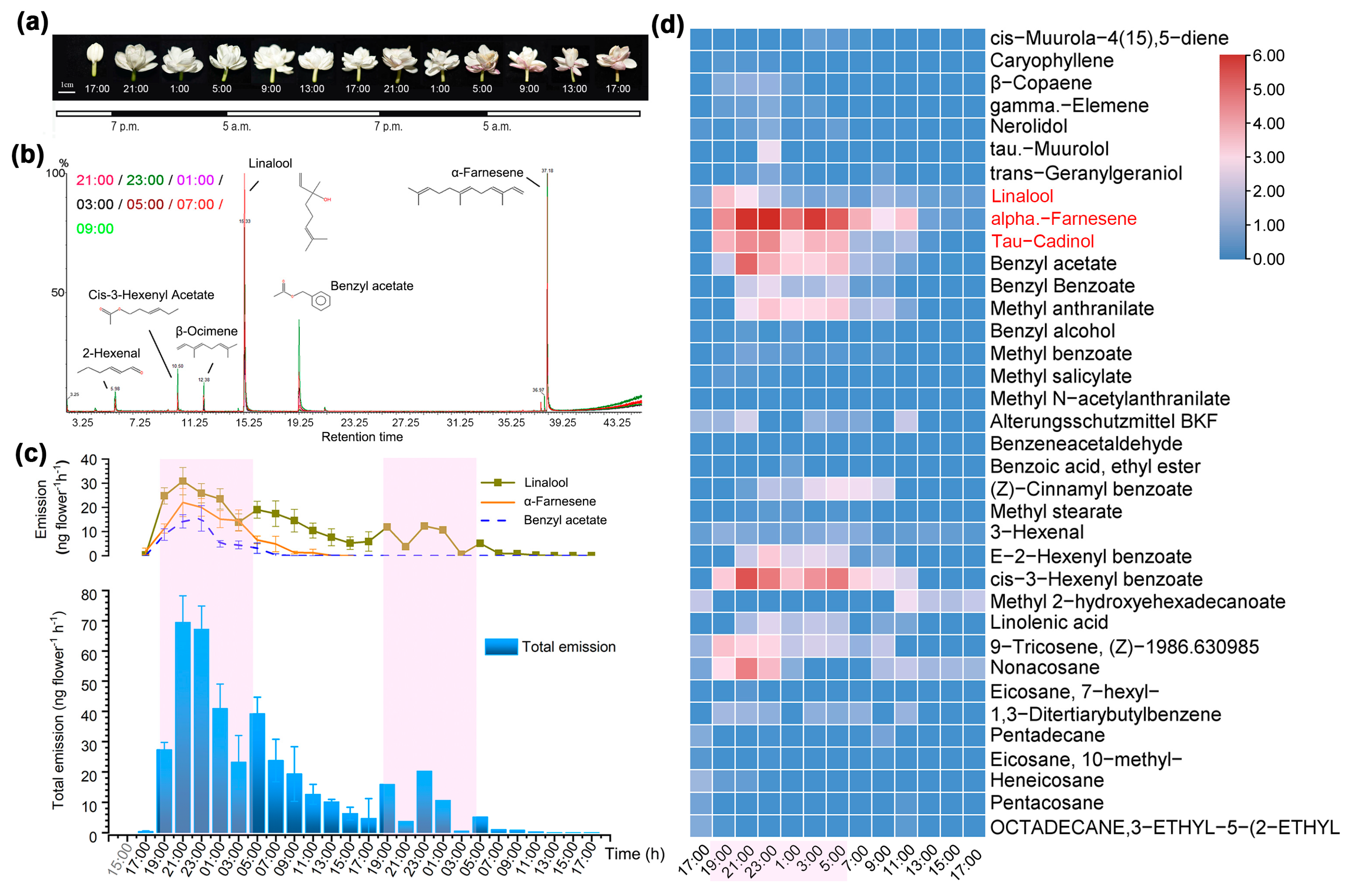
3.1. Circadian Emission of VOC in Flowers of J. sambac Dominated by Terpenoids
3.2. Identification and Cloning of Candidate TPS Genes Expressed in Petals
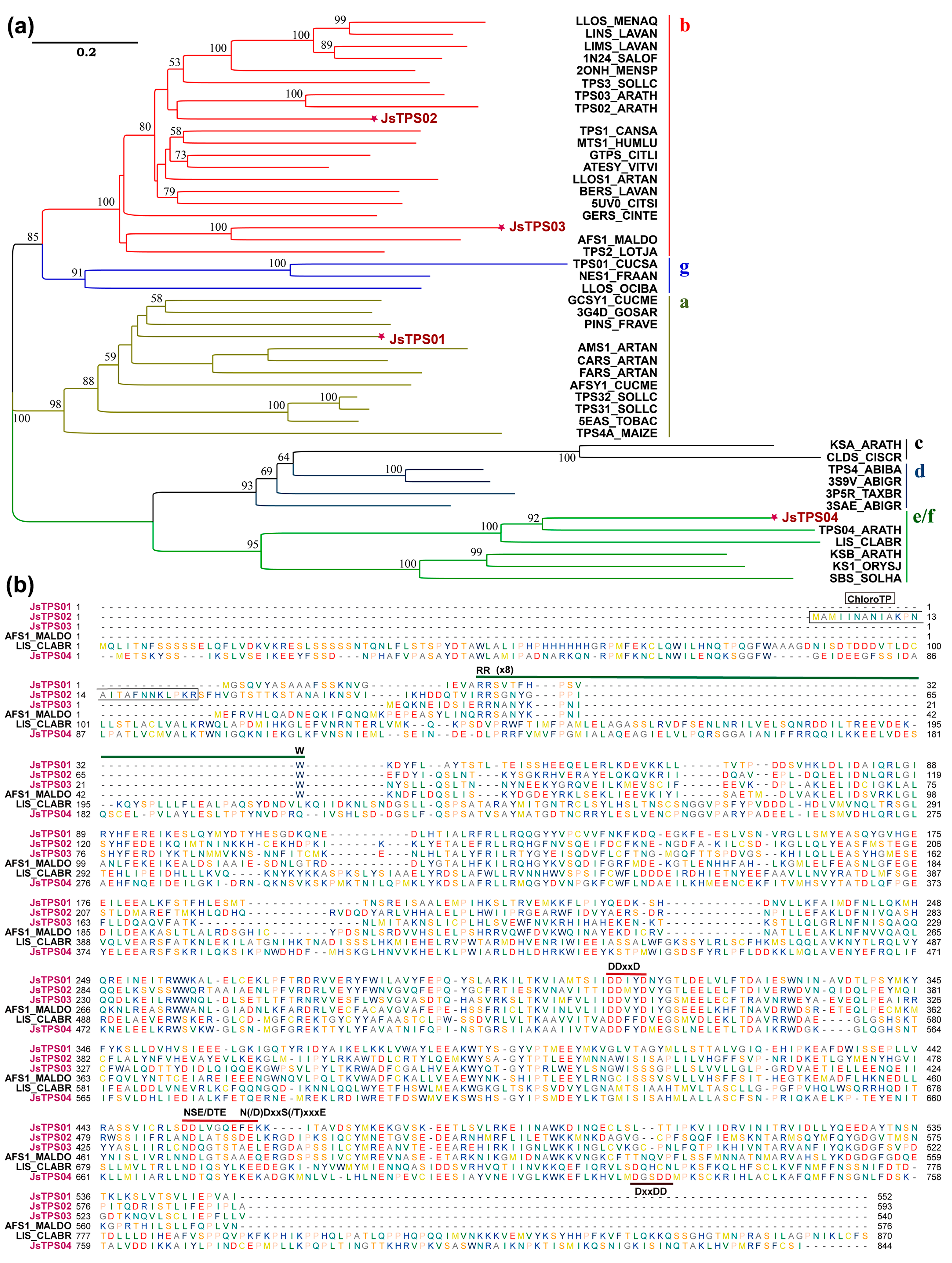
3.3. Subcellular Localization of the Four Terpene Synthase Proteins
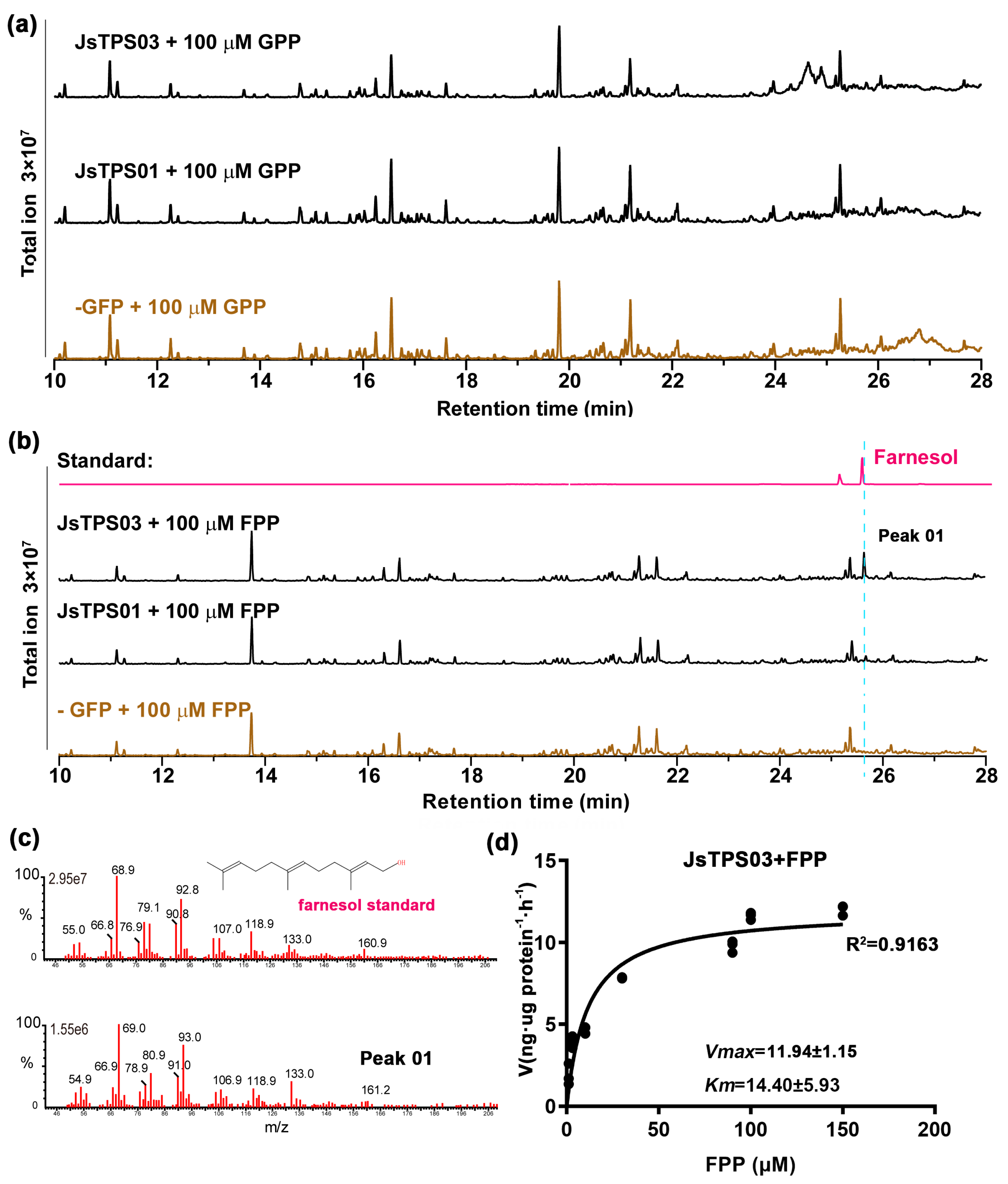
3.4. Enzyme Activities of the Four Jasminum Terpene Synthase Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pichersky, E.; Lewinsohn, E. Convergent Evolution in Plant Specialized Metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant Defense Against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Booij-James, I.S.; Dube, S.K.; Jansen, M.A.; Edelman, M.; Mattoo, A.K. Ultraviolet-B radiation impacts light-mediated turnover of the photosystem II reaction center heterodimer in Arabidopsis mutants altered in phenolic metabolism. Plant Physiol. 2000, 124, 1275–1284. [Google Scholar] [CrossRef]
- Amrad, A.; Moser, M.; Mandel, T.; de Vries, M.; Schuurink, R.C.; Freitas, L.; Kuhlemeier, C. Gain and Loss of Floral Scent Production through Changes in Structural Genes during Pollinator-Mediated Speciation. Curr. Biol. 2016, 26, 3303–3312. [Google Scholar] [CrossRef]
- Dixon, R.A.; Strack, D. Phytochemistry meets genome analysis, and beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar] [CrossRef]
- Leong, B.J.; Last, R.L. Promiscuity, impersonation and accommodation: Evolution of plant specialized metabolism. Curr. Opin. Struct. Biol. 2017, 47, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef]
- Gao, Y.; Honzatko, R.B.; Peters, R.J. Terpenoid synthase structures: A so far incomplete view of complex catalysis. Nat. Prod. Rep. 2012, 29, 1153–1175. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 2012, 51, 95–148. [Google Scholar] [CrossRef]
- Liang, P.; Ko, T.; Wang, A.H.J. Structure, mechanism and function of prenyltransferases. Eur. J. Biochem. 2002, 269, 3339–3354. [Google Scholar] [CrossRef]
- Kharel, Y.; Koyama, T. Molecular Analysis of cis-Prenyl Chain Elongating Enzymes. Nat. Prod. Rep. 2003, 34, 111–118. [Google Scholar] [CrossRef]
- Banerjee, A.; Hamberger, B. P450s controlling metabolic bifurcations in plant terpene specialized metabolism. Phytochem. Rev. 2018, 17, 81–111. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Magnard, J.-L.; Roccia, A.; Caissard, J.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrand-Saint Oyant, L.; Jullien, F.; Nicolè, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Alicandri, E.; Paolacci, A.R.; Osadolor, S.; Sorgonà, A.; Badiani, M.; Ciaffi, M. On the Evolution and Functional Diversity of Terpene Synthases in the Pinus Species: A Review. J. Mol. Evol. 2020, 88, 253–283. [Google Scholar] [CrossRef]
- Zhou, F.; Pichersky, E. The complete functional characterisation of the terpene synthase family in tomato. New Phytol. 2020, 226, 1341–1360. [Google Scholar] [CrossRef] [PubMed]
- Boutanaev, A.M.; Moses, T.; Zi, J.; Nelson, D.R.; Mugford, S.T.; Peters, R.J.; Osbourn, A. Investigation of terpene diversification across multiple sequenced plant genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E81–E88. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Rahfeld, P.; Luck, K.; Nagel, R.; Vogel, H.; Wielsch, N.; Irmisch, S.; Ramasamy, S.; Gershenzon, J.; Heckel, D.G.; et al. Novel family of terpene synthases evolved from trans-isoprenyl diphosphate synthases in a flea beetle. Proc. Natl. Acad. Sci. USA 2016, 113, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Li, G.; Köllner, T.G.; Fu, J.; Chen, X.; Xiong, W.; Crandall-Stotler, B.J.; Bowman, J.L.; Weston, D.J.; Zhang, Y.; et al. Microbial-type terpene synthase genes occur widely in nonseed land plants, but not in seed plants. Proc. Natl. Acad. Sci. USA 2016, 113, 12328–12333. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biology of Floral Scent, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Schiestl, F.P. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 2015, 206, 571–577. [Google Scholar] [CrossRef]
- Sas, C.; Müller, F.; Kappel, C.; Kent, T.V.; Wright, S.I.; Hilker, M.; Lenhard, M. Repeated Inactivation of the First Committed Enzyme Underlies the Loss of Benzaldehyde Emission after the Selfing Transition in Capsella. Curr. Biol. 2016, 26, 3313–3319. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; D’Auria, J.C.; Farooq, A.; Pichersky, E.; Gershenzon, J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Tholl, D.; Lee, S. Terpene Specialized Metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0143. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D.; Chen, F.; Petri, J.; Gershenzon, J.; Pichersky, E. Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J. 2005, 42, 757–771. [Google Scholar] [CrossRef]
- Falara, V.; Akhtar, T.A.; Nguyen, T.T.H.; Spyropoulou, E.A.; Bleeker, P.M.; Schauvinhold, I.; Matsuba, Y.; Bonini, M.E.; Schilmiller, A.L.; Last, R.L.; et al. The Tomato Terpene Synthase Gene Family. Plant Physiol. 2011, 157, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Raguso, R.A.; Wang, J.; Ross, J.R.; Pichersky, E. Floral scent production in Clarkia breweri: III. Enzymatic synthesis and emission of benzenoid esters. Plant Physiol. 1998, 116, 599–604. [Google Scholar] [CrossRef]
- Lücker, J.; Bouwmeester, H.J.; Schwab, W.; Blaas, J.; Van Der Plas, L.H.W.; Verhoeven, H.A. Expression of Clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-β-d-glucopyranoside. Plant J. 2001, 27, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D.; Kish, C.M.; Orlova, I.; Sherman, D.; Gershenzon, J.; Pichersky, E.; Dudareva, N. Formation of Monoterpenes in Antirrhinum majus and Clarkia breweri Flowers Involves Heterodimeric Geranyl Diphosphate Synthases. Plant Cell 2004, 16, 977–992. [Google Scholar] [CrossRef]
- Weiss, J.; Mühlemann, J.K.; RuizHernández, V.; Dudareva, N.; Egea-Cortines, M. Phenotypic Space and Variation of Floral Scent Profiles during Late Flower Development in Antirrhinum. Front. Plant Sci. 2016, 7, 1903. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bohman, B.; Wong, D.C.J.; RodriguezDelgado, C.; Scaffidi, A.; Flematti, G.R.; Phillips, R.D.; Pichersky, E.; Peakall, R. Complex Sexual Deception in an Orchid Is Achieved by Co-opting Two Independent Biosynthetic Pathways for Pollinator Attraction. Curr. Biol. 2017, 27, 1867–1877.e5. [Google Scholar] [CrossRef]
- Ramya, M.; Jang, S.; An, H.; Lee, S.; Park, P.; Park, P.H. Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. Int. J. Mol. Sci. 2020, 21, 1160. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Mostafa, S.; Lu, Z.; Du, R.; Cui, J.; Wang, Y.; Liao, Q.; Lu, J.; Mao, X.; Chang, B.; et al. The Jasmine (Jasminum sambac) Genome Provides Insight into the Biosynthesis of Flower Fragrances and Jasmonates. Genom. Proteom. Bioinf. 2023, 21, 127–149. [Google Scholar] [CrossRef]
- Thompson, J.D.; Dommée, B. Sequential variation in the components of reproductive success in the distylous Jasminum fruticans (Oleaceae). Oecologia 1993, 94, 480–487. [Google Scholar] [CrossRef]
- Qi, X.; Li, R.; Chen, S.; Feng, J.; Chen, H.; Liu, X.; Jin, Y.; Deng, Y. Identification of the FLA Gene Family and Functional Analysis of JsFLA2 in Jasminum sambac. Sci. Agric. Sin. 2025, 58, 3516–3530. [Google Scholar] [CrossRef]
- Ito, Y.; Sugimoto, A.; Kakuda, T.; Kubota, K. Identification of Potent Odorants in Chinese Jasmine Green Tea Scented with Flowers of Jasminum sambac. J. Agric. Food Chem. 2002, 50, 4878–4884. [Google Scholar] [CrossRef]
- Bera, P.; Kotamreddy, J.N.; Samanta, T.; Maiti, S.; Mitra, A. Inter-specific variation in headspace scent volatiles composition of four commercially cultivated jasmine flowers. Nat. Prod. Res. 2015, 29, 1328–1335. [Google Scholar] [CrossRef]
- Bera, P.; Mukherjee, C.; Mitra, A. Enzymatic production and emission of floral scent volatiles in Jasminum sambac. Plant Sci. 2017, 256, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Pragadheesh, V.S.; Chanotiya, C.S.; Rastogi, S.; Shasany, A.K. Scent from Jasminum grandiflorum flowers: Investigation of the change in linalool enantiomers at various developmental stages using chemical and molecular methods. Phytochemistry 2017, 140, 83–94. [Google Scholar] [CrossRef]
- Barman, M.; Mitra, A. Temporal relationship between emitted and endogenous floral scent volatiles in summer- and winter-blooming Jasminum species. Physiol. Plant. 2019, 166, 946–959. [Google Scholar] [CrossRef]
- Qi, X.; Wang, H.; Liu, S.; Chen, S.; Feng, J.; Chen, H.; Qin, Z.; Chen, Q.; Blilou, I.; Deng, Y. The chromosome-level genome of double-petal phenotype jasmine provides insights into the biosynthesis of floral scent. Hortic. Plant J. 2024, 10, 259–272. [Google Scholar] [CrossRef]
- Xu, S.; Ding, Y.; Sun, J.; Zhang, Z.; Wu, Z.; Yang, T.; Shen, F.; Xue, G. A high-quality genome assembly of Jasminum sambac provides insight into floral trait formation and Oleaceae genome evolution. Mol. Ecol. Resour. 2022, 22, 724–739. [Google Scholar] [CrossRef]
- Wang, P.; Fang, J.; Lin, H.; Yang, W.; Yu, J.; Hong, Y.; Jiang, M.; Gu, M.; Chen, Q.; Zheng, Y.; et al. Genomes of single- and double-petal jasmines (Jasminum sambac) provide insights into their divergence time and structural variations. Plant Biotechnol. J. 2022, 20, 1232–1234. [Google Scholar] [CrossRef]
- Fan, W.; Liao, Z.; Gu, M.; Zhang, Y.; Lei, W.; Zhang, Y.; Li, H.; Yan, J.; Xiao, Y.; Lin, H.; et al. Pan-Genome of Jasminum sambac Reveals the Genetic Diversity of Different Petal Morphology and Aroma-Related Genes. Mol. Ecol. Resour. 2025, 25, e70013. [Google Scholar] [CrossRef]
- Qi, X.; Wang, H.; Chen, S.; Feng, J.; Chen, H.; Qin, Z.; Blilou, I.; Deng, Y. The genome of single-petal jasmine (Jasminum sambac) provides insights into heat stress tolerance and aroma compound biosynthesis. Front. Plant Sci. 2022, 13, 1045194. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, X.; Wang, P.; Gu, M.; Gao, T.; Ye, N. Transcriptome Identification of Terpenoid Synthase Genes in Jasminum sambac and Their Expressions Responding to Exogenous Hormones. Biotechnol. Bull. 2022, 38, 41–49. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wan, C.; He, X.; Huang, J.; Lyu, M.; Yuan, Y.; Wu, B. Characterization of Two BAHD Acetyltransferases Highly Expressed in the Flowers of Jasminum sambac (L.) Aiton. Plants 2022, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Z.; Lyu, M.; Yuan, Y.; Wu, B. Characterization of JsWOX1 and JsWOX4 during Callus and Root Induction in the Shrub Species Jasminum sambac. Plants 2019, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Shen, J.; Fu, J.; Ma, J.; Wang, X.; Gao, C.; Zhuang, C.; Wan, J.; Jiang, L. Isolation, culture, and transient transformation of plant protoplasts. Curr. Protoc. Cell Biol. 2014, 63, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Cho, Y.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Wydro, M.; Kozubek, E.; Lehmann, P. Optimization of transient Agrobacterium-mediated gene expression system in leaves of Nicotiana benthamiana. Acta Biochim. Pol. 2006, 53, 289–298. [Google Scholar] [CrossRef]
- Jin, J.; Kim, M.J.; Dhandapani, S.; Tjhang, J.G.; Yin, J.L.; Wong, L.; Sarojam, R.; Chua, N.H.; Jang, I.C. The floral transcriptome of ylang ylang (Cananga odorata var. fruticosa) uncovers biosynthetic pathways for volatile organic compounds and a multifunctional and novel sesquiterpene synthase. J. Exp. Bot. 2015, 66, 3959–3975. [Google Scholar] [CrossRef]
- Wu, B.; Rambow, J.; Bock, S.; HolmBertelsen, J.; Wiechert, M.; Soares, A.B.; Spielmann, T.; Beitz, E. Identity of a Plasmodium lactate/H+ symporter structurally unrelated to human transporters. Nat. Commun. 2015, 6, 6284. [Google Scholar] [CrossRef]
- Dudareva, N.; Cseke, L.; Blanc, V.M.; Pichersky, E. Evolution of floral scent in Clarkia: Novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell 1996, 8, 1137–1148. [Google Scholar] [CrossRef]
- Ginglinger, J.F.; Boachon, B.; Höfer, R.; Paetz, C.; Köllner, T.G.; Miesch, L.; Lugan, R.; Baltenweck, R.; Mutterer, J.; Ullmann, P.; et al. Gene coexpression analysis reveals complex metabolism of the monoterpene alcohol linalool in Arabidopsis flowers. Plant Cell 2013, 25, 4640–4657. [Google Scholar] [CrossRef]
- Yahyaa, M.; Matsuba, Y.; Brandt, W.; Doron-Faigenboim, A.; Bar, E.; McClain, A.; Davidovich-Rikanati, R.; Lewinsohn, E.; Pichersky, E.; Ibdah, M. Identification, Functional Characterization, and Evolution of Terpene Synthases from a Basal Dicot. Plant Physiol. 2015, 169, 1683–1697. [Google Scholar] [CrossRef]
- Herde, M.; Gärtner, K.; Köllner, T.G.; Fode, B.; Boland, W.; Gershenzon, J.; Gatz, C.; Tholl, D. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. Plant Cell 2008, 20, 1152–1168. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.C.; Youn, B.; Zhao, Y.; Santhamma, B.; Coates, R.M.; Croteau, R.B.; Kang, C. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc. Natl. Acad. Sci. USA 2007, 104, 5360–5365. [Google Scholar] [CrossRef] [PubMed]
- Pechous, S.W.; Whitaker, B.D. Cloning and functional expression of an (E,E)-alpha-farnesene synthase cDNA from peel tissue of apple fruit. Planta 2004, 219, 84–94. [Google Scholar] [CrossRef]
- Fujii, T.; Nagasawa, N.; Iwamatsu, A.; Bogaki, T.; Tamai, Y.; Hamachi, M. Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1994, 60, 2786–2792. [Google Scholar] [CrossRef]
- Edris, A.E.; Chizzola, R.; Franz, C. Isolation and characterization of the volatile aroma compounds from the concrete headspace and the absolute of Jasminum sambac (L.) Ait. (Oleaceae) flowers grown in Egypt. Eur. Food Res. Technol. 2008, 226, 621–626. [Google Scholar] [CrossRef]
- Pragadheesh, V.; Yadav, A.; Chanotiya, C.S.; Rout, P.K.; Uniyal, G.C. Monitoring the emission of volatile organic compounds from flowers of Jasminum sambac using solid-phase micro-extraction fibers and gas chromatography with mass spectrometry detection. Nat. Prod. Commun. 2011, 6, 1333–1338. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents—A checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Cheng, S.S.; Wu, C.L.; Chang, H.T.; Kao, Y.T.; Chang, S.T. Antitermitic and antifungal activities of essential oil of Calocedrus formosana leaf and its composition. J. Chem. Ecol. 2004, 30, 1957–1967. [Google Scholar] [CrossRef]
- Jullien, F.; Moja, S.; Bony, A.; Legrand, S.; Petit, C.; Benabdelkader, T.; Poirot, K.; Fiorucci, S.; Guitton, Y.; Nicolè, F.; et al. Isolation and functional characterization of a τ-cadinol synthase, a new sesquiterpene synthase from Lavandula angustifolia. Plant Mol. Biol. 2014, 84, 227–241. [Google Scholar] [CrossRef]
- Ren, F.; Mao, H.; Liang, J.; Liu, J.; Shu, K.; Wang, Q. Functional characterization of ZmTPS7 reveals a maize τ-cadinol synthase involved in stress response. Planta 2016, 244, 1065–1074. [Google Scholar] [CrossRef]
- Raguso, R.A.; Pichersky, E. New Perspectives in Pollination Biology: Floral Fragrances. A day in the life of a linalool molecule: Chemical communication in a plant-pollinator system. Part 1: Linalool biosynthesis in flowering plants. Plant Species Biol. 1999, 14, 95–120. [Google Scholar] [CrossRef]
- FarréArmengol, G.; FernándezMartínez, M.; Filella, I.; Junker, R.R.; Peñuelas, J. Deciphering the Biotic and Climatic Factors That Influence Floral Scents: A Systematic Review of Floral Volatile Emissions. Front. Plant Sci. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, J.; Choi, E.; Kim, S. Microbial production of farnesol (FOH): Current states and beyond. Process Biochem. 2011, 46, 1221–1229. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Cheng, A.; Xiang, C.; Li, J.; Yang, C.; Hu, W.; Wang, L.; Lou, Y.; Chen, X. The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.; Kollner, T.G.; Gershenzon, J.; Degenhardt, J.R. The Maize Gene terpene synthase 1 Encodes a Sesquiterpene Synthase Catalyzing the Formation of (E)-β-Farnesene, (E)-Nerolidol, and (E,E)-Farnesol after Herbivore Damage. Plant Physiol. 2002, 130, 2049–2060. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Sun, J.; Wang, J.; Xun, H.; Tang, F. Cloning, expression and functional characterization of two sesquiterpene synthase genes from moso bamboo (Phyllostachys edulis). Protein Expr. Purif. 2016, 120, 1–6. [Google Scholar] [CrossRef]
- Manczak, T.; Simonsen, H.T. Insight into Biochemical Characterization of Plant Sesquiterpene Synthases. Anal. Chem. Insights 2016, 11, S40292. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Bhat, W.W.; Sadre, R.; Miller, G.P.; Garcia, A.S.; Hamberger, B. Promiscuous terpene synthases from Prunella vulgaris highlight the importance of substrate and compartment switching in terpene synthase evolution. New Phytol. 2019, 223, 323–335. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; He, Z.; Wang, F.; Yang, H.; Yan, Y.; Gao, M.; Gruber, M.Y.; Wan, X.; Wei, S. Implementation of CsLIS/NES in linalool biosynthesis involves transcript splicing regulation in Camellia sinensis. Plant Cell Environ. 2018, 41, 176–186. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Wu, Y.; Zhang, Y.; Li, X.; Li, J.; Zhu, W.; Ma, Z.; Li, W. Terpene synthases GhTPS6 and GhTPS47 participate in resistance to Verticillium dahliae in upland cotton. Plant Physiol. Biochem. 2024, 213, 108798. [Google Scholar] [CrossRef]
- Huang, M.; Abel, C.; Sohrabi, R.; Petri, J.; Haupt, I.; Cosimano, J.; Gershenzon, J.; Tholl, D. Variation of herbivore-induced volatile terpenes among Arabidopsis ecotypes depends on allelic differences and subcellular targeting of two terpene synthases, TPS02 and TPS03. Plant Physiol. 2010, 153, 1293–1310. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, S.; Tjhang, J.G.; Jang, I. Production of multiple terpenes of different chain lengths by subcellular targeting of multi-substrate terpene synthase in plants. Metab. Eng. 2020, 61, 397–405. [Google Scholar] [CrossRef]
- Pazouki, L.; Niinemets, Ü. Multi-Substrate Terpene Synthases: Their Occurrence and Physiological Significance. Front. Plant Sci. 2016, 7, 1019. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Cai, X.; Li, X.; Bian, L.; Luo, Z.; Li, Z.; Chen, Z.; Xin, Z. (E)-Nerolidol is a volatile signal that induces defenses against insects and pathogens in tea plants. Hortic. Res. 2020, 7, 52. [Google Scholar] [CrossRef]
- Cui, W.; Wang, B.; Guo, M.; Liu, Y.; JacquinJoly, E.; Yan, S.; Wang, G. A receptor-neuron correlate for the detection of attractive plant volatiles in Helicoverpa assulta (Lepidoptera: Noctuidae). Insect Biochem. Mol. Biol. 2018, 97, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, S.; Hua, J.; Li, D.; Ling, Y.; Luo, Q.; Li, S.H. Characterization of defensive cadinenes and a novel sesquiterpene synthase responsible for their biosynthesis from the invasive Eupatorium adenophorum. New Phytol. 2021, 229, 1740–1754. [Google Scholar] [CrossRef]
- Hallouti, A.; Ben El Caid, M.; Boubaker, H. Mediterranean fruit fly Ceratitis capitata (Wiedemann) management strategies and recent advances: A review. Int. J. Pest Manag. 2024, 1–13. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yuan, Y.; Hu, L.; He, X.; Li, J.; Wan, C.; Zhang, Y.; Wang, Y.; Wang, W.; Wu, B. Four Petal-Specific TPS Drive Nocturnal Terpene Scent in Jasminum sambac. Horticulturae 2026, 12, 10. https://doi.org/10.3390/horticulturae12010010
Yuan Y, Hu L, He X, Li J, Wan C, Zhang Y, Wang Y, Wang W, Wu B. Four Petal-Specific TPS Drive Nocturnal Terpene Scent in Jasminum sambac. Horticulturae. 2026; 12(1):10. https://doi.org/10.3390/horticulturae12010010
Chicago/Turabian StyleYuan, Yuan, Li Hu, Xian He, Jinan Li, Chao Wan, Yue Zhang, Yuting Wang, Wei Wang, and Binghua Wu. 2026. "Four Petal-Specific TPS Drive Nocturnal Terpene Scent in Jasminum sambac" Horticulturae 12, no. 1: 10. https://doi.org/10.3390/horticulturae12010010
APA StyleYuan, Y., Hu, L., He, X., Li, J., Wan, C., Zhang, Y., Wang, Y., Wang, W., & Wu, B. (2026). Four Petal-Specific TPS Drive Nocturnal Terpene Scent in Jasminum sambac. Horticulturae, 12(1), 10. https://doi.org/10.3390/horticulturae12010010





