Detection of Abscisic Acid and Jasmonates in Stigma Exudates and Their Role in Pollen Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Stigma Exudate Collection
2.2. Chromato-Mass-Spectrometric Detection of Phytohormones
2.3. Pollen Collection and Germination In Vitro
2.4. ROS and Ca2+ Level Measurements
2.5. Measuring the Stigma Surface Area
2.6. Data and Statistical Analyses
3. Results
3.1. Phytohormones Such as ABA and JA Are Present in Stigma Exudates of Some Dicot Plants
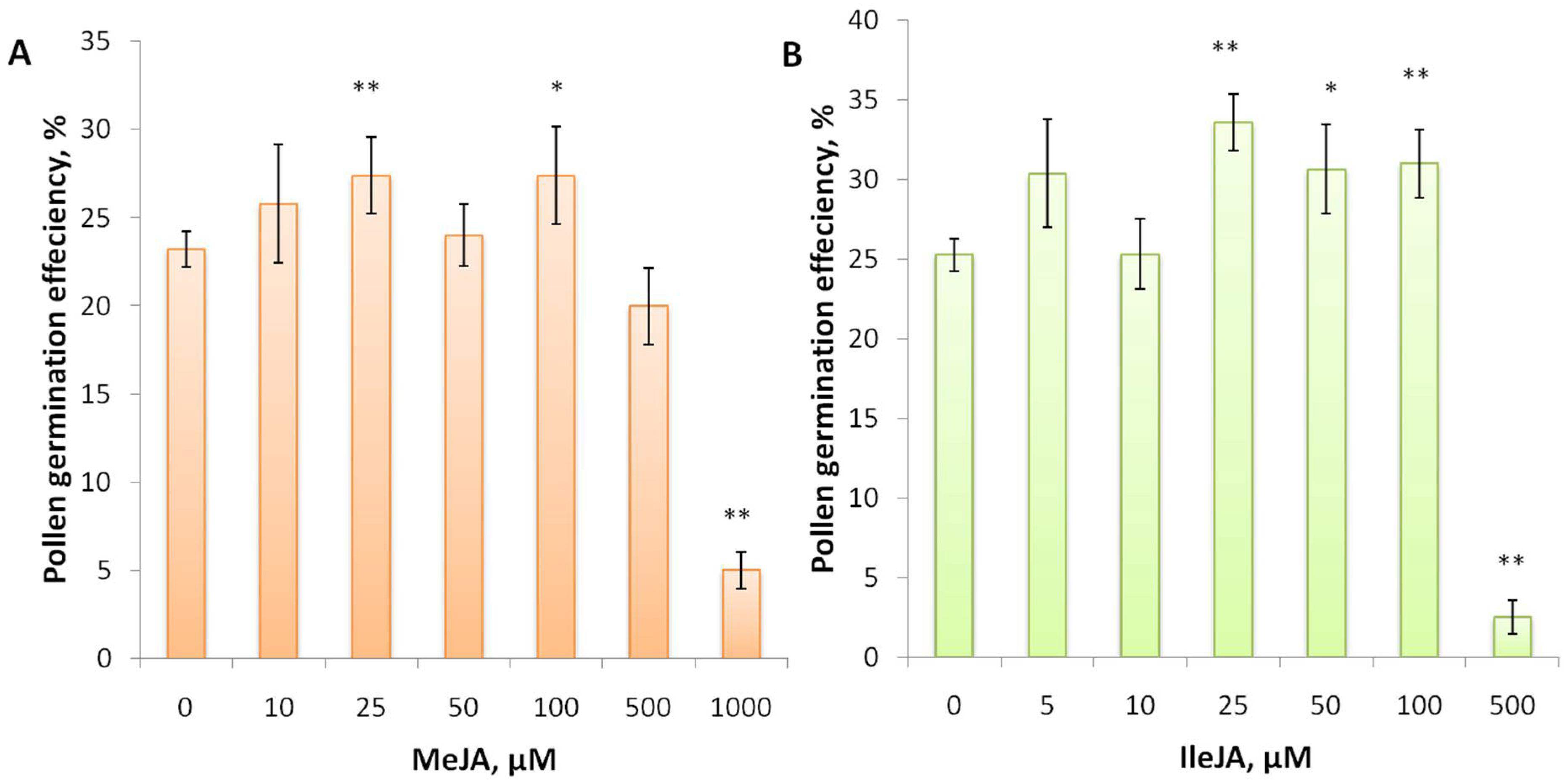
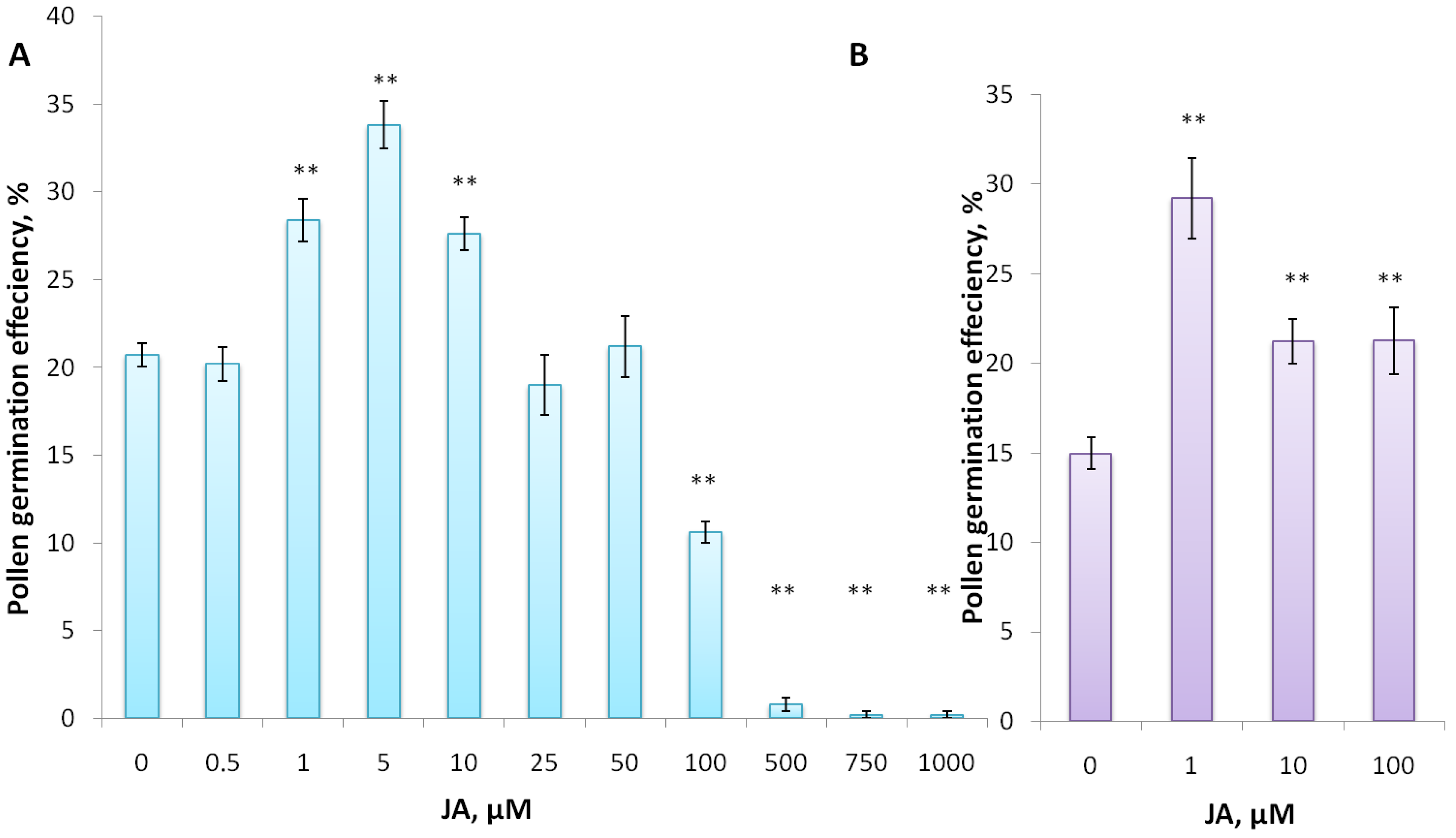
3.2. Jasmonic Acid and Its Conjugate Stimulate Pollen Germination
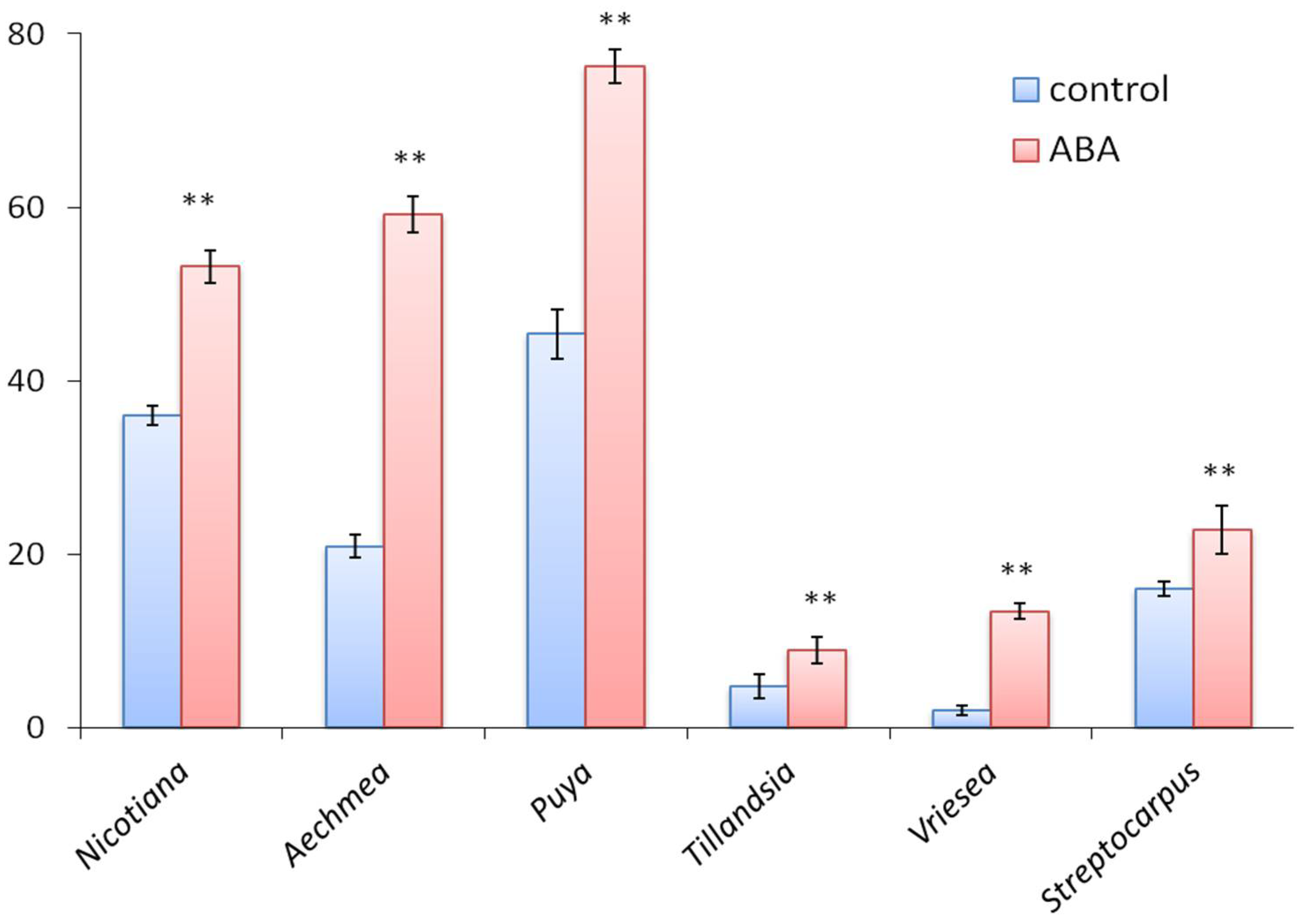
3.3. ABA Is an Effective Stimulator of Germination in All Studied Species
3.4. ABA and JA Affect H2O2 Production in Pollen Grains and Tubes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZT | Zeatin |
| ABA | Abscisic Acid |
| GA3 | Giberellic Acid 3 |
| IAA | Indole-3-acetic Acid |
| JA | Jasmonic Acid |
| MeJA | Methyl Jasmonic Acid |
| Ile-Ja | Isoleucine-Jasmonic Acid |
| SA | Salicylic Acid |
References
- He, Y.-J.; Xu, S.; Zhang, K.-M.; Zhang, Y.; Liu, X.-J.; Liu, C. Multiple Gatekeeping Steps in Pollination Lock Species Specificity. J. Exp. Bot. 2025, 76, 1510–1523. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Gogna, M.; Jain, P.; Singh, N.; Mukherjee, S.; Kalra, G. Signaling Mechanisms and Biochemical Pathways Regulating Pollen-Stigma Interaction, Seed Development and Seedling Growth in Sunflower under Salt Stress. Plant Signal. Behav. 2021, 16, 1958129. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, X.S.; Gao, X.-Q. ROS in the Male–Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 2020, 11, 177. [Google Scholar] [CrossRef]
- Ito, S.; Gorb, S.N. Attachment-Based Mechanisms Underlying Capture and Release of Pollen Grains. J. R. Soc. Interface 2019, 16, 20190269. [Google Scholar] [CrossRef]
- Qin, H.; Abhinandan, K.; Wang, M.; Chen, H.; Zhang, X.; Li, L.; Zhou, Z.; Wang, S.; Zhao, C.; Mu, W.; et al. Free Fatty Acid Biosynthesis Precursors Are Involved in Pollen-Stigma Interactions in Brassica. Hortic. Res. 2025, 12, uhaf147. [Google Scholar] [CrossRef]
- Nepi, M.; von Aderkas, P.; Pacini, E. Sugary Exudates in Plant Pollination. In Secretions and Exudates in Biological Systems; Springer: Berlin/Heidelberg, Germany, 2012; pp. 155–185. [Google Scholar]
- Heslop-Harrison, Y.; Shivanna, K.R. The Receptive Surface of the Angiosperm Stigma. Ann. Bot. 1977, 41, 1233–1258. [Google Scholar] [CrossRef]
- Miki-Hirosige, H.; Hoek, I.H.S.; Nakamura, S. Secretions from the Pistil of Lilium Longiflorum. Am. J. Bot. 1987, 74, 1709–1715. [Google Scholar] [CrossRef]
- Konar, R.N.; Linskens, H.F. Physiology and Biochemistry of the Stigmatic Fluid of Petunia Hybrida. Planta 1966, 71, 372–387. [Google Scholar] [CrossRef]
- Labarca, C.; Loewus, F. The Nutritional Role of Pistil Exudate in Pollen Tube Wall Formation in Lilium longiflorum: I. Utilization of Injected Stigmatic Exudate 1. Plant Physiol. 1972, 50, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kurilla, A.; Toth, T.; Dorgai, L.; Darula, Z.; Lakatos, T.; Silhavy, D.; Kerenyi, Z.; Dallmann, G. Nectar-and Stigma Exudate-Specific Expression of an Acidic Chitinase Could Partially Protect Certain Apple Cultivars against Fire Blight Disease. Planta 2020, 251, 20. [Google Scholar] [CrossRef] [PubMed]
- Rejón, J.D.; Delalande, F.; Schaeffer-Reiss, C.; Carapito, C.; Zienkiewicz, K.; de Dios Alché, J.; Rodríguez-García, M.I.; Van Dorsselaer, A.; Castro, A.J. The Plant Stigma Exudate: A Biochemically Active Extracellular Environment for Pollen Germination? Plant Signal. Behav. 2014, 9, e28274. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Luneva, O.; Babushkina, K.; Kushunina, M.; Lazareva, N.; Klimenko, E. Which Reactive Oxygen Species Are Produced on Wet Stigmas of Different Monocot Plants? Plant Growth Regul. 2025, 105, 1195–1205. [Google Scholar] [CrossRef]
- Breygina, M.; Luneva, O.; Schekaleva, O.; Lazareva, N.; Babushkina, K.; Kirilyuk, I.A. Pattern of ROS Generation and Interconversion on Wet Stigmas in Basal and Divergent Angiosperms. Plant Growth Regul. 2023, 101, 463–472. [Google Scholar] [CrossRef]
- Zafra, A.; Rodríguez-García, M.I.; Alché, J.D.D. Cellular Localization of ROS and NO in Olive Reproductive Tissues during Flower Development. BMC Plant Biol. 2010, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Kochkin, D.; Voronkov, A.; Ivanova, T.; Babushkina, K.; Klimenko, E. Plant Hormone and Fatty Acid Screening of Nicotiana Tabacum and Lilium Longiflorum Stigma Exudates. Biomolecules 2023, 13, 1313. [Google Scholar] [CrossRef]
- Kovaleva, L.; Voronkov, A.; Zakharova, E.; Minkina, Y.; Timofeeva, G.; Andreev, I. Regulation of Petunia Pollen Tube Growth by Phytohormones: Identification of Their Potential Targets. J. Agric. Sci. Technol. A 2016, 6, 239–254. [Google Scholar] [CrossRef][Green Version]
- Kovaleva, L.; Zakharova, E. Hormonal Status of the Pollen-Pistil System at the Progamic Phase of Fertilization after Compatible and Incompatible Pollination in Petunia hybrida L. Sex. Plant Reprod. 2003, 16, 191–196. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Voronkov, A.S.; Zakharova, E.V.; Minkina, Y.V.; Timofeeva, G.V.; Andreev, I.M. Exogenous IAA and ABA Stimulate Germination of Petunia Male Gametophyte by Activating Ca2+-Dependent K+-Channels and by Modulating the Activity of Plasmalemma H+-ATPase and Actin Cytoskeleton. Russ. J. Dev. Biol. 2016, 47, 109–121. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Minkina, Y.V.; Timofeeva, G.V.; Andreev, I.M. Germination and In Vitro Growth of Petunia Male Gametophyte Are Affected by Exogenous Hormones and Involve the Changes in the Endogenous Hormone Level. Russ. J. Plant Physiol. 2005, 52, 521–526. [Google Scholar] [CrossRef]
- Wu, J.; Qin, Y.; Zhao, J. Pollen Tube Growth Is Affected by Exogenous Hormones and Correlated with Hormone Changes in Styles in Torenia fournieri L. Plant Growth Regul. 2008, 55, 137–148. [Google Scholar] [CrossRef]
- Frascaroli, E.; Tuberosa, R. Effect of Abscisic Acid on Pollen Germination and Tube Growth of Maize Genotypes. Plant Breed. 1993, 110, 250–254. [Google Scholar] [CrossRef]
- Yildiz, K.; Yilmaz, H. Effect of Jasmonic Acid, ACC and Ethephon on Pollen Germination in Strawberry. Plant Growth Regul. 2002, 38, 145–148. [Google Scholar] [CrossRef]
- Muradoğlu, F.; Yildiz, K.; Balta, F. Methyl Jasmonate Influences of Pollen Germination and Pollen Tube Growth of Apricot (Prunus armeniaca L.). Yuz. Yıl Univ. J. Agric. Sci. 2010, 20, 183–188. [Google Scholar]
- Çetinbaş-Genç, A.; Vardar, F. Effect of Methyl Jasmonate on In-Vitro Pollen Germination and Tube Elongation of Pinus Nigra. Protoplasma 2020, 257, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Guo, X.; Xu, H.; Wu, Q.; Xie, A.; Zhao, Z.; Tian, R.; Gong, W.; Yuan, D. Methyl Jasmonate (MeJA) Promotes the Self-Pollen Tube Growth of Camellia Oleifera by Regulating Lignin Biosynthesis. Int. J. Mol. Sci. 2024, 25, 10720. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.J.; Fichman, Y.; Zandalinas, S.I.; Mittler, R. Jasmonic Acid and Salicylic Acid Modulate Systemic Reactive Oxygen Species Signaling during Stress Responses. Plant Physiol. 2023, 191, 862–873. [Google Scholar] [CrossRef]
- Munemasa, S.; Mori, I.C.; Murata, Y. Methyl Jasmonate Signaling and Signal Crosstalk between Methyl Jasmonate and Abscisic Acid in Guard Cells. Plant Signal. Behav. 2011, 6, 939–941. [Google Scholar] [CrossRef]
- Soares, A.M.d.S.; de Souza, T.F.; Jacinto, T.; Machado, O.L.T. Effect of Methyl Jasmonate on Antioxidative Enzyme Activities and on the Contents of ROS and H2O2 in Ricinus Communis Leaves. Braz. J. Plant Physiol. 2010, 22, 151–158. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D. Methyl Jasmonate Induces Production of Reactive Oxygen Species and Alterations in Mitochondrial Dynamics That Precede Photosynthetic Dysfunction and Subsequent Cell Death. Plant Cell Physiol. 2008, 49, 1092–1111. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The Roles of Methyl Jasmonate to Stress in Plants. Funct. Plant Biol. 2018, 46, 197–212. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Jakada, B.H.; Qin, H.; Zhan, Y.; Lan, X. Transcriptomics Reveal the Involvement of Reactive Oxygen Species Production and Sequestration during Stigma Development and Pollination in Fraxinus Mandshurica. For. Res. 2024, 4, e014. [Google Scholar] [CrossRef]
- Wang, J.-H.; Cao, J.; Yang, Z.-J.; Jin, Q.-J.; Wang, Y.-J.; Su, Q.; Wang, H.-Y.; Sun, C.-Q.; Xu, Y.-C. NcFER1 and NcRbohC1 Regulate Pollen Germination on Waterlily Stigma After Pollination. Physiol. Plant. 2025, 177, e70318. [Google Scholar] [CrossRef]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Geitmann, A. Reactive Oxygen Species Are Involved in Pollen Tube Initiation in Kiwifruit. Plant Biol. 2012, 14, 64–76. [Google Scholar] [CrossRef]
- Smirnova, A.; Matveyeva, N.; Yermakov, I. Reactive Oxygen Species Are Involved in Regulation of Pollen Wall Cytomechanics. Plant Biol. 2013, 16, 252–257. [Google Scholar] [CrossRef]
- Smirnova, A.V.; Matveyeva, N.P.; Polesskaya, O.G.; Yermakov, I.P. Generation of Reactive Oxygen Species during Pollen Grain Germination. Russ. J. Dev. Biol. 2009, 40, 345–353. [Google Scholar] [CrossRef]
- Nitsch, J.P. Deux Espaces Photoperiodiques de Jours Courts: Plumbago indica L. et P. zeyelanica. Bull. Soc. Bot. Fr. 1965, 112, 517–522. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Gu, M.; Yao, R.; Li, Y.; Chen, J.; Yang, M.; Tong, J.; Xiao, L.; Nan, F.; et al. Endogenous Bioactive Jasmonate Is Composed of a Set of (+)-7-Iso-JA-Amino Acid Conjugates. Plant Physiol. 2016, 172, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Brewbaker, J.L.; Kwack, B.H. The Essential Role of Calcium Ion in Pollen Germination and Pollen Tube Growth. Am. J. Bot. 1963, 50, 859. [Google Scholar] [CrossRef]
- Baptiste, F.J.; Fang, J.Y. Study on Pollen Viability and Stigma Receptivity throughout the Flowering Period in the Selected Taxa of the Gesneriaceae Family. Folia Hortic. 2023, 35, 123–133. [Google Scholar] [CrossRef]
- Souza, E.H.; Souza, F.V.D.; Rossi, M.L.; Packer, R.M.; Cruz-Barros, A.A.V.; Martinelli, A.P. Pollen Morphology and Viability in Bromeliaceae. An. Acad. Bras. Cienc. 2017, 89, 3067–3082. [Google Scholar] [CrossRef]
- Maeda, H.; Fukuyasu, Y.; Yoshida, S.; Fukuda, M.; Saeki, K.; Matsuno, H.; Yamauchi, Y.; Yoshida, K.; Hirata, K.; Miyamoto, K. Fluorescent Probes for Hydrogen Peroxide Based on a Non-Oxidative Mechanism. Angew. Chem.-Int. Ed. 2004, 43, 2389–2391. [Google Scholar] [CrossRef]
- Zakharova, E.V.; Khaliluev, M.R.; Kovaleva, L.V. Hormonal Signaling in the Progamic Phase of Fertilization in Plants. Horticulturae 2022, 8, 365. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Gao, X.; Dong, G.; Zeng, W.; Wang, W.; Sun, M.X. Transcriptome and Metabolome Analyses Reveal a Complex Stigma Microenvironment for Pollen Tube Growth in Tobacco. Int. J. Mol. Sci. 2024, 25, 12255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Lu, M.; Yang, J.; Tan, X. The Core Jasmonic Acid-Signalling Module CoCOI1/CoJAZ1/CoMYC2 Are Involved in Jas Mediated Growth of the Pollen Tube in Camellia oleifera. Curr. Issues Mol. Biol. 2022, 44, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Guo, L.; Cai, Q.; Ma, F.; Zhu, Q.-Y.; Zhang, Q. Sodmergen Arabidopsis Jingubang Is a Negative Regulator of Pollen Germination That Prevents Pollination in Moist Environments. Plant Cell 2016, 28, 2131–2146. [Google Scholar] [CrossRef]
- Breygina, M.; Voronkov, A.; Galin, I.; Akhiyarova, G.; Polevova, S.; Klimenko, E.; Ivanov, I.; Kudoyarova, G. Dynamics of Endogenous Levels and Subcellular Localization of ABA and Cytokinins during Pollen Germination in Spruce and Tobacco. Protoplasma 2022, 260, 237–248. [Google Scholar] [CrossRef]
- Záveská Drábková, L.; Pokorná, E.; Dobrev, P.I.; Kůrková, J.; Steinbachová, L.; Honys, D.; Motyka, V. Hormonome Dynamics During Microgametogenesis in Different Nicotiana Species. Front. Plant Sci. 2021, 12, 735451. [Google Scholar] [CrossRef]
- Chibi, F.; Angosto, T.; Matilla, A. Variations of the Patterns of Abscisic Acid and Proline during Maturation of Nicotiana Tabacum Pollen Grains. J. Plant Physiol. 1995, 147, 355–358. [Google Scholar] [CrossRef]
- Hsu, S.-W.; Cheng, C.-L.; Tzen, T.-C.J.; Wang, C.-S. Rop GTPase and Its Target Cdc42/Rac-Interactive-Binding Motif-Containing Protein Genes Respond to Desiccation during Pollen Maturation. Plant Cell Physiol. 2010, 51, 1197–1209. [Google Scholar] [CrossRef][Green Version]
- Knöfel, H.-D.; Sembdner, G. Jasmonates from Pine Pollen. Phytochemistry 1995, 38, 569–571. [Google Scholar] [CrossRef]
- Humplík, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To Stimulate or Inhibit? That Is the Question for the Function of Abscisic Acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- Kutasy, B.; Hegedűs, G.; Kiniczky, M.; Pallos, J.P.; Nagy, Á.; Pócsi, I.; Pákozdi, K.; Kállai, M.; Weingart, C.; Andor, K.; et al. Garlic Extracts Nanoliposome as an Enhancer of Bioavailability of ABA and Thiamine Content and as an Antifungal Agent Against Fusarium Oxysporum f. Sp. pisi Infecting Pisum sativum. Agronomy 2025, 15, 991. [Google Scholar] [CrossRef]
- Erb, M.; Meldau, S.; Howe, G.A. Role of Phytohormones in Insect-Specific Plant Reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef]
- Liu, H.; Senthilkumar, R.; Ma, G.; Zou, Q.; Zhu, K.; Shen, X.; Tian, D.; Hua, M.S.; Oelmüller, R.; Yeh, K.W. Piriformospora Indica-Induced Phytohormone Changes and Root Colonization Strategies Are Highly Host-Specific. Plant Signal. Behav. 2019, 14, 1632688. [Google Scholar] [CrossRef]
- Kirschner, G.K. It’s Not Witchcraft: The Role of Abscisic Acid in Striga Germination and Conditioning. Plant J. 2024, 117, 1303–1304. [Google Scholar] [CrossRef]
- Friero, I.; Alarcón, M.V.; Gordillo, L.; Salguero, J. Abscisic Acid Is Involved in Several Processes Associated with Root System Architecture in Maize. Acta Physiol. Plant. 2022, 44, 28. [Google Scholar] [CrossRef]
- Xie, Q.; Essemine, J.; Pang, X.; Chen, H.; Jin, J.; Cai, W. Abscisic Acid Regulates the Root Growth Trajectory by Reducing Auxin Transporter PIN2 Protein Levels in Arabidopsis Thaliana. Front. Plant Sci. 2021, 12, 632676. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Wu, Z.; Jia, Y.; Wang, H.; Sun, S.; Mao, C.; Wang, X. Abscisic Acid Regulates Auxin Homeostasis in Rice Root Tips to Promote Root Hair Elongation. Front. Plant Sci. 2017, 8, 1121. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Wang, L.; Yi, B.; Fu, T.; Ma, C.; Dai, C. Knockout of Stigmatic Ascorbate Peroxidase 1 (APX1) Delays Pollen Rehydration and Germination by Mediating ROS Homeostasis in Brassica napus L. Plant J. 2024, 119, 1258–1271. [Google Scholar] [CrossRef]
- Breygina, M.; Klimenko, E.; Shilov, E.; Podolyan, A.; Mamaeva, A.; Zgoda, V.; Fesenko, I. Hydrogen Peroxide in Tobacco Stigma Exudate Affects Pollen Proteome and Membrane Potential in Pollen Tubes. Plant Biol. 2021, 23, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The Interaction of ABA and ROS in Plant Growth and Stress Resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef]
- Grant, M.R.; Jones, J.D.G. Hormone (Dis) Harmony Moulds Plant Health and Disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase Dα1 and Phosphatidic Acid Regulate NADPH Oxidase Activity and Production of Reactive Oxygen Species in ABA-Mediated Stomatal Closure in Arabidopsis. Plant Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Zhao, Q.; Liu, J.; Cheng, F. Involvement of NADPH Oxidase Isoforms in the Production of O2− Manipulated by ABA in the Senescing Leaves of Early-Senescence-Leaf (Esl) Mutant Rice (Oryza Sativa). PLoS ONE 2018, 13, e0190161. [Google Scholar] [CrossRef]
- Ghassemian, M.; Lutes, J.; Chang, H.-S.; Lange, I.; Chen, W.; Zhu, T.; Wang, X.; Lange, B.M. Abscisic Acid-Induced Modulation of Metabolic and Redox Control Pathways in Arabidopsis Thaliana. Phytochemistry 2008, 69, 2899–2911. [Google Scholar] [CrossRef]


| Species/Variety | Phytohormone Content, nmol/mm2 Stigma Surface | ||
|---|---|---|---|
| Jasmonic Acid | Jasmonoyl-L-Isoleucine | Abscisic Acid | |
| Nicotiana tabacum cv. Petit Havana | 2.9 ± 0.7 | 1.0 ± 0.1 | 3.7 ± 0.4 |
| Nicotiana tabacum cv. Samsun | 1.3 ± 0.3 | traces * | 6.7 ± 1.0 |
| Lycopersicon esculentum cv. Pugovka | 211.5 ± 9.9 | traces | traces |
| Streptocarpus cv. Salmon Sunset | 73 ± 4.6 | 11.2 ± 1.9 | not found |
| Treatment | Fluorescence Intensity, rel.un. |
|---|---|
| Control | 74.4 ± 3.9 |
| ABA, 10 μM | 78.4 ± 4.1 |
| JA, 10 μM | 74.7 ± 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breygina, M.; Kochkin, D.V.; Podobedova, A.; Kushunina, M.; Afonin, D.; Klimenko, E. Detection of Abscisic Acid and Jasmonates in Stigma Exudates and Their Role in Pollen Germination. Horticulturae 2025, 11, 1146. https://doi.org/10.3390/horticulturae11091146
Breygina M, Kochkin DV, Podobedova A, Kushunina M, Afonin D, Klimenko E. Detection of Abscisic Acid and Jasmonates in Stigma Exudates and Their Role in Pollen Germination. Horticulturae. 2025; 11(9):1146. https://doi.org/10.3390/horticulturae11091146
Chicago/Turabian StyleBreygina, Maria, Dmitry V. Kochkin, Anna Podobedova, Maria Kushunina, Danil Afonin, and Ekaterina Klimenko. 2025. "Detection of Abscisic Acid and Jasmonates in Stigma Exudates and Their Role in Pollen Germination" Horticulturae 11, no. 9: 1146. https://doi.org/10.3390/horticulturae11091146
APA StyleBreygina, M., Kochkin, D. V., Podobedova, A., Kushunina, M., Afonin, D., & Klimenko, E. (2025). Detection of Abscisic Acid and Jasmonates in Stigma Exudates and Their Role in Pollen Germination. Horticulturae, 11(9), 1146. https://doi.org/10.3390/horticulturae11091146







