Study on the Motion Behavior of Charged Droplets near Plant Leaves
Abstract
1. Introduction
2. Materials and Methods
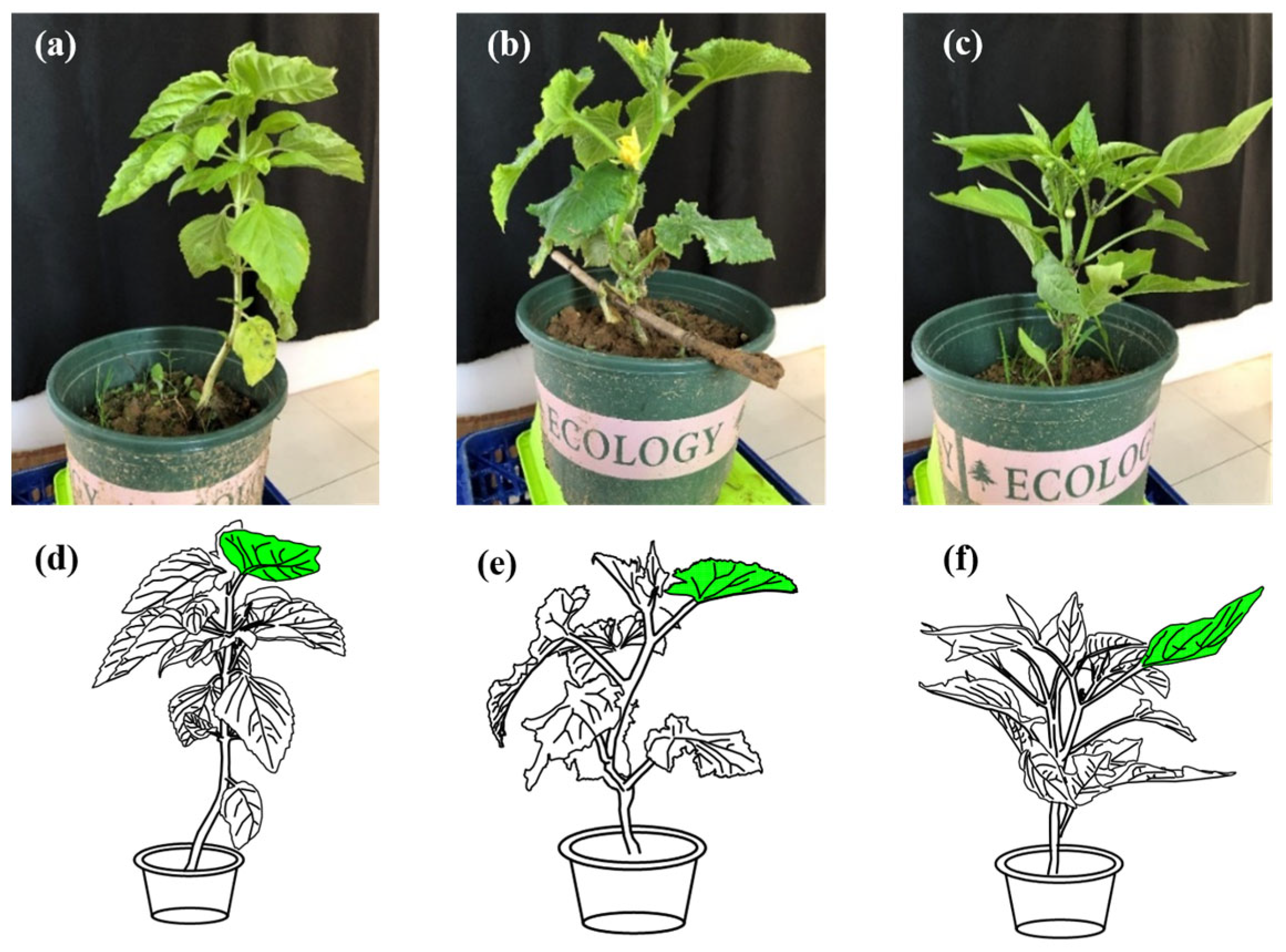
2.1. Crops
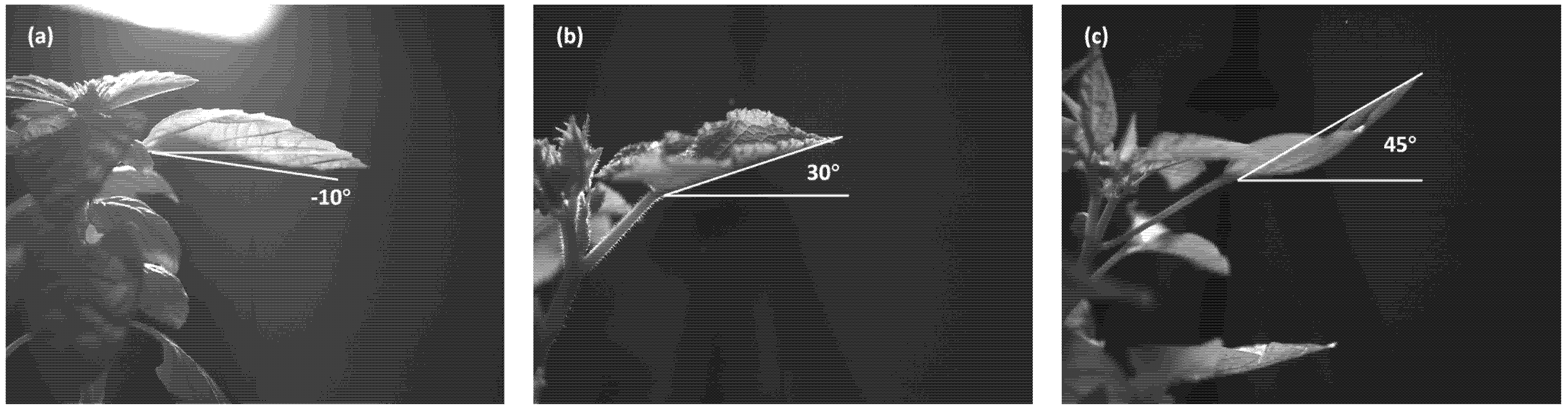
2.2. Physical Parameters of Target Leaf
2.3. Droplet-Capture System
2.4. Calibration of the High-Speed Imaging System
2.5. Statistical Method for Droplet Distribution on the Abaxial Leaf Surface
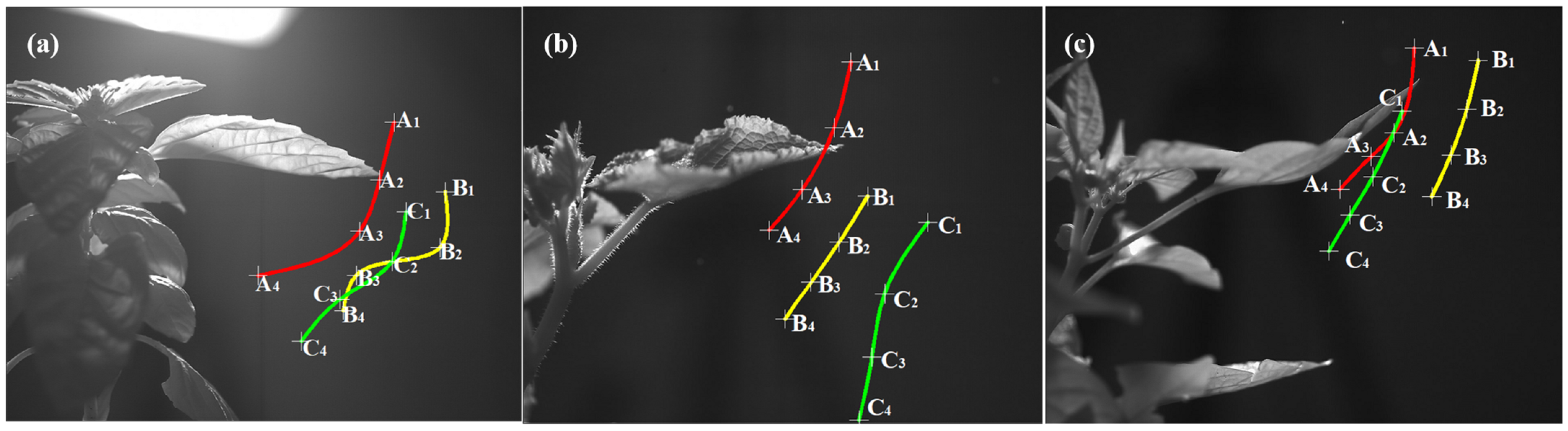
2.6. Selection and Characterization of Trackable Charged Droplets
2.6.1. Matching, Selection, and Labeling of Trackable Charged Droplets
2.6.2. Acquisition of Position Information for Trackable Charged Droplets
2.6.3. Calculation of Two-Dimensional Velocity of Trackable Charged Droplets
2.6.4. Determination of Trajectory Deflection Angle
3. Results and Discussion
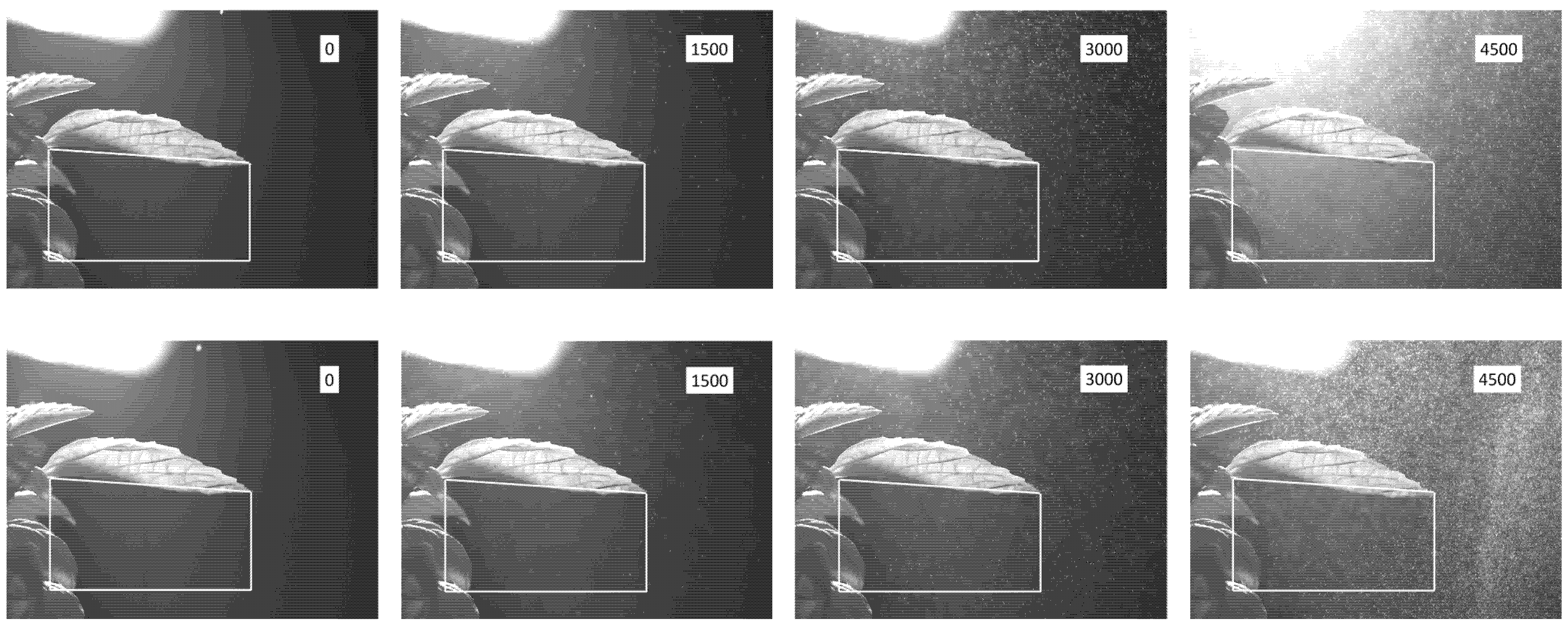
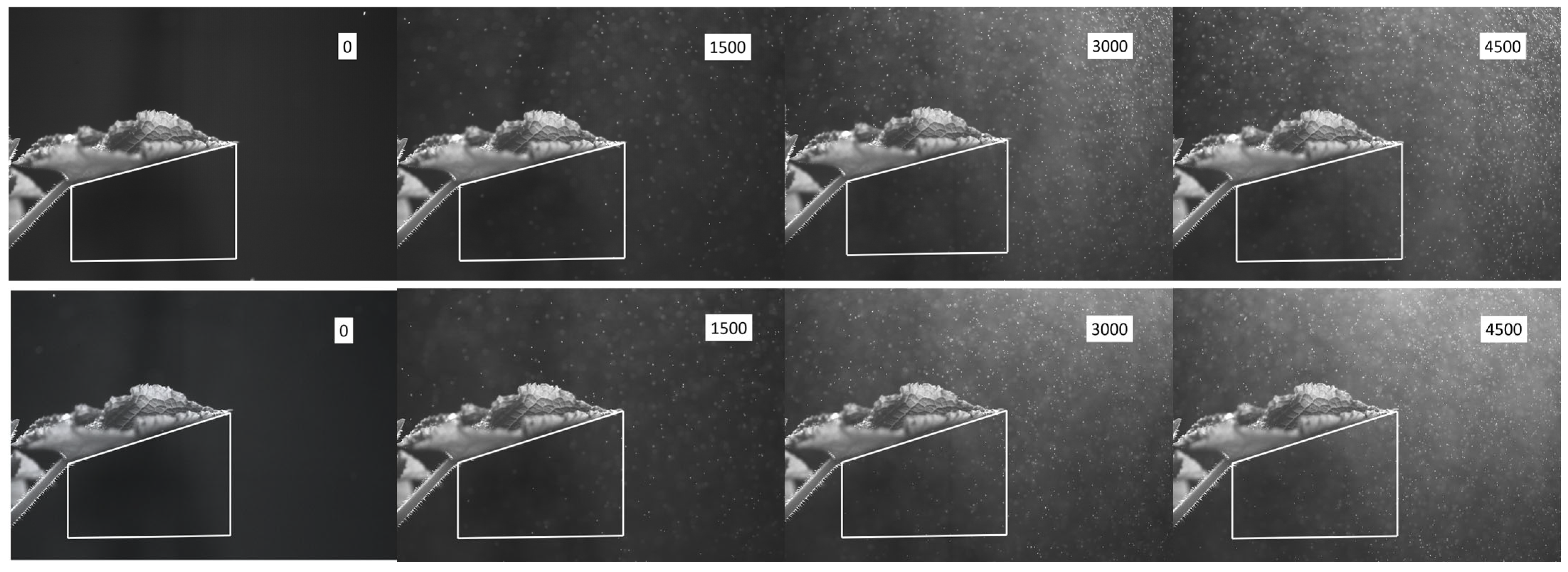
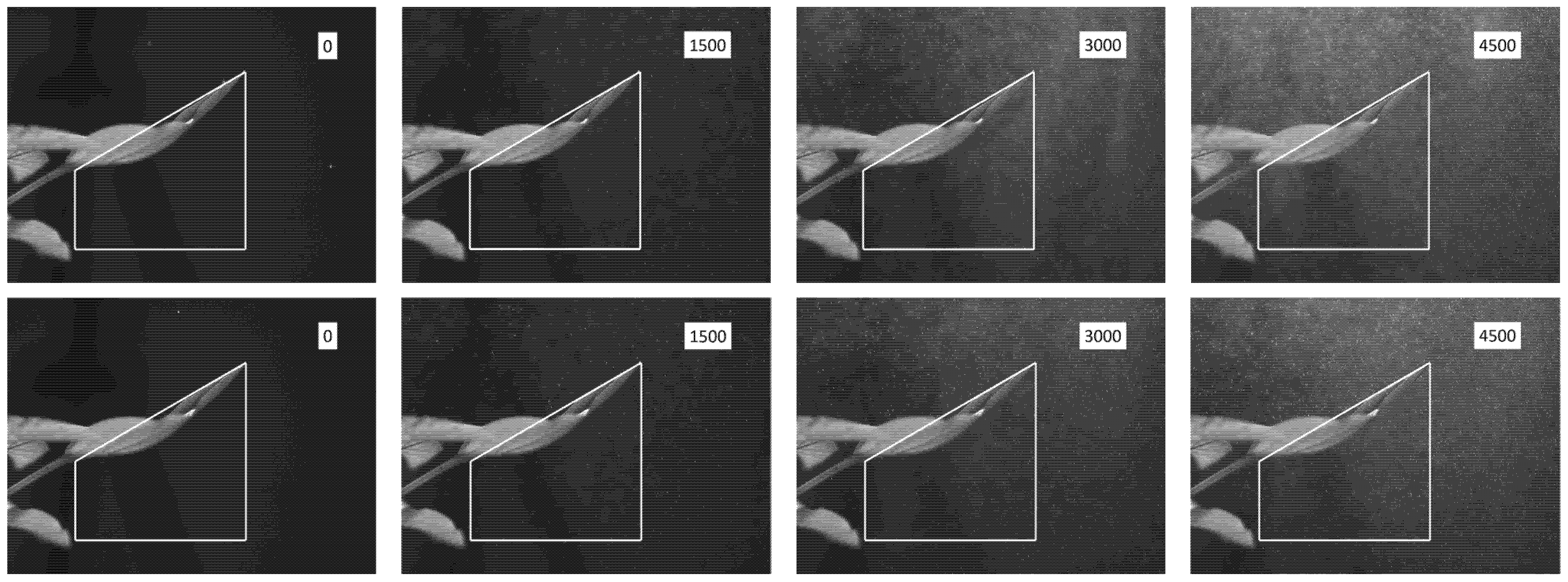
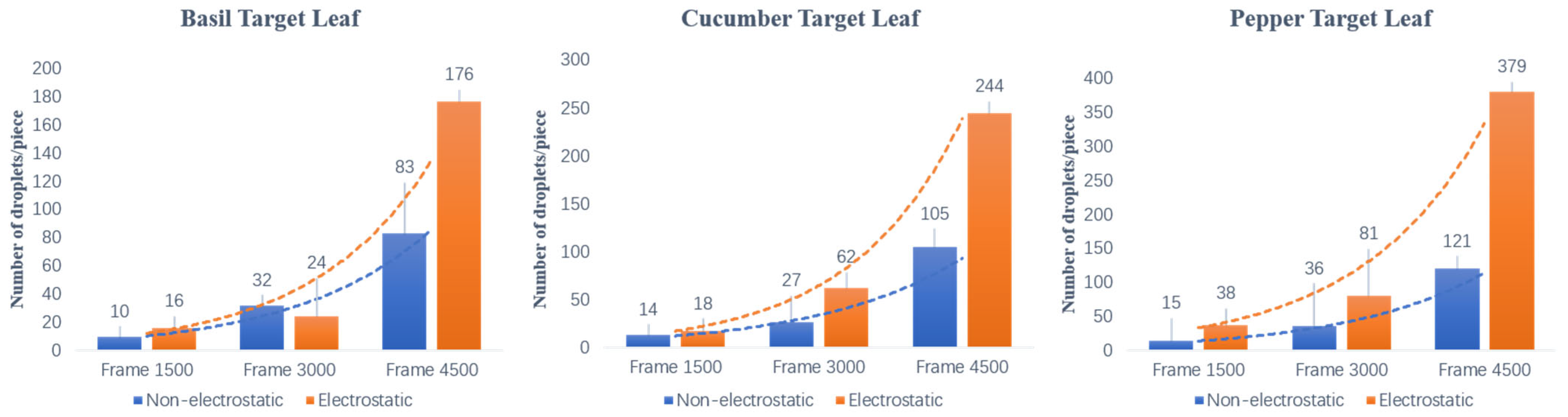
3.1. Droplet-Cluster Distribution on the Abaxial Leaf Surface
3.2. Near-Leaf Trajectories of Trackable Charged Droplets
3.3. Velocity and Deflection of Trackable Charged Droplets near the Leaf Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Law, S.E. Electrostatic Pesticide Spraying: Concepts and Practice. IEEE Trans. Ind. Appl. 1983, IA-19, 160–168. [Google Scholar] [CrossRef]
- Law, S.E. Agricultural Electrostatic Spray Application: A Review of Significant Research and Development during the 20th Century. J. Electrost. 2001, 51, 25–42. [Google Scholar] [CrossRef]
- Gossen, B.D.; Peng, G.; Wolf, T.M.; McDonald, M.R. Improving Spray Retention to Enhance the Efficacy of Foliar-Applied Disease- and Pest-Management Products in Field and Row Crops. Can. J. Plant Pathol. 2008, 30, 505–516. [Google Scholar] [CrossRef]
- Zhou, H.; Ou, M.; Dong, X.; Zhou, W.; Dai, S.; Jia, W. Spraying Performance and Deposition Characteristics of an Improved Air-Assisted Nozzle with Induction Charging. Front. Plant Sci. 2024, 15, 1309088. [Google Scholar] [CrossRef]
- Sánchez-Hermosilla, J.; Pérez-Alonso, J.; Martínez-Carricondo, P.; Carvajal-Ramírez, F.; Agüera-Vega, F. Evaluation of Electrostatic Spraying Equipment in a Greenhouse Pepper Crop. Horticulturae 2022, 8, 541. [Google Scholar] [CrossRef]
- Giles, D.; Blewett, T. Effects of Conventional and Reduced-Volume, Charged-Spray Application Techniques on Dislodgeable Foliar Residue of Captan on Strawberries. J. Agric. Food Chem. 1991, 39, 1646–1651. [Google Scholar] [CrossRef]
- Zhao, S.; Castle, G.S.P.; Adamiak, K. Factors Affecting Deposition in Electrostatic Pesticide Spraying. J. Electrost. 2008, 66, 594–601. [Google Scholar] [CrossRef]
- Appah, S.; Wang, P.; Ou, M.; Gong, C.; Jia, W. Review of Electrostatic System Parameters, Charged Droplets Characteristics and Substrate Impact Behavior from Pesticides Spraying. Int. J. Agric. Biol. Eng. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Almekinders, H.; Ozkan, H.E.; Reichard, D.L.; Carpenter, T.G.; Brazee, R.D. Deposition Efficiency of Air-Assisted, Charged Sprays in a Wind Tunnel. Trans. ASAE 1993, 36, 321–325. [Google Scholar] [CrossRef]
- Maski, D.; Durairaj, D. Effects of Charging Voltage, Application Speed, Target Height, and Orientation upon Charged Spray Deposition on Leaf Abaxial and Adaxial Surfaces. Crop Prot. 2010, 29, 134–141. [Google Scholar] [CrossRef]
- Western, N.M.; Hislop, E.C.; Dalton, W.J. Experimental Air-Assisted Electrohydrodynamic Spraying. Crop Prot. 1994, 13, 179–188. [Google Scholar] [CrossRef]
- Martin, D.E.; Latheef, M.A. Aerial Electrostatic Spray Deposition and Canopy Penetration in Cotton. J. Electrost. 2017, 90, 38–44. [Google Scholar] [CrossRef]
- Salcedo, R.; Llop, J.; Campos, J.; Costas, M.; Gallart, M.; Ortega, P.; Gil, E. Evaluation of Leaf Deposit Quality between Electrostatic and Conventional Multi-Row Sprayers in a Trellised Vineyard. Crop Prot. 2020, 127, 104964. [Google Scholar] [CrossRef]
- Urkan, E.; Guler, H.; Komekci, F. A Review of Electrostatic Spraying for Agricultural Applications. Tarım Makinaları Bilim. Derg. 2016, 12, 229–233. [Google Scholar]
- Lin, Z.; Xie, J.; Tian, S.; Wang, X.; Sun, W.; Mo, X. Research and Experiment of Electrostatic Spraying System for Agricultural Plant Protection Unmanned Vehicle. Front. Ecol. Evol. 2023, 11, 1138180. [Google Scholar] [CrossRef]
- Gao, J.; Guo, Y.; Tunio, M.H.; Chen, X.; Chen, Z. Design of a High-Voltage Electrostatic Ultrasonic Atomization Nozzle and Its Droplet Adhesion Effects on Aeroponically Cultivated Plant Roots. Int. J. Agric. Biol. Eng. 2023, 16, 30–37. [Google Scholar] [CrossRef]
- Kim, S.; Jung, M.; Choi, S.; Lee, J.; Lim, J.; Kim, M. Discharge Current of Water Electrospray with Electrical Conductivity under High-Voltage and High-Flow-Rate Conditions. Exp. Therm. Fluid Sci. 2020, 118, 110151. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Li, Y.; Zhao, S.; Li, Q.; Zhang, W.; Zhao, M. Numerical Simulation of the Trajectory of UAVs Electrostatic Droplets Based on VOF-UDF Electro-Hydraulic Coupling and High-Speed Camera Technology. Agronomy 2023, 13, 512. [Google Scholar] [CrossRef]
- Yue, J.; Chao, C.; Hong, L.; Qingjiang, X. Influences of Nozzle Parameters and Low-Pressure on Jet Breakup and Droplet Characteristics. Int. J. Agric. Biol. Eng. 2016, 9, 22–32. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Li, H.; Hua, L.; Yong, Y. Droplet Distribution Characteristics of Impact Sprinklers with Circular and Noncircular Nozzles: Effect of Nozzle Aspect Ratios and Equivalent Diameters. Biosyst. Eng. 2021, 212, 200–214. [Google Scholar] [CrossRef]
- Hua, L.; Jiang, Y.; Li, H.; Qin, L. Effects of Different Nozzle Orifice Shapes on Water Droplet Characteristics for Sprinkler Irrigation. Horticulturae 2022, 8, 538. [Google Scholar] [CrossRef]
- Liao, J.; Luo, X.; Wang, P.; Zhou, Z.; O’Donnell, C.C.; Zang, Y.; Hewitt, A.J. Analysis of the Influence of Different Parameters on Droplet Characteristics and Droplet Size Classification Categories for Air Induction Nozzle. Agronomy 2020, 10, 256. [Google Scholar] [CrossRef]
- Levia, D.F.; Michalzik, B.; Näthe, K.; Bischoff, S.; Richter, S.; Legates, D.R. Differential Stemflow Yield from European Beech Saplings: The Role of Individual Canopy Structure Metrics. Hydrol. Process. 2015, 29, 43–51. [Google Scholar] [CrossRef]
- Gonzalez-Ollauri, A.; Stokes, A.; Mickovski, S.B. A Novel Framework to Study the Effect of Tree Architectural Traits on Stemflow Yield and Its Consequences for Soil-Water Dynamics. J. Hydrol. 2020, 582, 124448. [Google Scholar] [CrossRef]
- Zhao, S.; Castle, G.S.P.; Adamiak, K. The Effect of Space Charge on the Performance of an Electrostatic Induction Charging Spray Nozzle. J. Electrost. 2005, 63, 261–272. [Google Scholar] [CrossRef]
- Pan, X.; Yang, S.; Gao, Y.; Wang, Z.; Zhai, C.; Qiu, W. Evaluation of Spray Drift from an Electric Boom Sprayer: Impact of Boom Height and Nozzle Type. Agronomy 2025, 15, 160. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; He, R.; Chen, X.; Tunio, M.H. Development and Experiments of Low Frequency Ultrasonic Electrostatic Atomizing Nozzle with Double Resonators. Int. J. Agric. Biol. Eng. 2022, 15, 39–48. [Google Scholar] [CrossRef]
- Liao, J.; Hewitt, A.J.; Wang, P.; Luo, X.; Zang, Y.; Zhou, Z.; Lan, Y.; O’Donnell, C. Development of Droplet Characteristics Prediction Models for Air Induction Nozzles Based on Wind Tunnel Tests. Int. J. Agric. Biol. Eng. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Ou, M.; Zhang, Y.; Wu, M.; Wang, C.; Dai, S.; Wang, M.; Dong, X.; Jiang, L. Development and Experiment of an Air-Assisted Sprayer for Vineyard Pesticide Application. Agriculture 2024, 14, 2279. [Google Scholar] [CrossRef]
- Zhang, Z. A Flexible New Technique for Camera Calibration. IEEE Trans. Pattern Anal. Mach. Intell. 2000, 22, 1330–1334. [Google Scholar] [CrossRef]
- Zhu, H.; Salyani, M.; Fox, R.D. A Portable Scanning System for Evaluation of Spray Deposit Distribution. Comput. Electron. Agric. 2011, 76, 38–43. [Google Scholar] [CrossRef]
- Cerruto, E.; Manetto, G.; Longo, D.; Failla, S.; Papa, R. A Model to Estimate the Spray Deposit by Simulated Water Sensitive Papers. Crop Prot. 2019, 124, 104861. [Google Scholar] [CrossRef]
- Sudheer, K.P.; Panda, R.K. Digital Image Processing for Determining Drop Sizes from Irrigation Spray Nozzles. Agric. Water Manag. 2000, 45, 159–167. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Ma, Y.; Cui, H.; Yang, Z.; Lu, H. Effects of Leaf Response Velocity on Spray Deposition with an Air-Assisted Orchard Sprayer. Int. J. Agric. Biol. Eng. 2021, 14, 123–132. [Google Scholar] [CrossRef]
- Gao, Z.-M.; Hu, W.; Dong, X.-Y.; Zhao, X.-Y.; Wang, S.; Chen, J.; Qiu, B.-J. Motion Behavior of Droplets on Curved Leaf Surfaces Driven by Airflow. Front. Plant Sci. 2024, 15, 1450831. [Google Scholar] [CrossRef] [PubMed]
- Bowker, G.E.; Crenshaw, H.C. Electrostatic Forces in Wind-Pollination—Part 2: Simulations of Pollen Capture. Atmos. Environ. 2007, 41, 1596–1603. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, J.; Jia, W.; Ou, M.; Zhou, H.; Dong, X.; Chen, H.; Wang, M.; Chen, Y.; Yang, S. Experimental Study on the Droplet Size and Charge-to-Mass Ratio of an Air-Assisted Electrostatic Nozzle. Agriculture 2022, 12, 889. [Google Scholar] [CrossRef]
- Erinin, M.A.; Néel, B.; Mazzatenta, M.T.; Duncan, J.H.; Deike, L. Comparison between Shadow Imaging and In-Line Holography for Measuring Droplet Size Distributions. Exp. Fluids 2023, 64, 96. [Google Scholar] [CrossRef]


| Equipment | Model | Specifications |
|---|---|---|
| High-voltage power supply | TCM6000 series, Taisman Technology, Dalian, China | Input voltage: AC 220 V ± 10% |
| Laptop computer | Dell Inspiron 15, Round Rock, TX, USA | \ |
| High-speed camera | OLYMPUS i-SPEED 3, Center Valley, PA, USA | 2000 fps, 15 μs, 1280 × 1040 |
| Light source | OSRAM, Munich, Germany | 230 V, 1000 W |
| Diaphragm pump | XTL-3210, Shenzhen, China | DC 24 V, 100 W, 1.1 MPa |
| Pressure regulator | Airtac BR-2000, Ningbo, China | 0–1.0 MPa |
| Electrostatic induction nozzle | MaxCharge nozzle, Beijing, China | inner diameter 45 mm, outer 55 mm |
| Capture Time | First Neighborhood | Second Neighborhood | Third Neighborhood | Fourth Neighborhood | |
|---|---|---|---|---|---|
| Basil leaves | Droplet A | (964, 284) | (928, 427) | (878, 554) | (828, 664) |
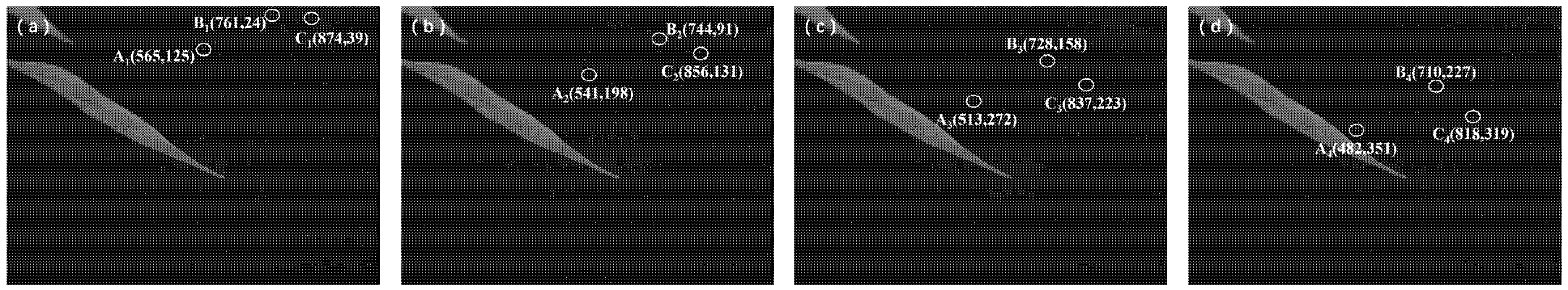
| Droplet B | (761, 24) | (744, 91) | (728, 158) | (710, 227) | |
| Droplet C | (874, 39) | (856, 131) | (837, 223) | (818, 319) | |
| Cucumber leaves | Droplet A | (805, 136) | (764, 297) | (685, 450) | (605, 549) |
| Droplet B | (846, 466) | (776, 579) | (707, 677) | (644, 767) | |
| Droplet C | (994, 530) | (888, 705) | (857, 860) | (825, 1015) | |
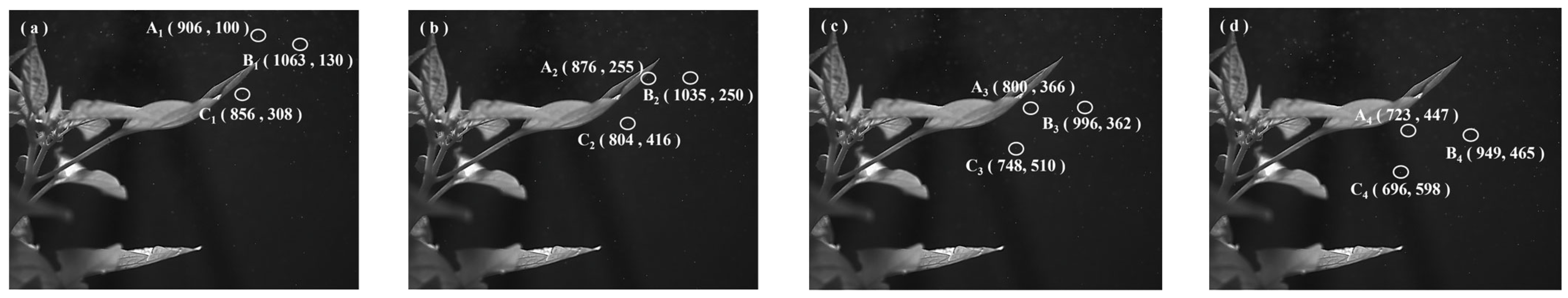
| Pepper leaves | Droplet A | (906, 100) | (876, 255) | (800, 366) | (723, 447) |
| Droplet B | (1063, 130) | (1035, 250) | (996, 362) | (949, 465) | |
| Droplet C | (856, 308) | (804, 416) | (748, 510) | (696, 598) | |
| Capture Time | First Neighborhood | Second Neighborhood | Third Neighborhood | Fourth Neighborhood |
|---|---|---|---|---|
| Droplet A | (−1.3, 10.3) | (−2.7, 10.4) | (−4.3, 10.6) | (−6.3, 10.8) |
| Droplet B | (0.5, 10.0) | (−0.4, 10.1) | (−1.3, 10.3) | (−2.3, 10.4) |
| Droplet C | (0.4, 10.1) | (−0.7, 10.2) | (−1.8, 10.3) | (−3.0, 10.4) |
| Capture Time | First Neighborhood | Second Neighborhood | Third Neighborhood |
|---|---|---|---|
| Droplet A | (−56.3, 6.5) | (−64.4, 7.4) | (−78.4, 9.1) |
| Droplet B | (−34.9, 4.1) | (−36.5, 4.2) | (−39.9, 6.7) |
| Droplet C | (−41.7, 4.8) | (−43.2, 5.1) | (−48.9, 5.5) |
| Trackable Droplet | Average Speed/(mm/s) | |
|---|---|---|
| Horizontal Direction | Vertical Direction | |
| Droplet A | 66.37 | 7.67 |
| Droplet B | 37.10 | 5.00 |
| Droplet C | 44.60 | 5.13 |
| Trackable Droplet | Number of Occupied Pixels (Count) | ||||
|---|---|---|---|---|---|
| First Neighborhood | Second Neighborhood | Third Neighborhood | Fourth Neighborhood | Average-e Value | |
| Droplet A | 12 | 12 | 11 | 10 | 11.25 |
| Droplet B | 21 | 20 | 18 | 18 | 19.25 |
| Droplet C | 16 | 16 | 15 | 14 | 15.25 |
| Trackable Droplet | Deflection Angle/(°) | |||
|---|---|---|---|---|
| Average Value | ||||
| Droplet A | 6.5858 | 6.5549 | 6.4208 | 6.4605 |
| Droplet B | 6.7003 | 6.5640 | 9.5322 | 7.5988 |
| Droplet C | 6.5663 | 6.7329 | 6.8173 | 6.7055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, T.; Wang, S.; Ma, J.; Wang, K.; Dong, L.; Qiu, B. Study on the Motion Behavior of Charged Droplets near Plant Leaves. Horticulturae 2025, 11, 1117. https://doi.org/10.3390/horticulturae11091117
Dong X, Wang T, Wang S, Ma J, Wang K, Dong L, Qiu B. Study on the Motion Behavior of Charged Droplets near Plant Leaves. Horticulturae. 2025; 11(9):1117. https://doi.org/10.3390/horticulturae11091117
Chicago/Turabian StyleDong, Xiaoya, Tao Wang, Shangfeng Wang, Jing Ma, Kaiyuan Wang, Lili Dong, and Baijing Qiu. 2025. "Study on the Motion Behavior of Charged Droplets near Plant Leaves" Horticulturae 11, no. 9: 1117. https://doi.org/10.3390/horticulturae11091117
APA StyleDong, X., Wang, T., Wang, S., Ma, J., Wang, K., Dong, L., & Qiu, B. (2025). Study on the Motion Behavior of Charged Droplets near Plant Leaves. Horticulturae, 11(9), 1117. https://doi.org/10.3390/horticulturae11091117







