Addition of Earthworms to Continuous Cropping Soil Inhibits the Fusarium Wilt in Watermelon: Evidence Under Both Field and Pot Conditions
Abstract
1. Introduction
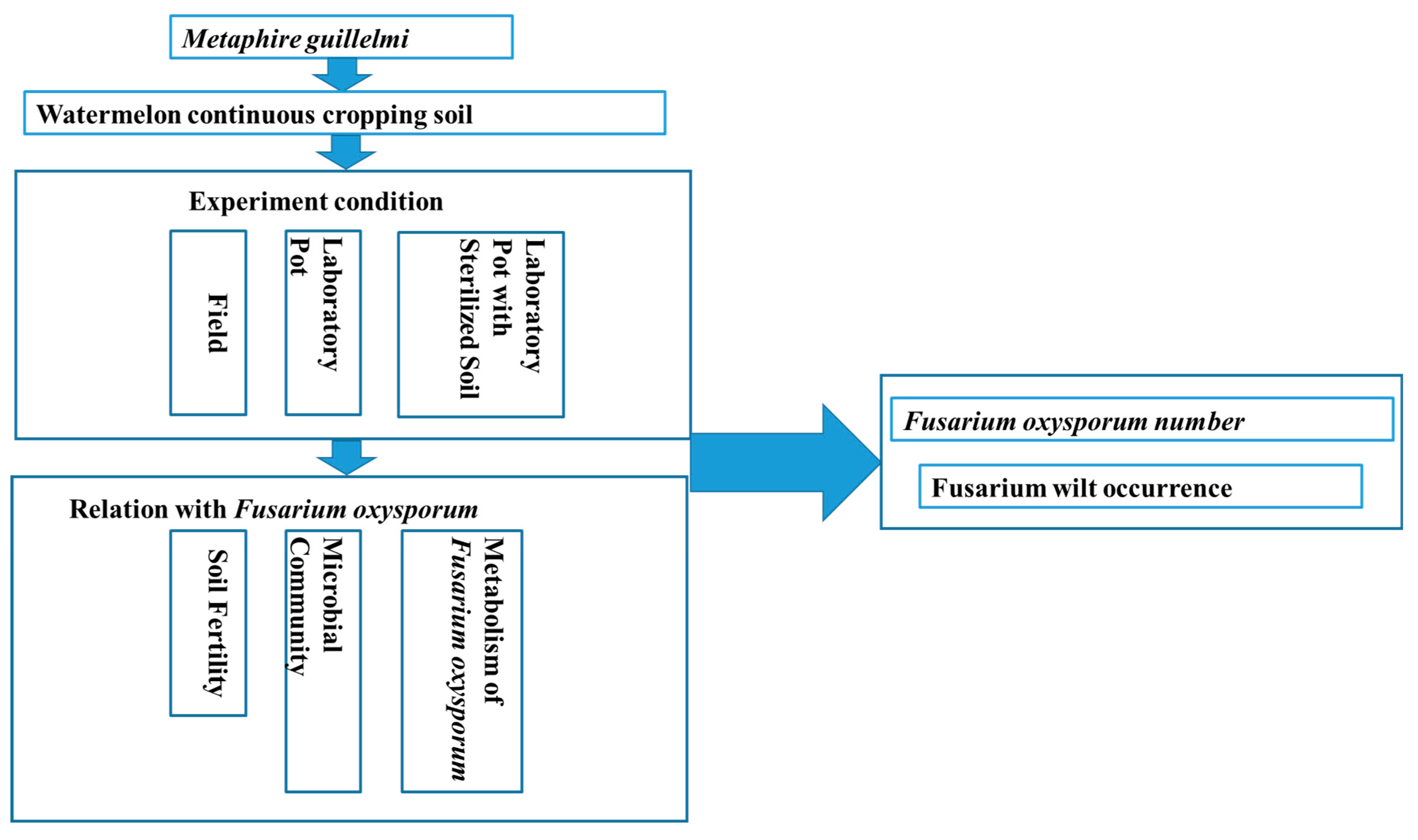
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Materials
2.2.1. Watermelon Varieties
2.2.2. Earthworm Source
2.2.3. Source of F. oxysporum
2.3. Fertilizer Regime for Watermelon Continuous Cropping
2.4. Experimental Design
2.4.1. Positioning Experiment of Facility Community
2.4.2. Laboratory Pot Experiment
2.4.3. Laboratory Pot with Sterilized Soil Test
2.5. Soil Sample Collection
2.6. Indicator Determination
2.6.1. Determination of Soil Fertility Indices
2.6.2. Microbial Community Determination
2.6.3. Quantity Determination of F. oxysporum
2.7. Data Processing and Analysis
3. Results
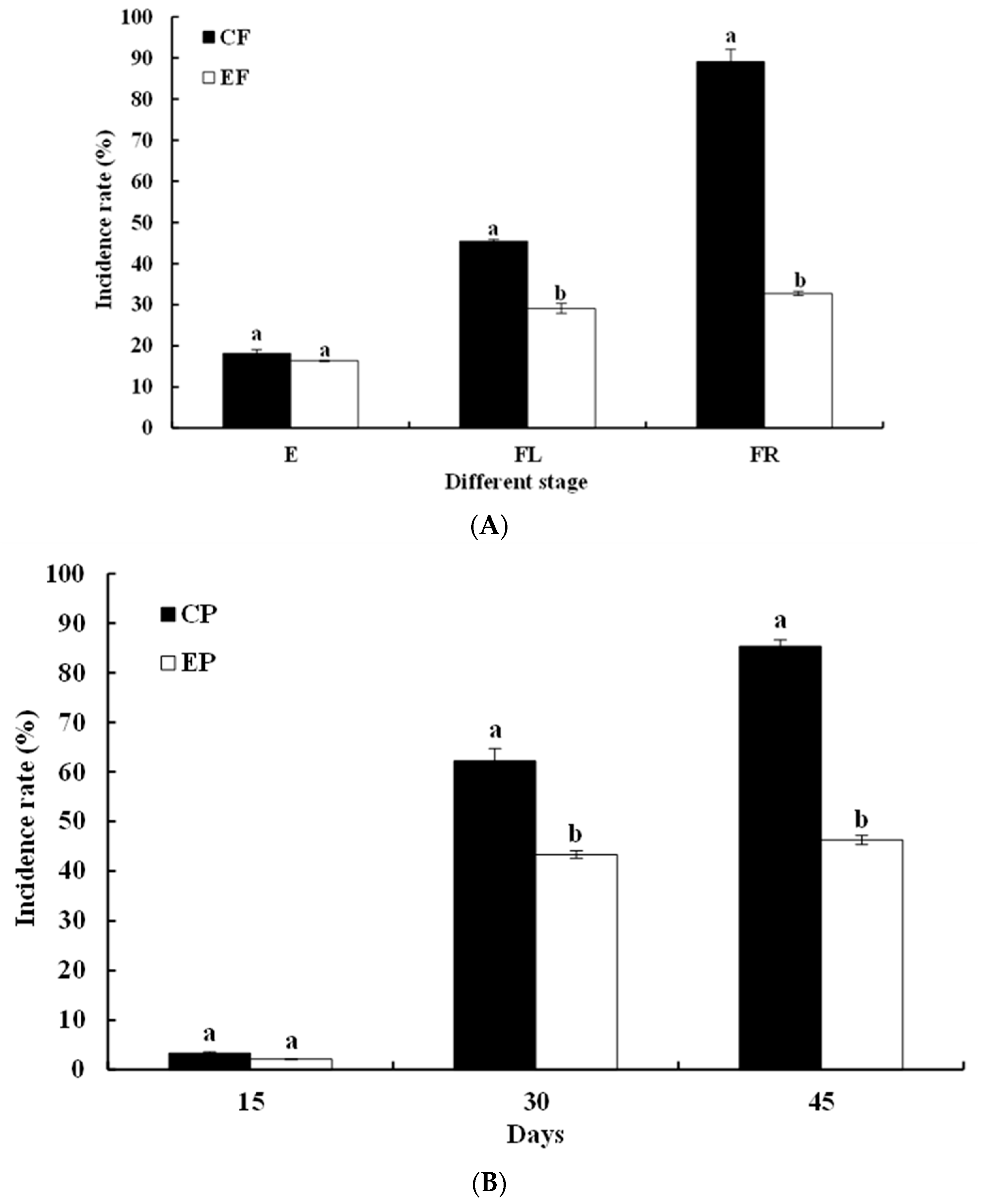
3.1. Effects of Earthworm on the Occurrence of Fusarium Wilt and Number of F. oxysporum in Continuous Cropping of Watermelon Soil
3.2. Impact Directions of Earthworm on the Number of F. oxysporum and Soil Fertility Indicators
3.3. Relationship Between Soil Fertility Indicators and Number of F. oxysporum
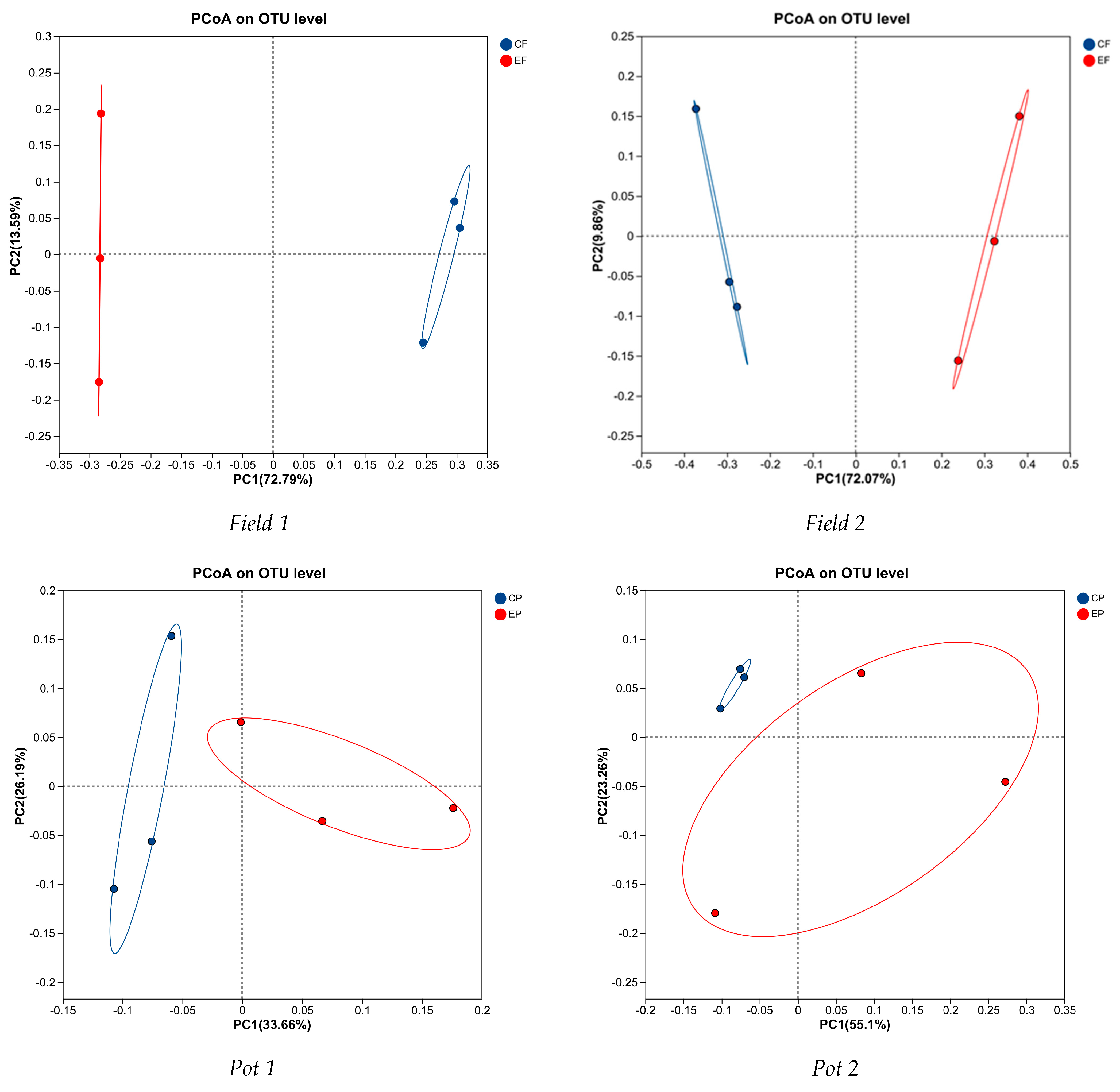
3.4. Effects of Earthworm on Microbial Diversity in Continuous Cropping Soil
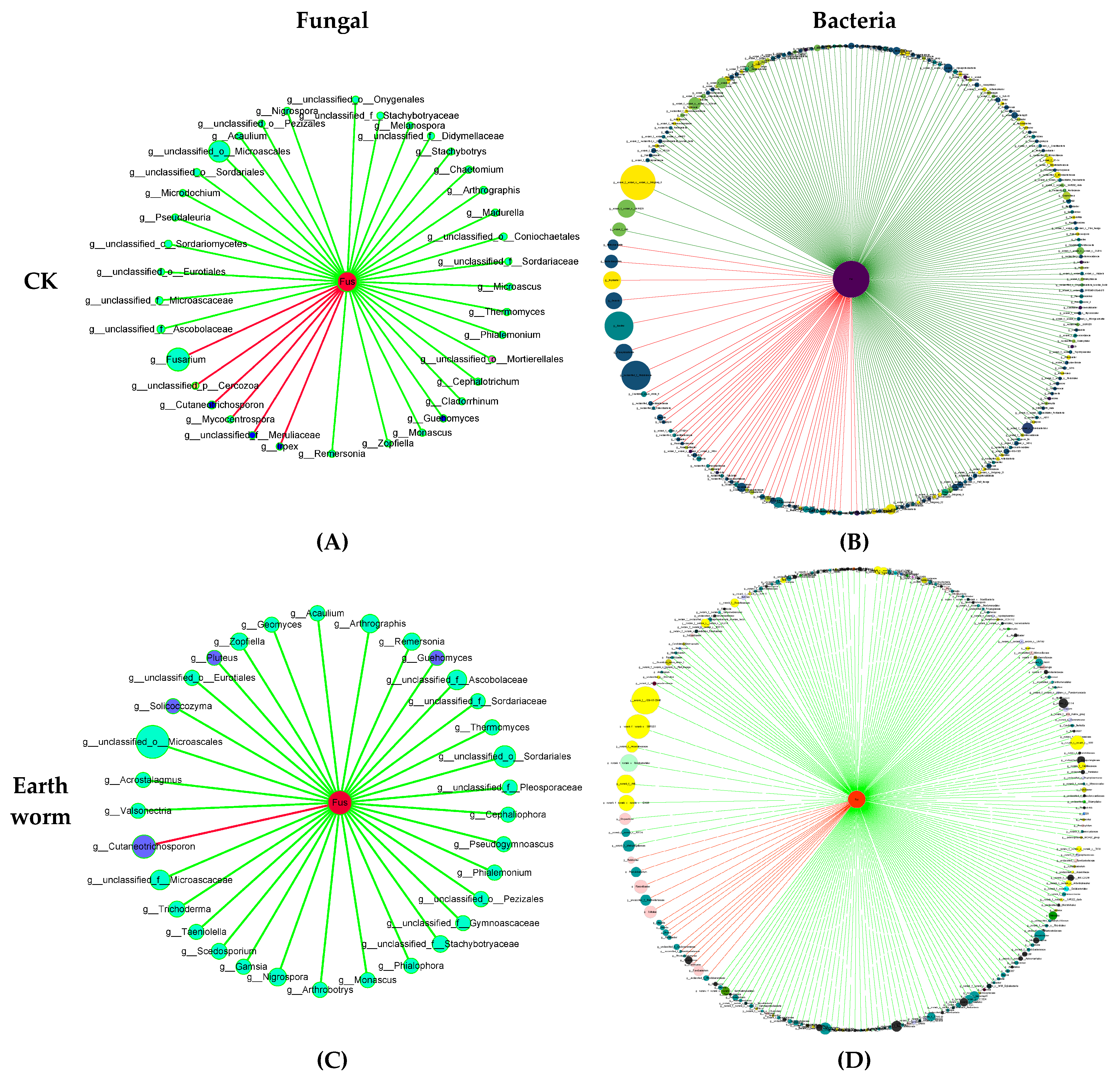
3.5. Effects of Earthworm on the Correlation Between Soil Microorganisms and F. oxysporum
3.6. Effects of Earthworm on the Changes of Metabolome of F. oxysporum in the Eco-Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- FAO. Area and Production of Watermelon. 2023. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 26 August 2023).
- Zamuz, S.; Munekata, P.E.S.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Zhang, P.P.; Xia, L.H.; Sun, Y.T.; Gao, S. Soil nutrients and enzyme activities based on millet continuous cropping obstacles. Sci. Rep. 2024, 14, 17329. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; Jonge, R.D.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef]
- Chen, S.; Qi, G.; Luo, T.; Zhang, H.; Jiang, Q.; Wang, R.; Zhao, X. Continuous-cropping tobacco caused variance of chemical properties and structure of bacterial network in soils. Land Degrad. Dev. 2018, 29, 4106–4120. [Google Scholar] [CrossRef]
- Chen, J.; Yang, X.; Zhong, D.; Huo, Z.; Sun, R.; Dong, H. Continuous Cropping Alters Soil Microbial Community Assembly and Co-Occurrence Network Complexity in Arid Cotton Fields. Agriculture 2025, 15, 1274. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xu, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep Pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, X.; Li, Z.; Wang, M.; Che, Y.; Zhang, L.; Jiang, Z.; Jie, S. Effects of continuous cropping on bacterial community and diversity in rhizosphere soil of industrial hemp: A five-year experiment. Diversity 2022, 14, 250. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Jiang, F.; Wang, X.; Xu, Y.; Shen, Q. Survival-virulence trade-off of soil-borne pathogenic bacteria. Acta Pedol. Sin. 2021, 59, 324–333, (In Chinses with English Abstract). [Google Scholar]
- Rahman, M.Z.; Ahmad, K.; Kutawa, A.B.; Siddiqui, Y.; Saad, N.; Geok Hun, T.; Hata, E.M.; Hossain, M.I. Biology, diversity, detection and management of Fusarium oxysporum f. sp. niveum causing vascular wilt disease of watermelon (Citrullus lanatus): A review. Agronomy 2021, 11, 1310. [Google Scholar]
- Ji, W.L.; Zhu, H.; Lu, X.; Zhao, S.; Liu, W. The mechanism of resistance to Fusarium oxysporum f. sp. nveum race 1 in tetraploid watermelon. Sci. Agric. Sin. 2018, 51, 3750–3765, (In Chinses with English Abstract). [Google Scholar]
- Biswas, A.; Wechter, P.; Ganaparthi, V.; Jarquin, D.; Kousik, S.; Graham, S.; Levi, A. Comparative genomic prediction of resistance to Fusarium wilt (Fusarium oxysporum f. sp. niveum race 2) in watermelon: Parametric and nonparametric approaches. Theor. Appl. Genet. 2025, 138, 35. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.Y.; Du, H.S.; Xiao, J.; Zhou, B.C.; Zheng, X.J.; Deng, Y.W.; Zheng, X.Q.; Chen, M. Watermelon wilt disease: Causes, harms, and control measures. Front. Microbiol. 2025, 16, 1601130. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Tymon, L.; Keinath, A.; Miles, C. Progress in grafting watermelon to manage Verticillium wilt. Plant Pathol. 2021, 70, 13344. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, B.; Zhang, R.; Zhou, L.; Huang, X.; Liu, L. Investigating Changes in the Soil Fungal Community Structure, Functions, and Network Stability with Prolonged Grafted Watermelon Cultivation. Horticulturae 2024, 10, 971. [Google Scholar] [CrossRef]
- Lv, H.F.; Yan, C.S. Effects of wheat intercropping on growth and occurrence of Fusarium wilt in watermelon. PeerJ 2024, 12, e17587. [Google Scholar] [CrossRef]
- Wang, K.H. Analysis of obstacles and overcoming measures of continuous watermelon cropping. China Fruit Veg. 2019, 39, 64–66, (In Chinses with English Abstract). [Google Scholar]
- Everts, K.L.; Egel, D.S.; Langston, D.; Zhou, X.-G. Chemical management of Fusarium wilt of watermelon. Crop Prot. 2014, 66, 114–119. [Google Scholar] [CrossRef]
- Ling, N.; Xue, C.; Huang, Q.; Yang, X.; Xu, Y.; Shen, Q. Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt. Biocontrol 2010, 55, 673–683. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Li, J.; Chen, Y.; Xie, Z.; Tian, Y.; Su, X.; Tian, T.; Shen, T. A Biocontrol Strain of Serratia plymuthica MM Promotes Growth and Controls Fusarium wilt in Watermelon. Agronomy 2023, 13, 2437. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, C.; Xiao, J.L.; Wei, L.; Tian, Y.; Liang, Z.H. Soil inoculation of Trichoderma asperellum M45a regulates rhizosphere microbes and triggers watermelon resistance to Fusarium wilt. AMB Express 2020, 10, 189. [Google Scholar] [CrossRef]
- Pang, B.W.; Li, Y.M.; Xu, K.L.; Fan, M.P. Research progress on effects of vermicompost on soil health and crop growth. Jiangsu Agric. Sci. 2020, 48, 29–35, (In Chinses with English Abstract). [Google Scholar]
- Batista, B.D.; Singh, B.K. Realities and hopes in the application of microbial tools in agriculture. Microb. Biotechnol. 2021, 14, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hua, Z.W.; Liang, W.Z.; Niu, Q.H.; Wang, X. The Prevention of Bio-Organic Fertilizer Fermented from Cow Manure Compost by Bacillus sp. XG-1 on Watermelon Continuous Cropping Barrier. Int. J. Environ. Res. Public Health 2020, 17, 5714. [Google Scholar] [CrossRef]
- Rodríguez, M.P.; Domínguez, A.; Gabbarini, L.A.; Escudero, H.J.; Wall, L.G.; Bedano, J.C. Earthworms mediate the effect of diversifying crop rotations on soil organic carbon incorporation, soil structure formation and microbial activity. Agric. Ecosyst. Environ. 2025, 391, 109751. [Google Scholar] [CrossRef]
- Singh, A.; Singh, D.P.; Tiwari, R.; Kumar, K.; Singh, R.V.; Singh, S.; Prasanna, R.; Saxena, A.K.; Nain, L. Taxonomic and functional annotation of gut bacterial communities of Eisenia foetida and Perionyx excavatus. Microbiol. Res. 2015, 175, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.E.; Yin, C.; Wu, Z.S.; Zhang, M.J.; Li, J.S.; Tian, Y.Q. Studies on in-situ vermicomposting in enhancing soil quality in a continuous monocropping of tomato. J. Plant Nutr. Fertil. 2022, 28, 247–259. [Google Scholar]
- Bai, Y.C.; Zhou, X.F.; Zhao, R.X.; Xue, R.X.; Zhou, G.G.; Qiao, X.Q. Solving successive cropping problems with the application of warm cast substrates in watermelon cultivation. Chin. Agric. Sci. Bull. 2011, 27, 212–216, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y.F.; Wang, C.C.; Wu, Z. Effects of sheep and earthworm manure on the agronomic traits and quality of tomato. North. Hortic. 2020, 51–55, (In Chinese with English Abstract). [Google Scholar]
- Wang, S.Q.; Xu, H.L.; Sun, C.X.; Li, X.H.; Chen, Z.H.; Jiang, N.; Zhang, Y.L. Effects of mushroom-residue vermicompost on soil physicochemical properties and bacterial diversity in strawberry cultivation. J. Jiangsu Agric. Sci. 2023, 51, 218–230. [Google Scholar]
- Bonkowski, M.; Griffiths, B.S.; Ritz, K. Food preferences of earthworms for soil fungi. Pedobiologia 2000, 44, 666–676. [Google Scholar] [CrossRef]
- Byzov, B.A.; Khomyakov, N.V.; Kharin, S.A.; Kurakov, A.V. Fate of soil bacteria and fungi in the gut of earthworms. Eur. J. Soil Biol. 2007, 43 (Suppl 1), S149–S156. [Google Scholar] [CrossRef]
- Plavšin, I.; Velki, M.; Ečimović, S.; Vrandečić, K.; Ćosić, J. Inhibitory effect of earthworm coelomic fluid on growth of the plant parasitic fungus Fusarium oxysporum. Eur. J. Soil Biol. 2017, 78, 1–6. [Google Scholar] [CrossRef]
- Goncharov, A.A.; Glebova, A.A.; Tiunov, A.V. Trophic interactions between Fusarium species and Soil fauna: A meta anaysis of experiment studies. Appl. Soil Ecol. 2020, 145, 103302. [Google Scholar] [CrossRef]
- Moody, S.A.; Briones, M.J.I.; Piearce, T.G.; Dighton, J. Selective consumption of decompsing wheat straw by earthworms. Soil Biol. Biochem. 1995, 27, 1209–1213. [Google Scholar] [CrossRef]
- Moody, S.A.; Piearce, T.G.; Dighton, J. Fate of some fungal spores asscociated with wheat straw decomposition on passage through the guts of Lumbricus terrestris and Aporrectodea Longa. Soil Biol. Biochem. 1996, 28, 533–537. [Google Scholar] [CrossRef]
- Brown, G.G.; Barois, I.; Lavelle, P. Regulation of sol organic matter dynamics and microbial activity in the drilosphere and role of interactions with other edaphic functional domains. Eur. J. Soil Biol. 1995, 36, 177–198. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Razavi, D.S. Evaluation of chemical and biological consequences of soil sterilization methods. Casp. J. Environ. Sci. 2007, 5, 87–91. [Google Scholar]
- Trevors, J.T. Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 1996, 26, 53–59. [Google Scholar] [CrossRef]
- Swenson, T.L.; Northen, T.R. Untargeted Soil metabolomics using liquid chromatography–mass spectrometry and gas chromatography–mass spectrometry. In Methods and Protocols; Springer: New York, NY, USA, 2019; pp. 97–109. [Google Scholar]
- Montesinos, D. Trade-offs involved in the choice of pot vs field experiments. New Phycol. 2025, 245, 1808–1809. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, L.; Wu, C.; Zhang, H.; Deng, Y.; Chen, M.; Zheng, X.; Lv, W.; Chen, J.; Ge, T. Promotion of the symbiotic interaction between phagotrophcic protists and beneficial bacteria, mediated via a balancing of soil nutrients, reduces the incidence of watermelon Fusarium wilt. Pedosphere 2025, 35, 352–363. [Google Scholar] [CrossRef]
- Bi, Y.M.; Tian, G.L.; Wang, C.; Zhang, Y.; Wang, D.N.; Zhang, F.F.; Zhang, L.S.; Sun, Z.J. Differential effects of two earthworm species on Fusarium wilt of strawberry. Appl. Soil Ecol. 2018, 126, 174–181. [Google Scholar] [CrossRef]
- Bertrand, M.; Blouin, M.; Barot, S.; Charlier, A.; Marchand, D.; Roger-Estrade, J. Biocontrol of eyespot disease on two winter wheat cultivars by an anecic earthworm (Lumbricus terrestris). Appl. Soil Ecol. 2015, 96, 33–41. [Google Scholar] [CrossRef]
- Meyer-Wolfarth, F.; Schrader, S.; Olderburg, E.; Weinert, J.; Brunotte, J. Biocontrol of the toxigenic plant pathogen Fusarium clumorum by soil fauna in an agroecosystem. Mycotoxin Res. 2017, 33, 237–244. [Google Scholar] [CrossRef]
- Gudeta, K.; Bhagat, A.; Julka, J.M.; Sinha, R.; Verma, R.; Kumar, A.; Kumari, S.; Ameen, F.; Bhat, S.A.; Amarowicz, R.; et al. Vermicompost and its derivatives against phytopathogenic fungi in the soil: A review. Horticulure 2022, 8, 311. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, Z.; Zhang, N.; Deng, X.; Thomashow, L.S.; Lidbury, I.; Liu, H.; Li, R.; Shen, Q.; Kowalchuk, G.A. Phosphorus availability influences disease-suppressive soil microbiome through plant-microbe interactions. Microbiome 2024, 12, 185. [Google Scholar] [CrossRef]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between nutrition, plant diseases and pests. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Science Press: Beijing, China, 2012; pp. 283–298. [Google Scholar]
- Veresoglou, S.D.; Barto, E.K.; Menexes, G.; Rillig, M.C. Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathol. 2013, 62, 961–969. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Gao, F.; Ye, L.; Mu, X.; Xu, L.; Shi, Z.; Luo, Y. Synergistic effects of earthworms and cow manure under reduced chemical fertilization modified microbial community structure to mitigate continuous cropping effects on Chinese flowering cabbage. Front. Microbiol. 2023, 14, 1285464. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, P.; Wang, Y.; Li, W.; Cui, X.; Zhou, J.; Peng, F.; Dai, L. Earthworm activity optimized the rhizosphere bacterial community structure and further alleviated the yield loss in continuous cropping lily (Lilium lancifolium Thunb.). Sci. Rep. 2021, 11, 20840. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Raiesi, F.; Hosseinpur, A. The combined effects of earthworms and arbuscular mycorrhizal fungi on microbial biomass and enzyme activities in a calcareous soil spiked with cadmium. Appl. Soil Ecol. 2014, 75, 33–42. [Google Scholar] [CrossRef]
- Aira, M.; Sampedro, L.; Monroy, F.; Domiguez, J. Detritivorous earthworms directly modify the structure, thus altering the functioning of a microdecomposer food web. Soil Biol. Biochem. 2008, 40, 2511–2516. [Google Scholar] [CrossRef]
- Zhao QXiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-term coffee monoculture alters soil chemical properties and microbial communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Carrión, V.J.; Revillini, D.; Yin, S.; Dong, Y.; Zhang, T.; Wang, X.; Delgado-Baquerizo, M. Acidification suppresses the natural capacity of soil microbiome to fight pathogenic Fusarium infections. Nat. Commun. 2023, 14, 5090. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; He, X.; Gao, N.; Li, Q.; Qiu, Z.; Hou, Y.; Shen, W. Soil pH amendment alters the abundance, diversity, and composition of microbial communities in two contrasting agricultural soils. Microbiol. Spectr. 2024, 12, e04165-23. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Zhou, Z.; Takaya, N.; Shoun, H. Multi-energy metabolic mechanisms of the fungus Fusarium oxysporum in low oxygen envrionment. Biosci. Biotechnol. Biochem. 2010, 74, 2431–2437. [Google Scholar] [CrossRef]



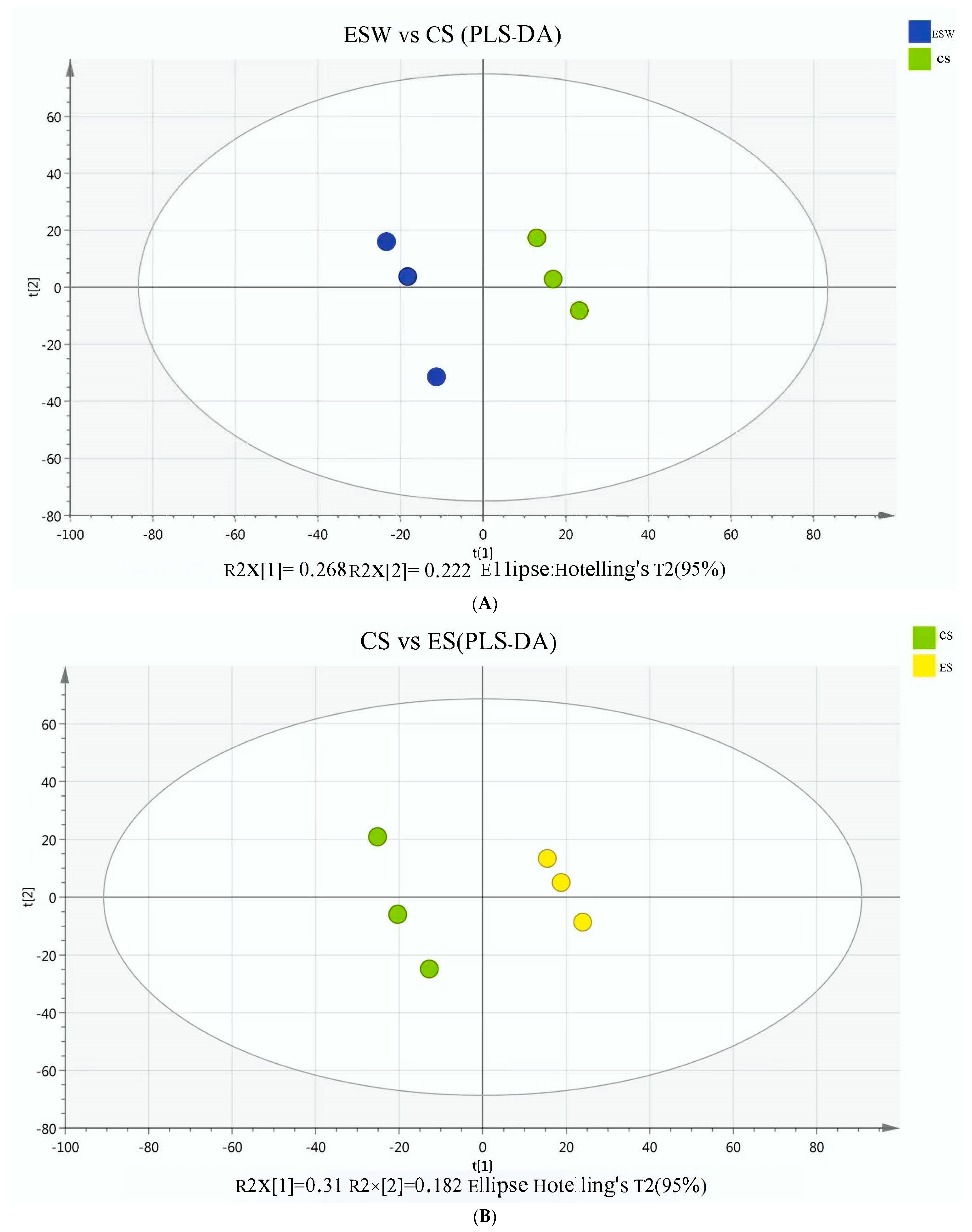
| Experimental Condition | |||
|---|---|---|---|
| Fertility Indicators | Field | Pot | Sterilization |
| SOM | −0.235 | −0.922 ** | −0.760 |
| TN | 0.193 | −0.384 | −0.384 |
| TP | −0.311 | −0.671 | 0.703 |
| TK | −0.020 | −0.144 | 0.742 |
| AN | −0.094 | −0.001 | −0.917 * |
| AP | −0.362 | −0.674 | −0.577 |
| AK | 0.057 | −0.768 | −0.711 |
| pH | −0.239 | 0.969 ** | 0.715 |
| Group | Chao1 (Mean ± SD) | Shannon (Mean ± SD) | Simpson (Mean ± SD) | |
|---|---|---|---|---|
| Field | CF | 2796.17 ± 93.28 | 6.21 ± 0.21 | 0.007 ± 0.002 |
| EF | 2563.01 ± 92.33 | 5.48 ± 0.41 | 0.03 ± 0.01 | |
| p-value | 0.037 * | 0.051 | 0.044 * | |
| Pot | CP | 3161.28 ± 93.84 | 6.8 ± 0.06 | 0.003 ± 0.001 |
| EP | 3232.21 ± 62.72 | 6.88 ± 0.01 | 0.002 ± 0 | |
| p-value | 0.338 | 0.103 | 0.341 | |
| Sterilization | CS | 1053.49 ± 52.78 | 5.11 ± 0.11 | 0.016 ± 0.003 |
| ES | 930.68 ± 30.12 | 4.82 ± 0.06 | 0.02 ± 0.002 | |
| p-value | 0.025 * | 0.015 * | 0.097 | |
| Group | Chao1 (Mean ± SD) | Shannon (Mean ± SD) | Simpson (Mean ± SD) | |
|---|---|---|---|---|
| Field | CF | 224.15 ± 49.53 | 3.43 ± 0.47 | 0.07 ± 0.038 |
| EF | 305.63 ± 46.89 | 3.43 ± 0.13 | 0.138 ± 0.018 | |
| p-value | 0.108 | 0.999 | 0.048 * | |
| Pot | CP | 352.8 ± 0.93 | 2.06 ± 0.1 | 0.408 ± 0.032 |
| EP | 412.99 ± 23.08 | 2.7 ± 0.46 | 0.269 ± 0.125 | |
| p-value | 0.011 * | 0.076 | 0.138 | |
| Sterilization | CS | 50.23 ± 23.39 | 1.29 ± 0.53 | 0.448 ± 0.144 |
| ES | 84.67 ± 17.61 | 1.75 ± 0.93 | 0.347 ± 0.211 | |
| p-value | 0.111 | 0.502 | 0.537 | |
| Metabolite Name | Fold Change | VIP | p-Value |
|---|---|---|---|
| Indole | 1.65664 | 1.46069 | 0.00101 |
| Octadecanamine | 5.05523 | 2.69404 | 0.00180 |
| Stearamine | 2.85919 | 2.09360 | 0.00420 |
| Palmitamide | 1.81636 | 1.54632 | 0.00926 |
| Bis (2-ethylhexyl) phthalate | 1.52966 | 1.28161 | 0.02067 |
| Didrovaltrate | 56.13264 | 1.82532 | 0.01117 |
| Oleic acid | 0.34096 | 1.43336 | 0.01895 |
| Metabolite Name | Fold Change | VIP | p-Value |
|---|---|---|---|
| Kynurenic acid | 1.97728 | 1.57168 | 0.01096 |
| Maraniol | 1.47630 | 1.18960 | 0.01267 |
| Bis(2-ethylhexyl) phthalate | 1.75434 | 1.42175 | 0.0195 |
| Palmitamide | 1.64786 | 1.30955 | 0.0296 |
| Cyprodenate | 1.47528 | 1.14116 | 0.0419 |
| 2-Dodecylbenzenesulfonic acid | 5.68887 | 2.21543 | 0.0497 |
| Linoleamide | 0.47435 | 1.59435 | 0.0497 |
| Myristyl sulfate | 0.38569 | 1.54697 | 0.0020 |
| 4-Undecylbenzenesulfonic acid | 0.24038 | 1.88692 | 0.0104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zheng, L.; Liu, D.; Song, K.; Lu, P.; Yang, Y.; Yang, L.; Li, X.; Li, Y.; Zhang, Y.; et al. Addition of Earthworms to Continuous Cropping Soil Inhibits the Fusarium Wilt in Watermelon: Evidence Under Both Field and Pot Conditions. Horticulturae 2025, 11, 1088. https://doi.org/10.3390/horticulturae11091088
Zhao X, Zheng L, Liu D, Song K, Lu P, Yang Y, Yang L, Li X, Li Y, Zhang Y, et al. Addition of Earthworms to Continuous Cropping Soil Inhibits the Fusarium Wilt in Watermelon: Evidence Under Both Field and Pot Conditions. Horticulturae. 2025; 11(9):1088. https://doi.org/10.3390/horticulturae11091088
Chicago/Turabian StyleZhao, Xin, Liang Zheng, Dong Liu, Ke Song, Ping Lu, Yefeng Yang, Lijuan Yang, Xiaoxiao Li, Yinsheng Li, Yue Zhang, and et al. 2025. "Addition of Earthworms to Continuous Cropping Soil Inhibits the Fusarium Wilt in Watermelon: Evidence Under Both Field and Pot Conditions" Horticulturae 11, no. 9: 1088. https://doi.org/10.3390/horticulturae11091088
APA StyleZhao, X., Zheng, L., Liu, D., Song, K., Lu, P., Yang, Y., Yang, L., Li, X., Li, Y., Zhang, Y., Lv, W., & Zheng, X. (2025). Addition of Earthworms to Continuous Cropping Soil Inhibits the Fusarium Wilt in Watermelon: Evidence Under Both Field and Pot Conditions. Horticulturae, 11(9), 1088. https://doi.org/10.3390/horticulturae11091088






