Effects of Different Agricultural Wastes on the Growth of Photinia × fraseri Under Natural Low-Temperature Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Chemical Properties of Substrates
2.3. Measurement of Morphological Indicators of P. fraseri
2.4. Measurement of Physiological and Photosynthetic Indicators
2.5. Comprehensive Evaluation
2.6. Statistical Analysis
3. Results
3.1. Analysis of Chemical Properties of Different Combined Cultivation Substrates
3.2. Effects of Different Cultivation Substrates on the Growth and Development of P. fraseri
3.3. Determination of Physiological Indicators of P. fraseri
3.4. Detection of Photosynthetic-Related Indicators of P. fraseri
3.5. Comprehensive Evaluation Analysis
3.6. Correlation Analysis Between Different Indicators
4. Discussion
4.1. Impact of Substrates on the Growth of P. fraseri
4.2. Photosynthetic Indicators Affect the Growth and Development of P. fraseri
4.3. Impact of Substrates on the Physiological Status of P. fraseri
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Chl | chlorophyll |
| LWC | leaf water content |
| Ci | intercellular CO2 concentration |
| Gs | stomatal conductance |
| Pn | net photosynthetic rate |
| Tr | transpiration rate |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| Pro | proline |
| SP | soluble protein |
| OM | organic matter |
| AHN | alkali-hydrolyzed nitrogen |
| APH | available phosphorus |
| APO | available potassium |
| TN | total nitrogen |
| TPH | total phosphorus |
| TPO | total potassium |
| PH | plant height |
| SD | stem diameter |
| BN | branch number |
| BL | branch length |
| BD | branch diameter |
| NS | new shoots |
| MP | membrane permeability |
| FW | fresh weight |
| DW | dry weigh |
References
- Yu, X.; Kou, Y.; Jia, R.; Zhao, X.; Zha, N.; Guo, Y.; Zhang, Q.; Ge, H.; Yang, S. Advances in overwintering physiological responses and molecular regulation mechanism of cold tolerance in woody plants. Mol. Plant Breed. 2022, 1–19. Available online: https://link.cnki.net/urlid/46.1068.S.20220712.1651.010 (accessed on 29 August 2025).
- Lu, L.; Chen, Y.; Xue, C.; Huang, X.; Zhao, Y. Overexpression of the tea plant CsWRKY29 transcription factor influences cold tolerance and growth development in transgenic tobacco. Genom. Appl. Biol. 2025, 44, 527–536. [Google Scholar]
- Zhao, T.; Zhao, J.; Zhang, D.; Chen, C. Comparative study on the anatomical and physiological characteristics and cold resistance of 8 almond varieties. J. Arid Land Resour. Environ. 2024, 38, 182–190. [Google Scholar]
- Sun, G.; Chen, F.; Meng, X.; Huang, D.; Meng, Q. Study on the anatomic structure of leaves of nine evergreen broad-leaved plants. N. Horticult. 2024, 5, 53–61. [Google Scholar]
- Deng, C.; Liu, X.; Liao, F.; Chen, S.; Yang, L.; Yin, P. Light intensity plays the key role in the regulation of leaf color, anthocyanin and polyphenol profiles, as well as antioxidant activity of Photinia × fraseri leaves. Arab. J. Chem. 2024, 17, 106046. [Google Scholar] [CrossRef]
- Si, M.; Mu, Y. Comparison of carbon sequestration and cooling benefits of plants in different landscaped green sites based on GGE biplot. Chin. J. Appl. Ecol. 2024, 36, 682–692. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, J.; Chen, S.; Zhang, J.; Ren, Y.; Jin, X.; Chen, J. Effects of periodic drought with severe exhaust exposure on particle retention capacity and physiological responses of Photinia × fraseri Dress. Ecotoxicol. Environ. Saf. 2022, 241, 113807. [Google Scholar] [CrossRef]
- Wang, F.; Liu, N.; Chen, K.; Xie, Z. Growth performance of two Photinia × fraseri varieties introduced to Jinzhong region. J. Shanxi Agric. Sci. 2023, 51, 69–73. [Google Scholar] [CrossRef]
- Detti, C.; Gori, A.; Azzini, L.; Nicese, F.P.; Alderotti, F.; Lo Piccolo, E.; Stella, C.; Ferrini, F.; Brunetti, C. Drought tolerance and recovery capacity of two ornamental shrubs: Combining physiological and biochemical analyses with online leaf water status monitoring for the application in urban settings. Plant Physiol. Biochem. 2024, 216, 109208. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Yuan, X.; Wei, S.; Zhang, N.; Xiao, Y.; Huo, G.; Cui, C. First report of Pseudopestalotiopsis ixorae causing leaf spot of Photinia × fraseri in China. Plant Dis. 2024, 108. [Google Scholar] [CrossRef]
- Wu, H.; Yang, S.; Chen, J.; Wang, B.; Liu, M.; Shen, J.; Zheng, G. Adsorption capacity and photosynthetic response of Photinia fraseri on different particle size. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2018, 47, 600–606. [Google Scholar]
- Shen, S.; Wang, J.; Zhou, T.; Ma, Y.; Wang, B. Physiological responses of typical subtropical landscape shrubs to artificial light at night. Chin. J. Appl. Ecol. 2023, 34, 2321–2329. [Google Scholar]
- Hu, X.; Yuan, Q.; Zhan, M.; Zhou, S.; Hu, W. Effects of low temperature in winter on photoinhibition of sun leaves and shade leaves in three evergreen broad-leaved plants. J. Plant Resour. Environ. 2023, 32, 65–72. [Google Scholar]
- Hu, W.; Xiao, Y.; Yan, X.; Ye, Z.; Zeng, J.; Li, X. Photoprotective mechanisms under low temperature and high light stress of Photinia × fraseri and Osmanthus fragrans during overwintering. Bull. Bot. Res. 2021, 41, 938–946. [Google Scholar] [CrossRef]
- Gusta, L.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013, 147, 4–14. [Google Scholar] [CrossRef]
- Xu, C. Research progress on the mechanism of improving plant cold hardiness. Acta Ecol. Sin. 2012, 32, 7966–7980. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, A.; Zhang, Y.; Yang, X.; Yang, S.; Zhao, K. Effects of progressive drought stress on the growth, ornamental values, and physiological properties of Begonia semperflorens. Horticulturae 2024, 10, 405. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Li, Q.; Zhang, L.; He, J.; Li, J.; Zhang, H.; Li, Y.; Gu, Y.; Luo, H.; Lu, M.; Lu, K.; et al. Effects of different shade treatments on Melaleuca seedling growth and physiological properties. BMC Plant Biol. 2025, 25, 203. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Tian, R.; Gao, Z.; Chen, L.; Hu, Y. Evaluation of heat resistance of new wheat advanced lines at seedling stage. J. Triticeae Crops 2024, 44, 279–1286. [Google Scholar]
- You, L.; Zhu, X.; Zhang, Q.; Li, Y.; Chen, X.; Xue, H.; Zhu, P.; Zhang, K. Characteristics of formula substrates added agricultural wastes and their effects on the growth of Paeonia suffruticosa. J. Plant Resour. Environ. 2024, 33, 36–49. [Google Scholar]
- Ding, H.; Li, P.; Sun, W.; Wu, L.; Yuan, X.; Zou, S. Effect of different planting patterns on the growth of Camellia and soil physicochemical properties. Jiangsu Agric. Sci. 2018, 46, 106–110. [Google Scholar]
- Zhang, Q.; Zhang, S.; Li, L. Effects of organic material-improved purple soil on the growth and development of blueberry. J. Southwest. Univ. (Nat. Sci. Ed.) 2018, 40, 21–28. [Google Scholar]
- Yin, Z.; Zhang, L.; Bai, Y. Replacing peat with garden waste compost in Cosmos bipinnata cultivation. J. Zhejiang AF Univ. 2022, 5, 1045–1051. [Google Scholar]
- Bi, M.; Liang, R.; Wang, J.; Qu, Y.; Liu, X.; Cao, Y.; He, G.; Yang, Y.; Yang, P.; Xu, L.; et al. Multifaceted roles of LhWRKY44 in promoting anthocyanin accumulation in Asiatic hybrid lilies (Lilium spp.). Hortic. Res. 2023, 10, uhad167. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H. Research progress on effect of soil pH on plant growth. Guizhou Agric. Sci. 2022, 67, 4039–4040. [Google Scholar]
- Inoue, T.; Sunaga, M.; Ito, M.; Yuchen, Q.; Yamori, W. Minimizing VPD fluctuations maintains higher stomatal conductance and photosynthesis, resulting in improvement of plant growth in lettuce. Front. Plant Sci. 2021, 12, 646144. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zhao, W.; Cui, C.; Zhang, X.; Zhao, X.; Liu, Y.; Wang, C.; Zhang, H.; Li, S. Physiological indices of five hybrid larch seedlings under low-temperature stress. Forests 2024, 15, 2026. [Google Scholar] [CrossRef]
- Lin, Z.; Gan, Y.; Lin, W.; Wang, B.; Guo, C.; Liu, L.; Wang, H. Effects of low temperature stress on the adversity physiology and total flavonoid content of Cibotium barometz leaves. Chin. J. Trop. Crops 2025, 46, 1441–1448. [Google Scholar]
- Yang, Z.; Zhang, Y.; Ding, Q.; Xing, H.; Wang, H.; Meng, X.; Fan, H.; Yu, Y.; Cui, N. The role of TOR in response to chilling stress in Solanum lycopersicum L. Plant Growth Regul. 2025, 105, 1–15. [Google Scholar] [CrossRef]
- Ouyang, Z.; Shi, J.; Jia, X.; Teng, W.; Liu, X. Effect of exogenous growth regulators on physiological characteristics of cold resistance of Rhizophora stylosa seedlings with different ages under low temperature stress. Chin. J. Appl. Ecol. 2025, 36, 780–790. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Y.; Sun, N.; Zhang, Y.; Wang, D.; Guo, C. The role of proline in plant resistances to oxidative stress induced by abiotic stresses. J. Plant Genet. Res. 2025, 1–15. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, Y.; Guo, Y. The relationship between the shape of Cartagena’s root and free proliferating line. J. Sichuan Univ. (Nat. Sci. Ed.) 2018, 55, 1121–1126. [Google Scholar]
- Chen, G.; Zhang, Q.; Tang, S.; Cai, H.; Chen, F.; Liu, G. Physiological changes and cold resistance evaluation of different Dendrobenthamia plants under low temperature stress. Chin. Wild Plant Resour. 2025, 44, 43–49. [Google Scholar]
- Wei, X.; Yu, X.; Mo, S.; Lu, S.; Luo, X.; Yi, S.; Liao, Y.; Zhang, J.; Yang, G. The influences of exogenous 2,4-epibrassinolide on physiological characteristics of Dendrobium hybrid seedlings under low-temperature stress. Chin. J. Trop. Crops 2025, 46, 1405–1415. [Google Scholar]
- Liu, N.; Lin, S.; Shen, Y. The response of leaf osmolyte content to low temperature in autumn, and its relationship with chilling injury in centipede grass. Acta Pratacult. Sin. 2019, 28, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Zhong, P.; Liu, J.; Tang, Z.; Gao, Y.; Yu, H.; Guo, W. Effect of low-temperature stress and gibberellin on seed germination and seedling physiological responses in peanut. Acta Agron. Sin. 2019, 45, 118–130. [Google Scholar] [CrossRef]
- Han, S.; Li, J.; Huang, D.; Zhang, X. Effect of exogenous ABA on the cold resistance of Ligustrum lucidum under the condition of natural decreasing in temperature. For. Ecol. Sci. 2025, 40, 229–238. [Google Scholar]
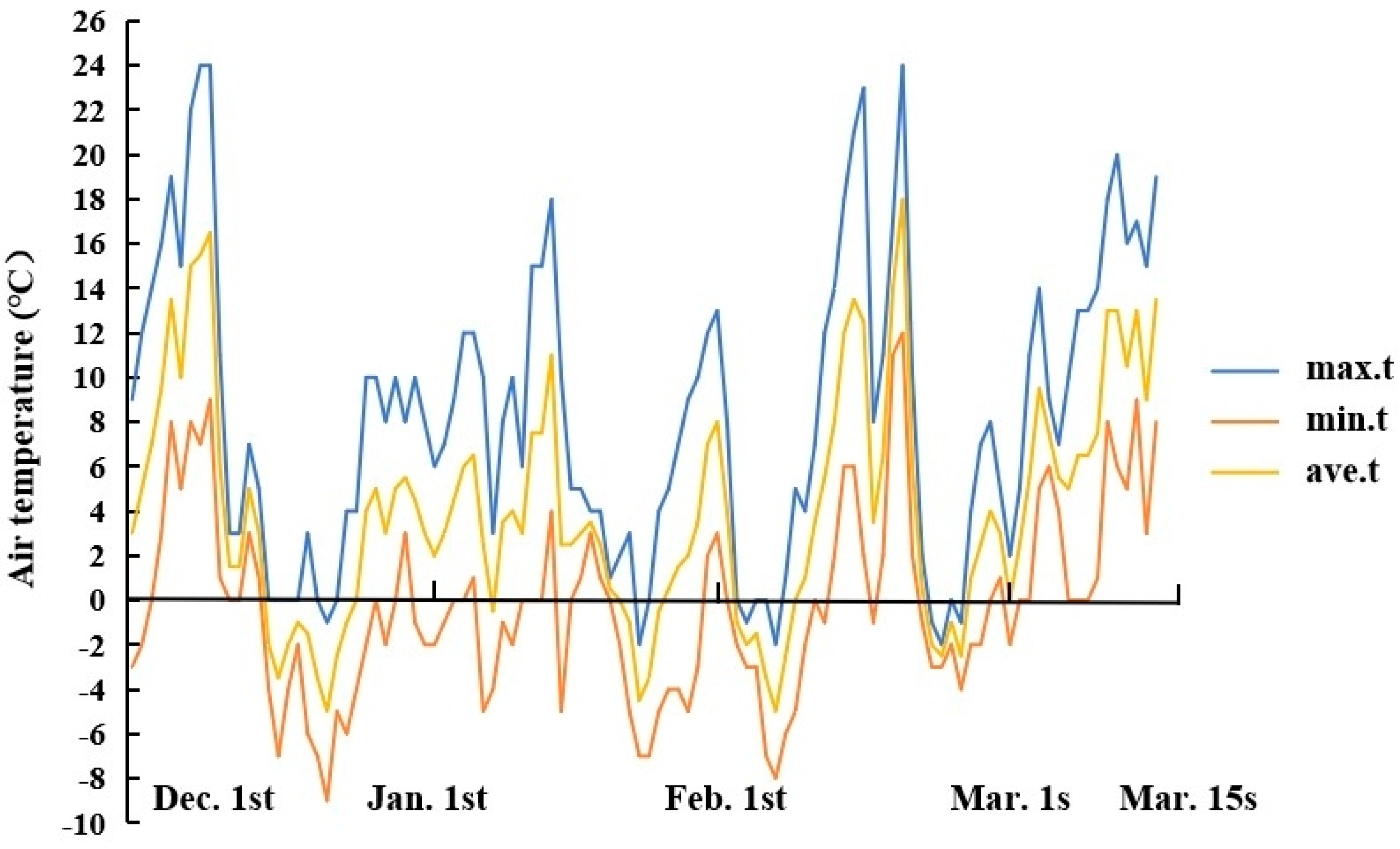



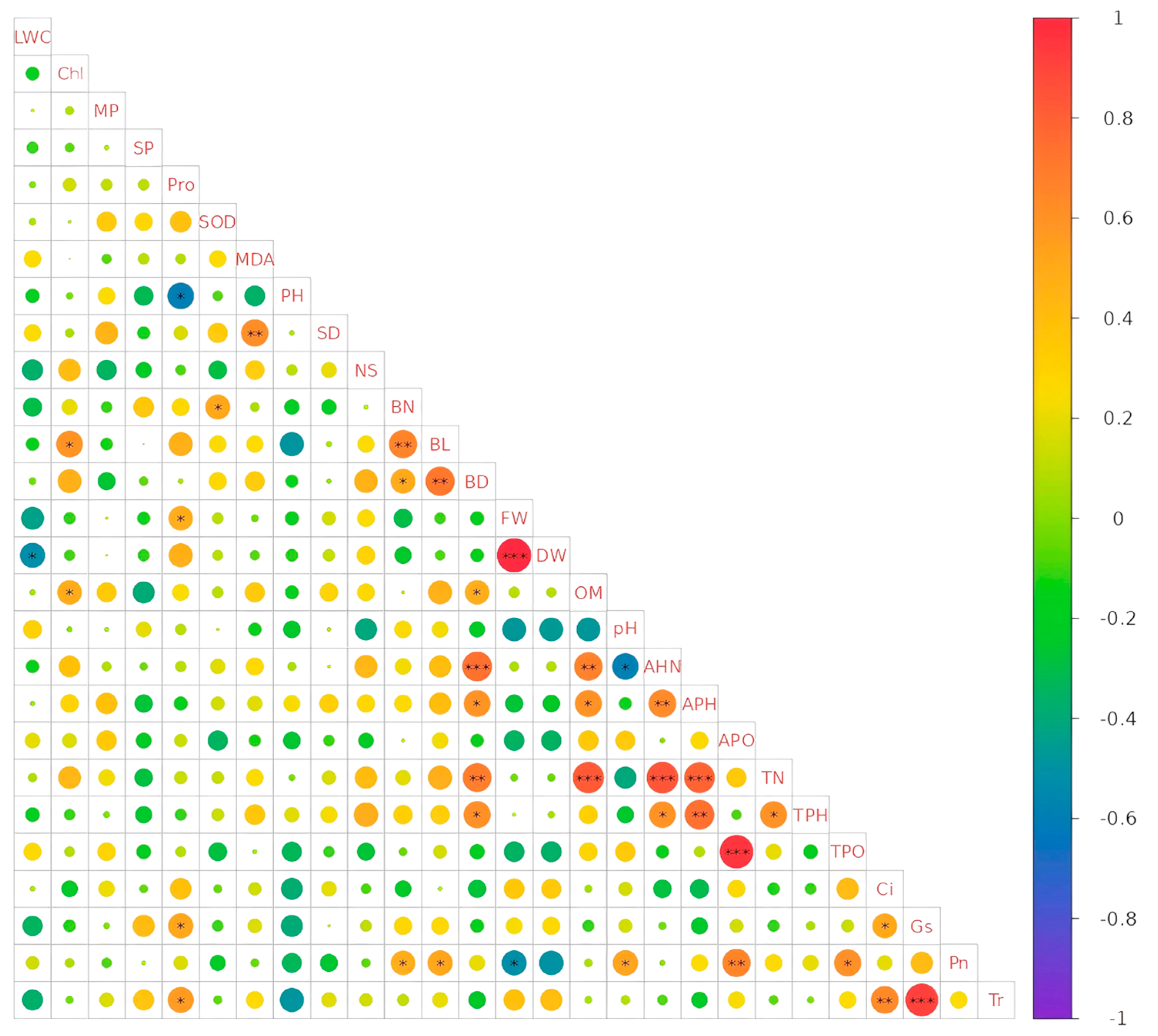
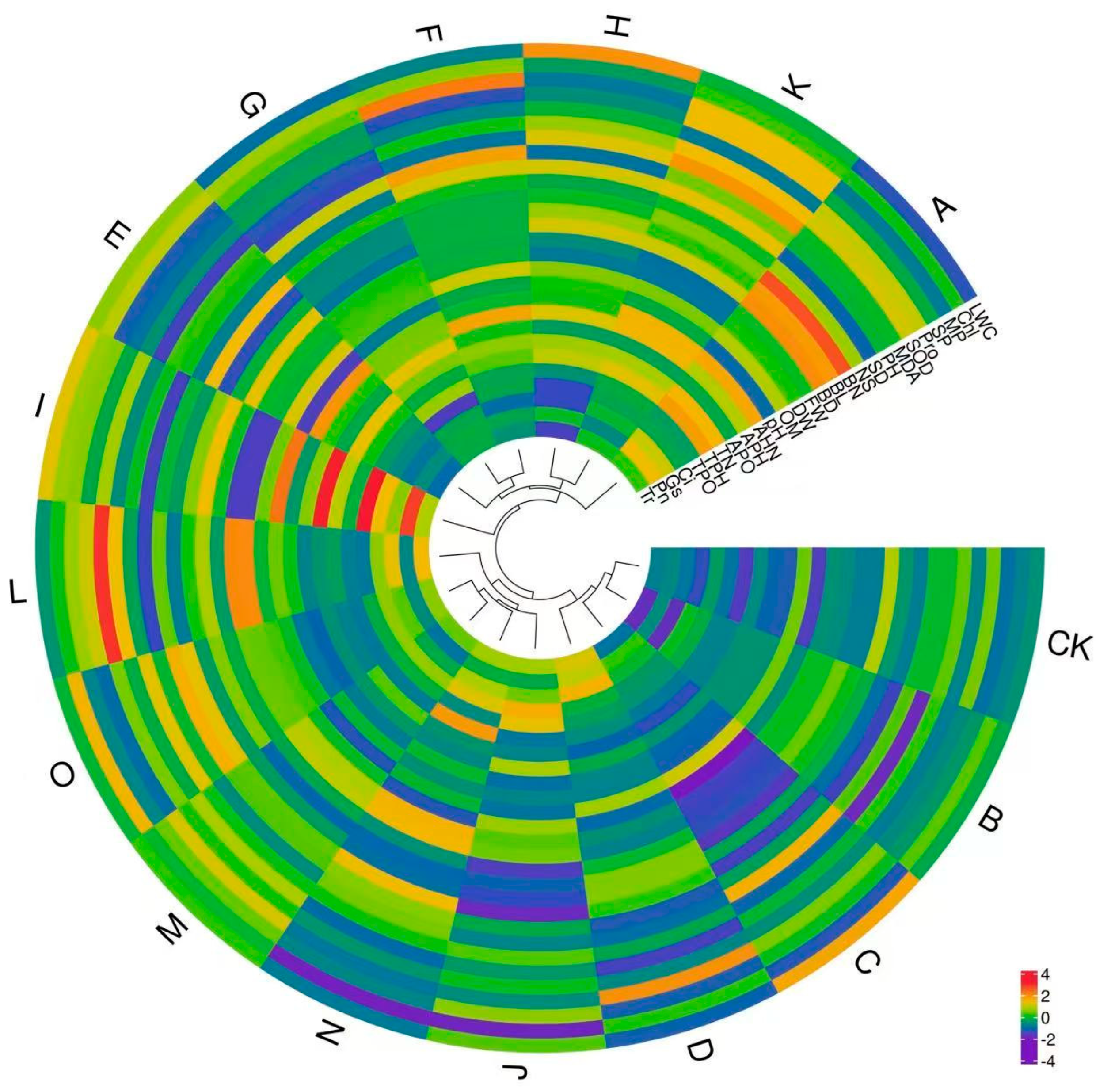
| Treatment | Substrate Species | Volume Ratio |
|---|---|---|
| CK | Garden soil/coir | 9:1 |
| A | Garden soil/coir/municipal sludge | 7:1:2 |
| B | Garden soil/coir/cyanobacterial mud | 7:1:2 |
| C | Garden soil/coir/edible fungus residue | 7:1:2 |
| D | Garden soil/coir/vermicompost | 7:1:2 |
| E | Garden soil/coir/pig manure | 7:1:2 |
| F | Garden soil/coir/cow manure | 7:1:2 |
| G | Garden soil/coir/chicken manure | 7:1:2 |
| H | Garden soil/coir/duck manure | 7:1:2 |
| I | Garden soil/coir/tobacco residue | 7:1:2 |
| J | Garden soil/coir/wheat straw | 7:1:2 |
| K | Garden soil/coir/cassava residue | 7:1:2 |
| L | Garden soil/coir/pear residue | 7:1:2 |
| M | Garden soil/coir/distiller’s grains | 7:1:2 |
| N | Garden soil/coir/sawdust | 7:1:2 |
| O | Garden soil/coir/pond sediment | 7:1:2 |
| Treatment | Organic Matter (g/kg) | pH | Alkali-Hydrolyzed N (mg/kg) | Available P (mg/kg) | Available K (mg/kg) | Total N (g/kg) | Total P (g/kg) | Total K (g/kg) |
|---|---|---|---|---|---|---|---|---|
| CK | 16.24 j | 8.35 bc | 40.47 n | 16.20 o | 209.29 l | 0.59 h | 0.42 n | 12.08 de |
| A | 32.62 bc | 7.52 efg | 438.12 b | 207.73 d | 358.38 e | 2.59 a | 4.59 b | 12.14 de |
| B | 22.71 g | 8.34 bc | 129.31 i | 72.38 i | 247.22 k | 1.16 e | 0.71 j | 12.63 d |
| C | 13.49 k | 8.648 c | 48.49 m | 21.40 n | 264.39 j | 0.77 fg | 0.54 l | 11.86 def |
| D | 19.89 h | 8.47 bc | 103.72 k | 56.37 j | 360.45 e | 0.9 f | 0.89 i | 12.13 de |
| E | 34.48 a | 7.24 g | 466.84 a | 150.50 g | 359.02 e | 2.48 a | 2.13 f | 12.07 de |
| F | 33.81 ab | 7.92 cdef | 208.53 f | 290.60 a | 528.46 d | 2.06 bc | 2.47 e | 12.28 de |
| G | 29.318 e | 7.63 defg | 278.23 d | 179.38 f | 981.55 b | 2.01 bc | 1.49 h | 14.62 c |
| H | 30.92 b | 8.22 bcd | 229.74 e | 240.39 c | 303.43 g | 2.13 b | 3.27 d | 11.82 def |
| I | 30.19 de | 9.27 a | 140.59 h | 184.62 e | 1705.55 a | 2.21 b | 1.59 g | 19.90 a |
| J | 31.44 cd | 7.59 cde | 107.30 j | 30.11 m | 794.32 c | 1.18 de | 0.47 m | 15.91 b |
| K | 30.66 d | 7.95 defg | 415.51 c | 268.57 b | 323.01 f | 2.15 b | 3.72 c | 12.57 de |
| L | 26.42 f | 8.33 bc | 228.64 e | 53.61 k | 266.62 i | 1.38 d | 0.60 k | 11.15 f |
| M | 27.36 f | 7.38 fg | 201.63 g | 46.35 l | 284.81 h | 1.90 c | 0.59 k | 12.74 d |
| N | 18.33 i | 8.04 bcde | 129.19 i | 123.65 h | 207.57 l | 1.30 de | 4.64 a | 11.65 ef |
| O | 29.14 e | 8.10 bcde | 87.49 l | 15.79 o | 246.49 k | 1.16 e | 0.54 l | 12.57 de |
| Treatment | Plant Height (cm) | Stem Diameter (mm) | Branch Number | Branch Length (cm) | Branch Diameter (mm) | No. New Shoots |
|---|---|---|---|---|---|---|
| CK | 122.33 de | 17.28 fg | 48 b | 7.73 g | 3.39 j | 3.57 ef |
| A | 122.17 de | 16.38 gh | 64 a | 15.70 a | 4.53 a | 4.94 b |
| B | 123.67 cd | 15.36 h | 38 de | 10.53 e | 3.93 d | 3.06 f |
| C | 128.00 ab | 18.41 ef | 37 ef | 5.13 i | 3.17 k | 2.41 g |
| D | 118.67 fg | 16.15 ef | 45 b | 11.90 d | 3.72 ef | 4.46 bc |
| E | 122.83 d | 17.29 fg | 28 h | 9.87 f | 4.24 b | 5.72 a |
| F | 128.67 a | 22.60 cd | 38 de | 9.67 f | 3.66 fg | 4.24 cd |
| G | 126.00 bc | 17.28 fg | 34 f | 8.33 g | 3.37 j | 4.45 bc |
| H | 118.83 fg | 22.64 ab | 41 cd | 12.27 cd | 4.18 bc | 3.80 de |
| I | 116.67 g | 19.34 de | 46 b | 14.33 b | 3.76 e | 3.65 e |
| J | 119.67 f | 18.69 ef | 27 h | 6.67 h | 3.08 i | 2.09 g |
| K | 124.17 cd | 23.09 a | 45 b | 11.07 e | 4.16 c | 4.22 cd |
| L | 117.00 g | 20.81 cd | 42 c | 12.60 c | 3.54 hi | 3.22 ef |
| M | 120.00 ef | 20.52 cd | 36 de | 6.93 h | 3.59 gh | 4.61 bc |
| N | 124.17 cd | 21.24 bc | 31 g | 6.93 h | 3.49 i | 5.70 a |
| O | 122.17 de | 23.80 a | 36 de | 12.00 cd | 3.62 g | 5.54 a |
| Treatment | Membrane Permeability (%) | Soluble Protein (mg/g) | Proline (mg/g) | Superoxide Dismutase (U/g) | Malondialdehyde (µmol/g) | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|---|---|---|
| CK | 46.33 j | 82.57 e | 3.75 kl | 219.70 d | 0.02 cde | 0.57 fg | 0.27 de |
| A | 47.93 i | 67.40 f | 19.69 b | 254.74 b | 0.02 cde | 0.68 de | 0.34 bc |
| B | 52.16 g | 59.33 i | 3.24 l | 212.01 e | 0.01 h | 0.68 de | 0.33 cd |
| C | 59.20 f | 83.25 e | 5.15 ij | 194.49 f | 0.01 fg | 0.44 hi | 0.20 fg |
| D | 43.78 k | 110.75 a | 4.361 jk | 118.34 k | 0.02 de | 0.55 fg | 0.27 de |
| E | 43.62 k | 53.30 i | 5.33 ij | 118.34 k | 0.02 c | 0.67 de | 0.31 cd |
| F | 83.78 a | 45.85 k | 3.96 kl | 206.70 e | 0.01 f | 0.64 ef | 0.31 cd |
| G | 62.72 e | 63.15 h | 7.24 h | 134.00 j | 0.01 gh | 0.70 de | 0.34 bc |
| H | 47.70 i | 60.22 i | 9.61 g | 231.64 c | 0.03 b | 0.57 fg | 0.25 e |
| I | 65.11 d | 65.93 g | 17.75 c | 164.78 h | 0.02 cd | 0.42 i | 0.19 g |
| J | 65.32 d | 60.08 i | 11.41 f | 171.94 h | 0.02 c | 0.75 bc | 0.35 bc |
| K | 74.29 b | 100.30 b | 2.92 l | 268.52 a | 0.03 a | 0.52 gh | 0.25 ef |
| L | 66.50 c | 92.13 d | 37.40 a | 266.41 a | 0.02 e | 0.99 a | 0.48 a |
| M | 63.50 e | 94.50 c | 12.49 e | 237.31 c | 0.02 c | 0.82 bc | 0.38 b |
| N | 49.37 h | 55.08 j | 5.83 i | 144.08 j | 0.02 c | 0.90 b | 0.44 a |
| O | 49.54 h | 52.32 k | 15.73 d | 184.51 g | 0.03 b | 0.75 cd | 0.36 bc |
| Treatment | LWC (%) | Chl (mg/g) | Ci (mg/m3) | Gs (mmol∙m−2∙s−1) | Pn (µmol∙m−2∙s−1) | Tr (mmol∙m−2∙s−1) |
|---|---|---|---|---|---|---|
| CK | 51.72 abcd | 4.49 h | 343.63 i | 41.57 g | 1.52 hi | 0.90 h |
| A | 50.50 fg | 6.66 ef | 391.39 fg | 81.24 ab | 7.33 b | 1.28 de |
| B | 52.17 fg | 7.20 d | 409.85 e | 23.44 h | 1.71 ghi | 0.54 i |
| C | 55.46 abc | 3.261 i | 384.53 g | 59.50 f | 4.29 d | 0.85 h |
| D | 50.72 bcde | 6.85 de | 398.32 f | 91.13 a | 6.02 c | 1.58 a |
| E | 53.68 efg | 8.43 b | 375.81 h | 41.11 g | 1.67 ghi | 0.81 h |
| F | 51.42 defg | 7.67 c | 391.66 fg | 38.60 g | 2.43 fgh | 0.97 gh |
| G | 51.01 a | 7.95 c | 333.20 j | 52.94 f | 2.77 ef | 1.09 fg |
| H | 55.82 a | 5.25 g | 340.56 ij | 30.28 h | 3.64 de | 0.63 i |
| I | 54.69 efg | 7.88 c | 469.52 b | 72.77 bcd | 11.30 a | 1.46 bc |
| J | 53.26 bcde | 1.80 j | 477.59 a | 69.51 de | 2.54 gf | 1.45 bcd |
| K | 52.44 fg | 6.98 de | 390.61 fg | 59.17 f | 1.55 hi | 1.22 ef |
| L | 51.79 cdef | 6.58 ef | 435.23 d | 86.23 a | 1.47 hi | 1.67 a |
| M | 53.14 g | 6.18 f | 435.61 d | 53.66 f | 0.76 i | 1.33 cde |
| N | 51.25 g | 1.68 j | 460.54 c | 76.23 cd | 2.79 ef | 1.44 bcd |
| O | 52.56 ab | 9.78 a | 434.93 d | 67.75 e | 1.60 ghi | 1.39 cd |
| Treatment | Plant Height | Stem Diameter | Branch Length | Branch Diameter | Branch Number | No. New Shoots | Composite Index | Ranking |
|---|---|---|---|---|---|---|---|---|
| CK | 0.49 | 0.28 | 0.25 | 0.07 | 0.57 | 0.39 | 0.34 | 10 |
| A | 0.48 | 0.20 | 0.92 | 0.32 | 0.97 | 0.74 | 0.60 | 1 |
| B | 0.58 | 0.11 | 0.49 | 0.19 | 0.33 | 0.26 | 0.33 | 11 |
| C | 0.87 | 0.39 | 0.03 | 0.03 | 0.30 | 0.10 | 0.28 | 12 |
| D | 0.24 | 0.18 | 0.60 | 0.15 | 0.51 | 0.62 | 0.38 | 7 |
| E | 0.52 | 0.29 | 0.43 | 0.26 | 0.08 | 0.93 | 0.42 | 5 |
| F | 0.91 | 0.77 | 0.41 | 0.13 | 0.33 | 0.56 | 0.52 | 3 |
| G | 0.73 | 0.29 | 0.30 | 0.07 | 0.23 | 0.61 | 0.37 | 8 |
| H | 0.26 | 0.77 | 0.63 | 0.25 | 0.39 | 0.45 | 0.46 | 4 |
| I | 0.11 | 0.47 | 0.81 | 0.16 | 0.52 | 0.41 | 0.41 | 6 |
| J | 0.31 | 0.41 | 0.16 | 0.01 | 0.06 | 0.02 | 0.16 | 13 |
| K | 0.61 | 0.82 | 0.53 | 0.24 | 0.51 | 0.56 | 0.54 | 2 |
| L | 0.13 | 0.61 | 0.66 | 0.11 | 0.43 | 0.31 | 0.37 | 8 |
| M | 0.33 | 0.58 | 0.18 | 0.12 | 0.28 | 0.65 | 0.36 | 9 |
| N | 0.61 | 0.65 | 0.18 | 0.10 | 0.16 | 0.93 | 0.44 | 6 |
| O | 0.48 | 0.88 | 0.61 | 0.12 | 0.28 | 0.88 | 0.54 | 2 |
| Treatment | LWC | Chl | Ci | Gs | Pn | Tr | MP | SP | Pro | SOD | MDA | FW | DW | Composite Index | Ranking |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 0.04 | 0.35 | 0.09 | 0.28 | 0.08 | 0.37 | 0.92 | 0.33 | 0.04 | 0.67 | 0.50 | 0.30 | 0.33 | 0.33 | 11 |
| A | 0.34 | 0.61 | 0.40 | 0.83 | 0.58 | 0.65 | 0.88 | 0.20 | 0.49 | 0.88 | 0.48 | 0.49 | 0.53 | 0.57 | 2 |
| B | 0.05 | 0.68 | 0.53 | 0.03 | 0.09 | 0.10 | 0.78 | 0.12 | 0.03 | 0.62 | 0.96 | 0.49 | 0.49 | 0.38 | 7 |
| C | 0.10 | 0.20 | 0.36 | 0.53 | 0.32 | 0.33 | 0.61 | 0.34 | 0.08 | 0.51 | 0.76 | 0.10 | 0.09 | 0.33 | 11 |
| D | 0.02 | 0.64 | 0.45 | 0.97 | 0.47 | 0.87 | 0.98 | 0.59 | 0.06 | 0.04 | 0.58 | 0.28 | 0.32 | 0.48 | 4 |
| E | 0.07 | 0.83 | 0.30 | 0.28 | 0.09 | 0.30 | 0.99 | 0.07 | 0.08 | 0.04 | 0.42 | 0.47 | 0.45 | 0.34 | 10 |
| F | 0.03 | 0.74 | 0.41 | 0.24 | 0.15 | 0.42 | 0.01 | 0.00 | 0.05 | 0.59 | 0.72 | 0.42 | 0.44 | 0.32 | 12 |
| G | 0.03 | 0.77 | 0.02 | 0.44 | 0.18 | 0.50 | 0.52 | 0.16 | 0.14 | 0.14 | 0.86 | 0.52 | 0.54 | 0.37 | 8 |
| H | 0.11 | 0.44 | 0.07 | 0.13 | 0.26 | 0.17 | 0.89 | 0.13 | 0.20 | 0.74 | 0.19 | 0.31 | 0.26 | 0.30 | 13 |
| I | 0.09 | 0.76 | 0.92 | 0.71 | 0.93 | 0.78 | 0.46 | 0.18 | 0.43 | 0.33 | 0.47 | 0.07 | 0.08 | 0.48 | 4 |
| J | 0.07 | 0.02 | 0.97 | 0.67 | 0.16 | 0.77 | 0.46 | 0.13 | 0.26 | 0.37 | 0.40 | 0.60 | 0.56 | 0.42 | 5 |
| K | 0.05 | 0.65 | 0.40 | 0.53 | 0.08 | 0.60 | 0.24 | 0.49 | 0.02 | 0.97 | 0.02 | 0.22 | 0.24 | 0.35 | 9 |
| L | 0.04 | 0.60 | 0.69 | 0.90 | 0.07 | 0.94 | 0.43 | 0.42 | 0.98 | 0.95 | 0.59 | 0.98 | 0.95 | 0.66 | 1 |
| M | 0.06 | 0.55 | 0.70 | 0.45 | 0.01 | 0.68 | 0.50 | 0.44 | 0.29 | 0.77 | 0.45 | 0.71 | 0.67 | 0.48 | 4 |
| N | 0.03 | 0.99 | 0.86 | 0.76 | 0.19 | 0.76 | 0.85 | 0.09 | 0.10 | 0.20 | 0.44 | 0.84 | 0.84 | 0.53 | 3 |
| O | 0.05 | 0.01 | 0.69 | 0.64 | 0.08 | 0.73 | 0.84 | 0.06 | 0.38 | 0.45 | 0.18 | 0.59 | 0.58 | 0.41 | 6 |
| Treatment | Morphology | Physiology | Composite Index | Ranking |
|---|---|---|---|---|
| CK | 0.34 | 0.33 | 0.34 | 11 |
| A | 0.60 | 0.57 | 0.59 | 1 |
| B | 0.33 | 0.38 | 0.36 | 10 |
| C | 0.28 | 0.33 | 0.31 | 12 |
| D | 0.38 | 0.48 | 0.43 | 6 |
| E | 0.42 | 0.34 | 0.38 | 8 |
| F | 0.52 | 0.32 | 0.42 | 7 |
| G | 0.37 | 0.37 | 0.37 | 9 |
| H | 0.46 | 0.30 | 0.38 | 8 |
| I | 0.41 | 0.48 | 0.45 | 5 |
| J | 0.16 | 0.42 | 0.29 | 13 |
| K | 0.54 | 0.35 | 0.45 | 5 |
| L | 0.37 | 0.66 | 0.52 | 2 |
| M | 0.36 | 0.48 | 0.42 | 7 |
| N | 0.44 | 0.53 | 0.49 | 3 |
| O | 0.54 | 0.41 | 0.48 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, J.; Liu, A.; Zhang, Y.; Zhao, K. Effects of Different Agricultural Wastes on the Growth of Photinia × fraseri Under Natural Low-Temperature Conditions. Horticulturae 2025, 11, 1055. https://doi.org/10.3390/horticulturae11091055
Li X, Li J, Liu A, Zhang Y, Zhao K. Effects of Different Agricultural Wastes on the Growth of Photinia × fraseri Under Natural Low-Temperature Conditions. Horticulturae. 2025; 11(9):1055. https://doi.org/10.3390/horticulturae11091055
Chicago/Turabian StyleLi, Xiaoye, Jie Li, Airong Liu, Yuanbing Zhang, and Kunkun Zhao. 2025. "Effects of Different Agricultural Wastes on the Growth of Photinia × fraseri Under Natural Low-Temperature Conditions" Horticulturae 11, no. 9: 1055. https://doi.org/10.3390/horticulturae11091055
APA StyleLi, X., Li, J., Liu, A., Zhang, Y., & Zhao, K. (2025). Effects of Different Agricultural Wastes on the Growth of Photinia × fraseri Under Natural Low-Temperature Conditions. Horticulturae, 11(9), 1055. https://doi.org/10.3390/horticulturae11091055






