Physiological Responses and Determination of Harvest Maturity in ‘Daehong’ Peach According to Days After Full Bloom
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. External Quality
2.3. Internal Quality
2.4. Analysis of Reducing Sugars, Non-Reducing Sugars, and Organic Acid
2.5. Respiration Rate and Ethylene Production Rate
2.6. Hunter a* Analysis of Exocarp and Mesocarp with Anthocyanin Analysis
2.7. Accumulated Temperature
2.8. Statistical Analysis
3. Results and Discussions
3.1. External Quality
3.2. Internal Quality
3.3. Analyses of Reducing Sugars, Non-Reducing Sugars, and Organic Acid
3.4. Respiration Rate and Ethylene Production Rate
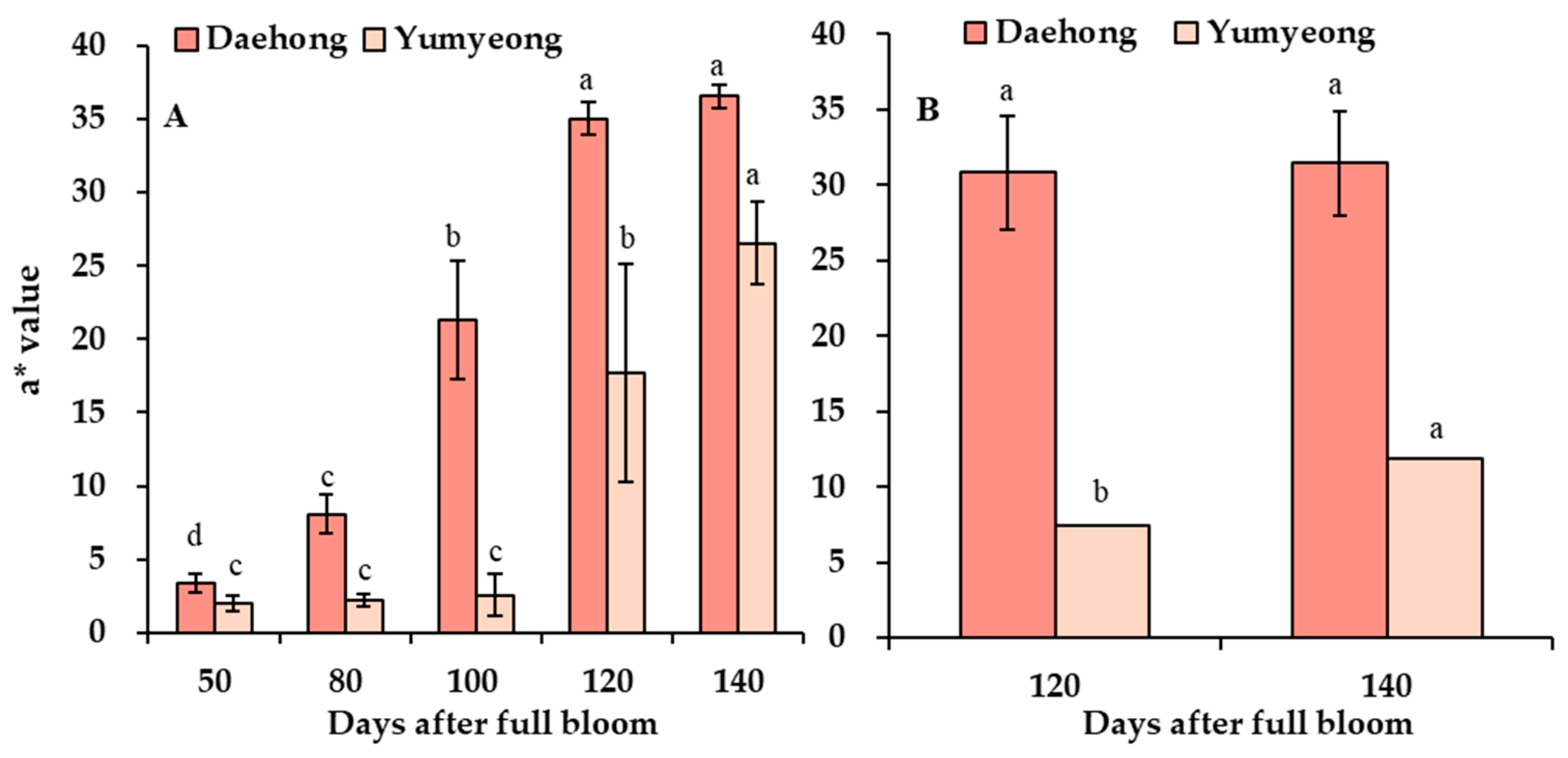
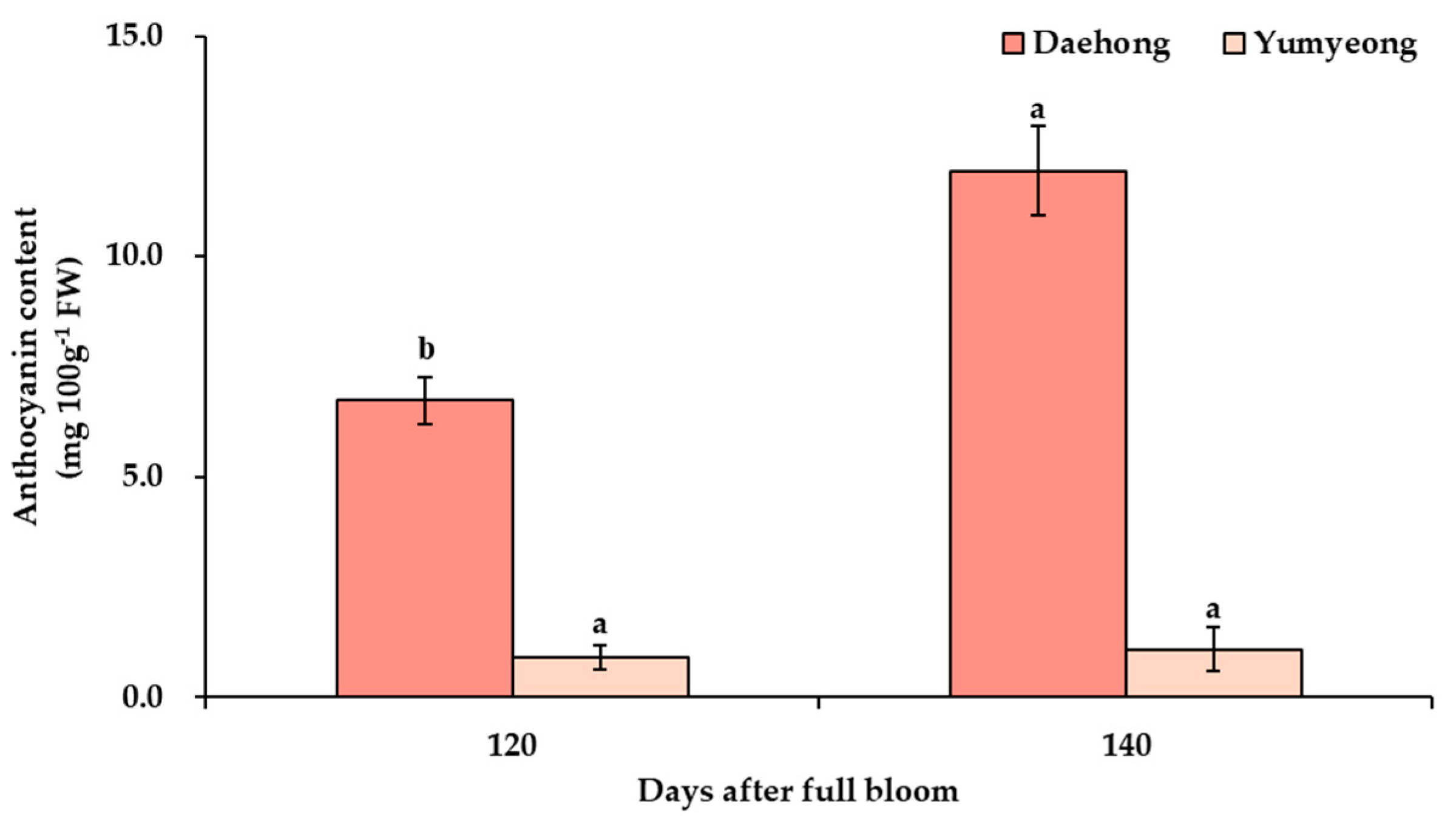
3.5. Analysis of Exocarp and Mesocarp Hunter a* Values with Anthocyanin Content Analysis
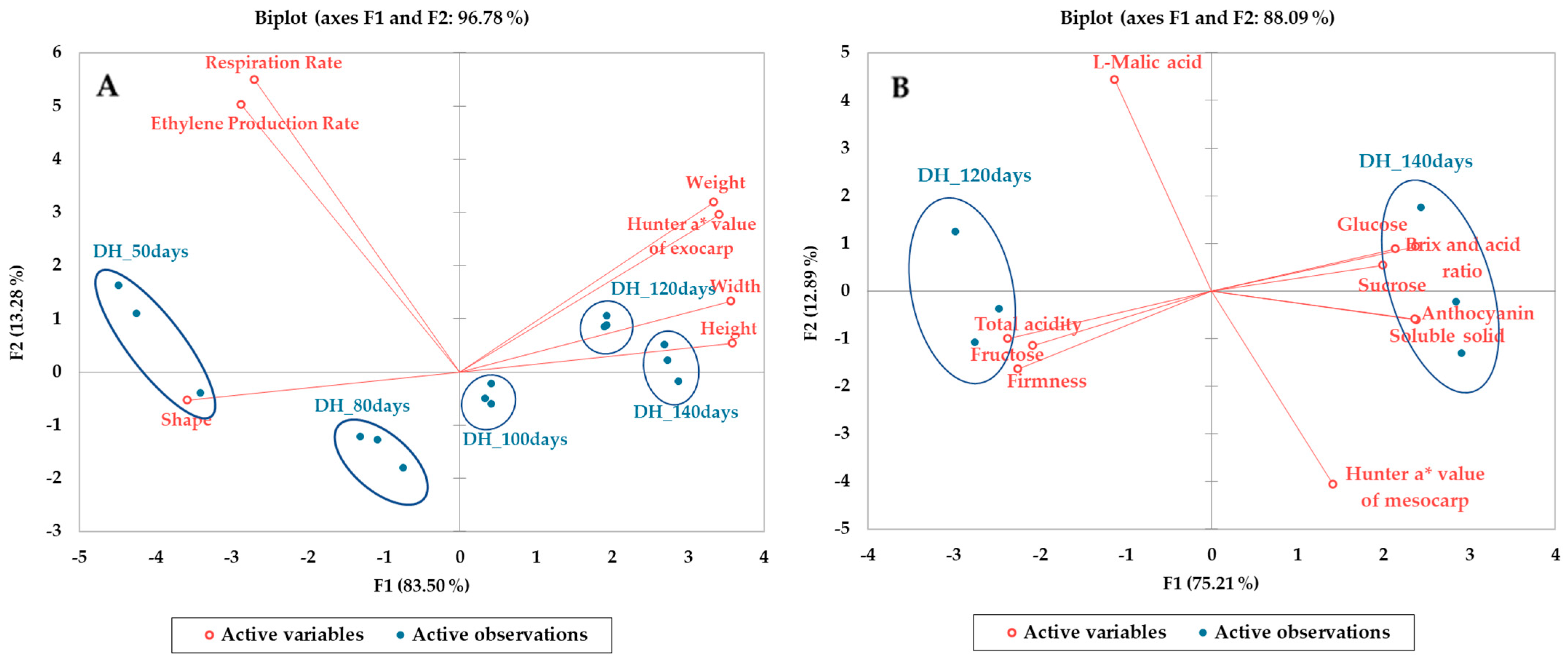
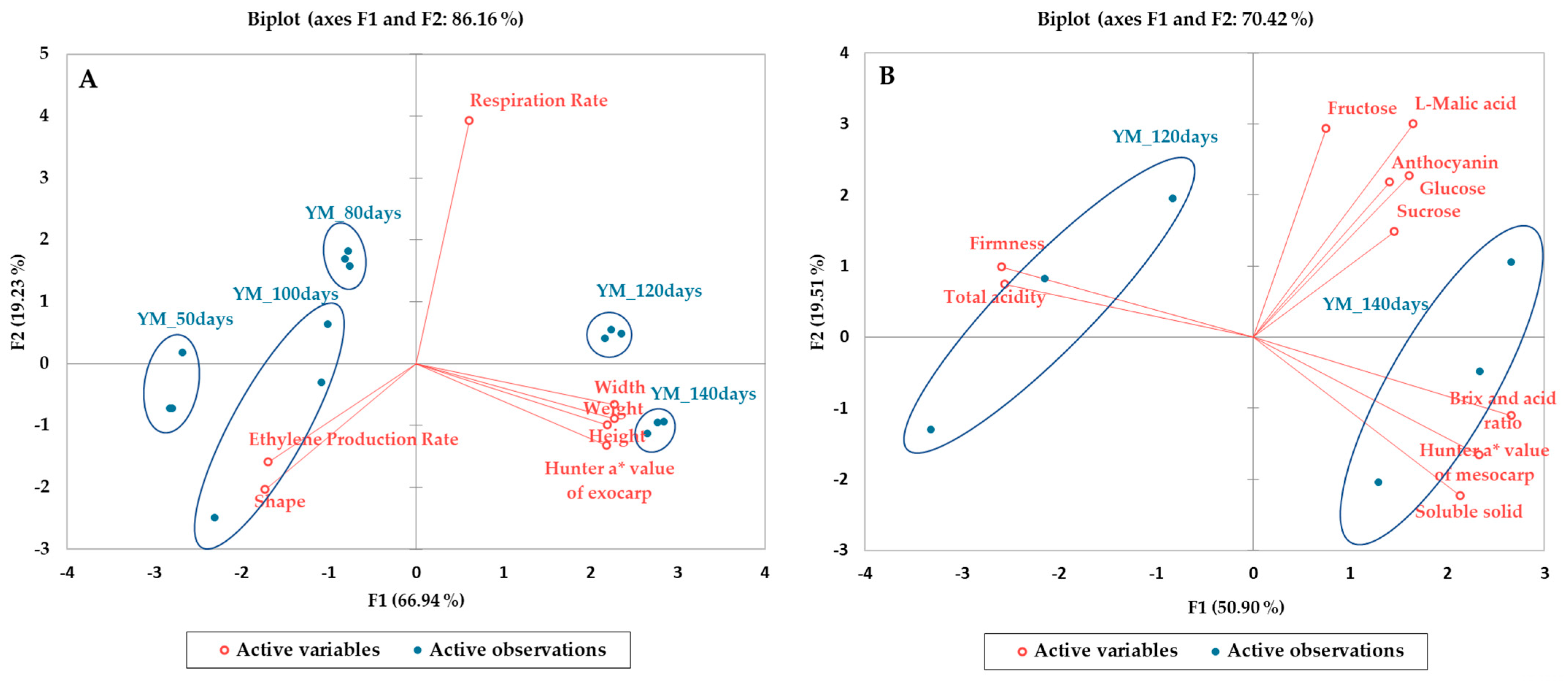
3.6. Principal Component Analysis
3.7. Accumulated Temperature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korean Statistical Information Serveice (KOSIS). Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1ET0014&conn_path=I2 (accessed on 29 June 2023).
- Roh, Y.H.; Lee, J.H.; Kwon, Y.B.; Choi, I.L.; Jeong, H.N.; Kang, H.M. Effect of 1-Methylcyclopropene (1-MCP) Treatment and MAP on Quality Changes of Peach ‘Daehong’ during Cold Storage. J. Bio-Environ. Control 2023, 32 (Suppl. S4), 267–277. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, H.N.; Lee, J.C.; Lee, K.O.; Lee, J.Y.; Choi, J.S.; Kwon, H.J.; Won, J.H.; Choi, J.Y. Effects of physical properties of bagging papers on coloration and quality of ‘Daehong’ peach cultivar during rainy season. Hortic. Sci. Technol. 2022, 40 (Suppl. S2), 119–120. [Google Scholar]
- Kim, Y.B.; Noh, H.S.; Shin, D.H.; Ahn, Y.J.; Jeong, J.S. Use the results of market tests of new varieties of peach ‘Daehong’ as marketing materials. Hortic. Sci. Technol. 2019, 37 (Suppl. S2), 119–120. [Google Scholar]
- Cho, M.D.; Park, H.S.; Kim, Y.K. Changes of Fruit Structure and Sugar Contents during the Fruit Growth and Development in ‘Yumyeong’ Peach [Prunus persica (L.) Batsch]. Hortic. Sci. Technol. 2000, 18 (Suppl. S3), 353–359. [Google Scholar]
- Anthony, B.M.; Sterle, D.G.; Minas, I.S. Robust non-destructive individual cultivar models allow for accurate peach fruit quality and maturity assessment following customization in phenotypically similar cultivars. Postharvest Biol. Technol. 2023, 195, 112148. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.; Xu, F.; Wang, H.; Chen, M.; Shao, X. Changes in the chlorophyll absorbance index (I AD) are related to peach fruit maturity. N. Z. J. Crop Hortic. Sci. 2020, 48, 34–46. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Anthony, B.M.; Minas, I.S. Redefining the impact of preharvest factors on peach fruit quality development and metabolism: A review. Sci. Hortic. 2022, 297, 110919. [Google Scholar] [CrossRef]
- Verma, P.; Singh, J.; Sharma, S.; Thakur, H. Phenological growth stages and growing degree days of peach [Prunus persica (L.) Batsch] in sub-temperate climatic zone of North-Western Himalayan region using BBCH scale. Ann. Appl. Biol. 2022, 182, 284–294. [Google Scholar] [CrossRef]
- Choi, I.L.; Lee, J.H.; Choi, D.H.; Wang, L.X.; Kang, H.M. Evaluation of the Storage Characteristics in Maintaining the Overall Quality of Whole and Fresh-Cut Romaine Lettuce during MA Storage. Horticulturae 2021, 7, 461. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, L.; Wang, Q.; Wang, R.; Li, C.; Qiao, Y.; Liu, H. Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review. Plants 2025, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qian, M.; Song, C.; Li, J.; Zhao, C.; Li, G.; Wang, A.; Han, M. Down-Regulation of PpBGAL10 and PpBGAL16 Delays Fruit Softening in Peach by Reducing Polygalacturonase and Pectin Methylesterase Activity. Front. Plant Sci. 2018, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Deng, L.; Wang, X.; Wang, Y.; Pan, L.; Liu, H.; Niu, L.; Lu, Z.; Cui, G.; Zeng, W.; et al. Expression patterns of genes involved in sugar metabolism and accumulation during peach fruit development and ripening. Sci. Hortic. 2019, 257, 108633. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.J.; Ogutu, C.; Ntini, C.; Zhang, X.; Han, Y. Assessment of organic acid accumulation and its related genes in peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Korea Meteorological Administration (KMA). 2022. Available online: http://www.weather.go.kr (accessed on 17 April 2025).
- Pinzón Sandoval, E.H.; Balaguera López, H.E.; Becerra González, M.E. Phenological and physicochemical changes during fruit development in two peach cultivars in the high tropics. Rev. UDCA Actual. Divulg. Cient. 2022, 25, e1942. [Google Scholar] [CrossRef]
- Cirilli, M.; Rossini, L. Many candidates for a single chair: A critical review of the genetic determinant of flat fruit shape trait in peach (Prunus persica L. Batsch). Tree Genet. Genomes 2021, 17, 34. [Google Scholar] [CrossRef]
- Martínez-González, M.E.; Balois-Morales, R.; Alia-Tejacal, I.; Cortes-Cruz, M.A.; Palomino-Hermosillo, Y.A.; López-Gúzman, G.G. Poscosecha de frutos: Maduración, ablandamiento y control transcripcional. Rev. Mex. Cienc. Agríc. 2017, 19, 4089–4101. [Google Scholar]
- Africano-Pérez, K.L.; Balaguera-López, H.E.; Almanza-Merchán, P.J.; Cárdenas-Hernández, J.F.; Herrera-Arévalo, A. Caracterizacion poscosecha del fruto de durazno [Prunus persica (L.) Bastch] cv. Dorado producido bajo condiciones de tropico alto. Rev. Colomb. Cienc. Hortic. 2016, 10, 232–240. [Google Scholar] [CrossRef]
- Hayama, H.; Shimada, T.; Fujii, H.; Ito, A.; Kashimura, Y. Ethylene-regulation of fruit softening and softening-related genes in peach. J. Exp. Bot. 2006, 57, 4071–4077. [Google Scholar] [CrossRef]
- Payasi, A.; Mishra, N.N.; Chaves, A.L.S.; Singh, R. Biochemistry of fruit softening: An overview. Physiol. Mol. Biol. Plants 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Xu, G.; Li, C.; Qin, S.; Xiao, W.; Fu, X.; Chen, X.; Li, D. Changes in Sucrose and Sorbitol Metabolism Cause Differences in the Intrinsic Quality of Peach Fruits Cultivated in Field and Greenhouse Environments. Agronomy 2022, 12, 2877. [Google Scholar] [CrossRef]
- Kroger, M.; Meister, K.; Kava, R. Low-calorie sweeteners and other sugar substitutes: A review of the safety issues. Compr. Rev. Food Sci. Food Saf. 2006, 5, 35–47. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. Hortic. Rev. 2018, 45, 371–430. [Google Scholar] [CrossRef]
- Famiani, F.; Baldicchi, A.; Moscatello, S.; Battistelli, A.; Walker, R.P. The contribution of stored malate and citrate to the substrate requirements of metabolism of ripening peach (Prunus persica L. Batsch) flesh is negligible. Implications for the occurrence of phosphoenolpyruvate carboxykinase and gluconeogenesis. Plant Physiol. Biochem. 2020, 151, 313–324. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, L.; Yang, S.; Zhang, S.; Wang, Y. Comparative network analysis reveals the dynamics of organic acid diversity during fruit ripening in peach (Prunus persica L. Batsch). BMC Plant Biol. 2023, 23, 240. [Google Scholar] [CrossRef]
- Kays, S.J.; Paull, E.R. Postharvest Biology; Exon Press: Athens, GA, USA, 2004. [Google Scholar]
- Tatsuki, M.; Nakajima, N.; Fujii, H.; Shimada, T.; Nakano, M.; Hayashi, K.; Hayama, H.; Yoshioka, H.; Nakamura, Y. Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch). J. Exp. Bot. 2013, 64, 1049–1059. [Google Scholar] [CrossRef]
- Pan, L.; Zeng, W.; Niu, L.; Lu, Z.; Liu, H.; Cui, G.; Wang, Z. PpYUC11, a strong candidate gene for the stony hard phenotype in peach (Prunus persica L. Batsch), participates in IAA biosynthesis during fruit ripening. J. Exp. Bot. 2015, 66, 7031–7044. [Google Scholar] [CrossRef]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 2018, 153, 11–23. [Google Scholar] [CrossRef]
- Predieri, S.; Ragazzini, P.; Rondelli, R. Sensory evaluation and peach fruit quality. VI Int. Peach Symp. 2005, 713, 429–434. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Changes in phenolics and antioxidant property of peach fruit during ripening and responses to 1-methylcyclopropene. Postharvest Biol. Technol. 2015, 108, 111–118. [Google Scholar] [CrossRef]
- Cho, J.H.; Yang, U.; Wi, S.G.; Lee, B.R.; Oh, S.W.; Kim, M.S.; Lee, S.H. Potential Effects of Temperature Differences on the Soluble Sugar Content in Pear Fruit during the Growing Seasons of 2018 and 2019. Hortic. Sci. Technol. 2021, 39, 560–571. [Google Scholar] [CrossRef]
- Amaral, M.H.; McConchie, C.; Dickinson, G.; Walsh, K.B. Growing degree day targets for fruit development of australian mango cultivars. Horticulturae 2023, 9, 489. [Google Scholar] [CrossRef]
- Marra, F.P.; DeJong, T.M.; Marini, R.P. Predicting peach maturity with thermal time models. Acta Hortic. 2002, 592, 465–472. [Google Scholar] [CrossRef]
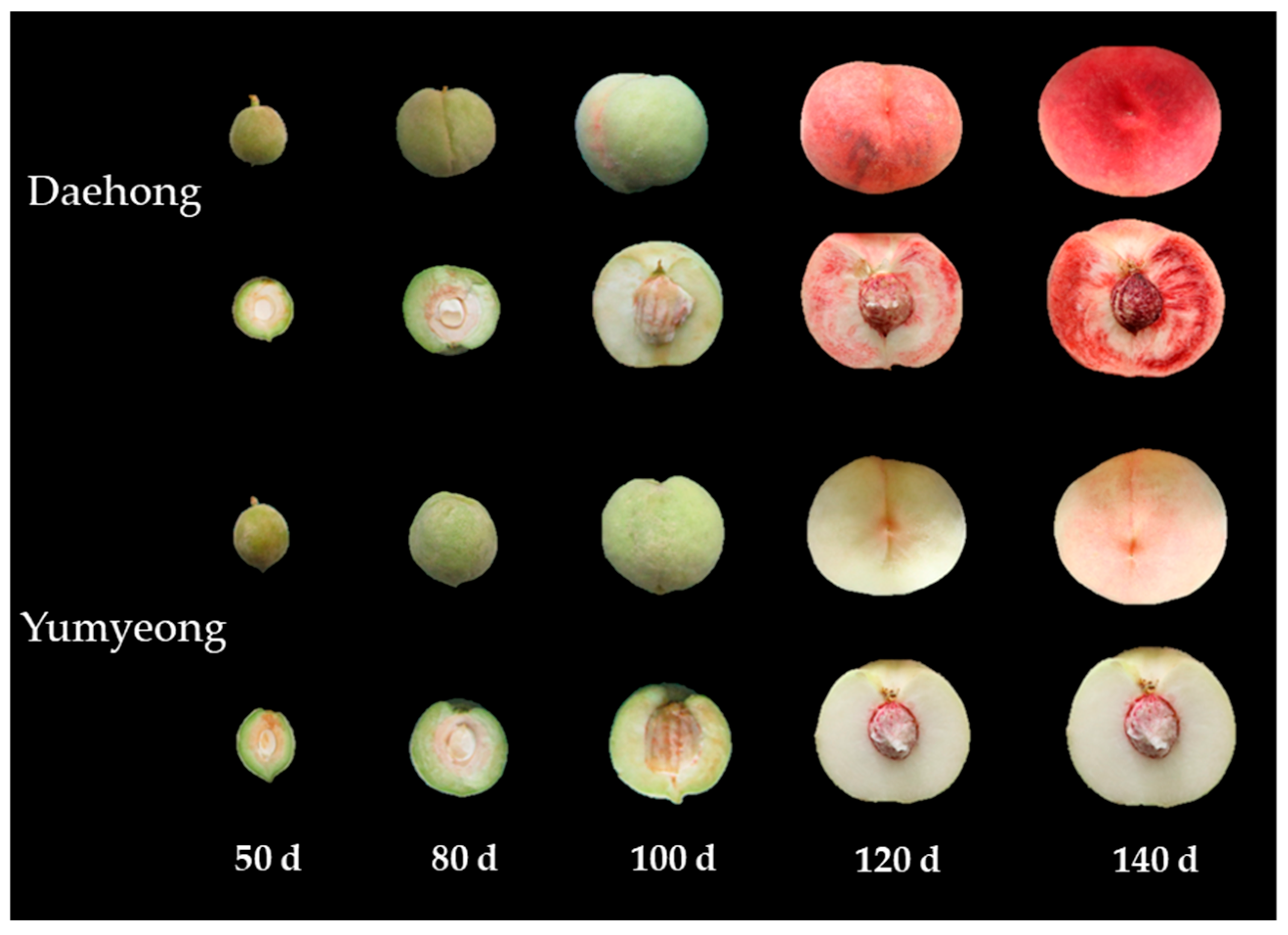
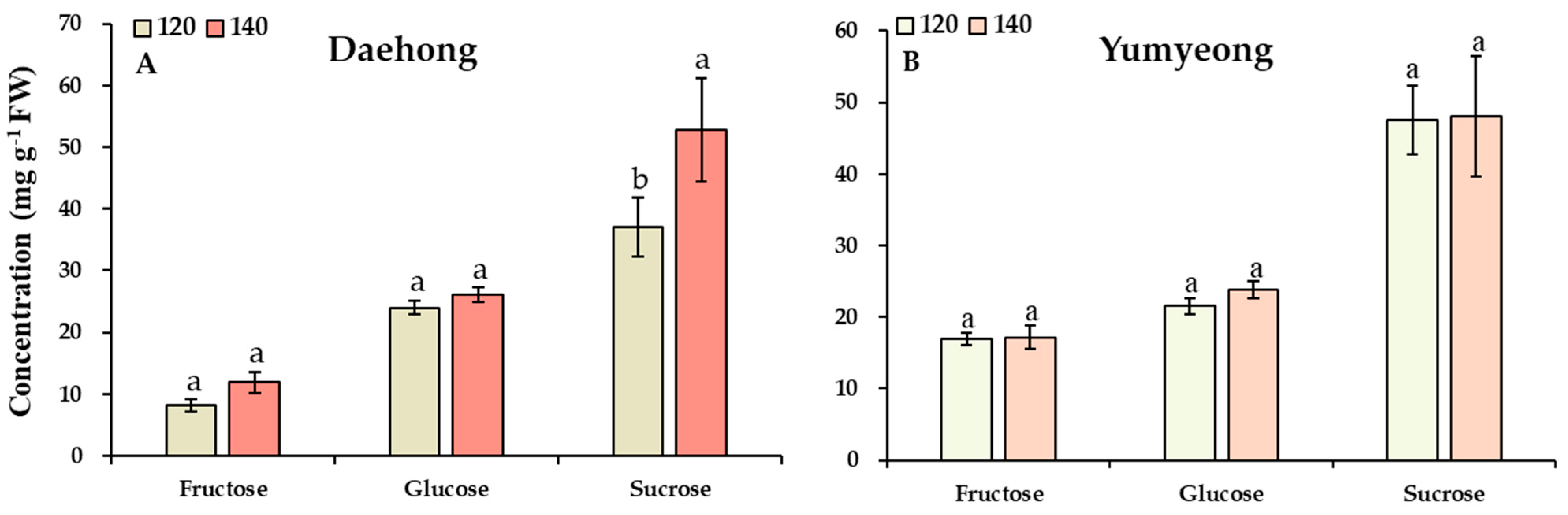
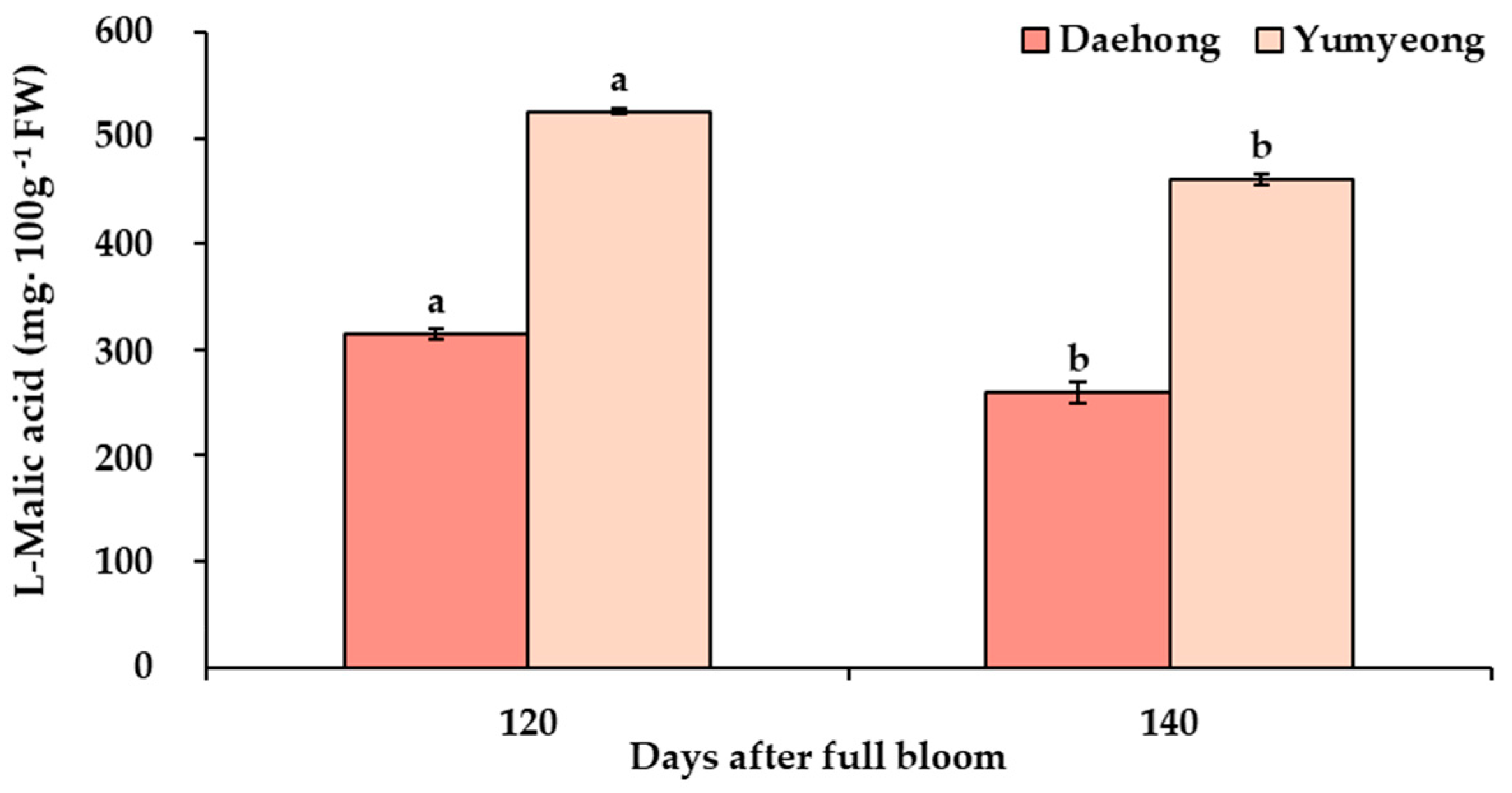
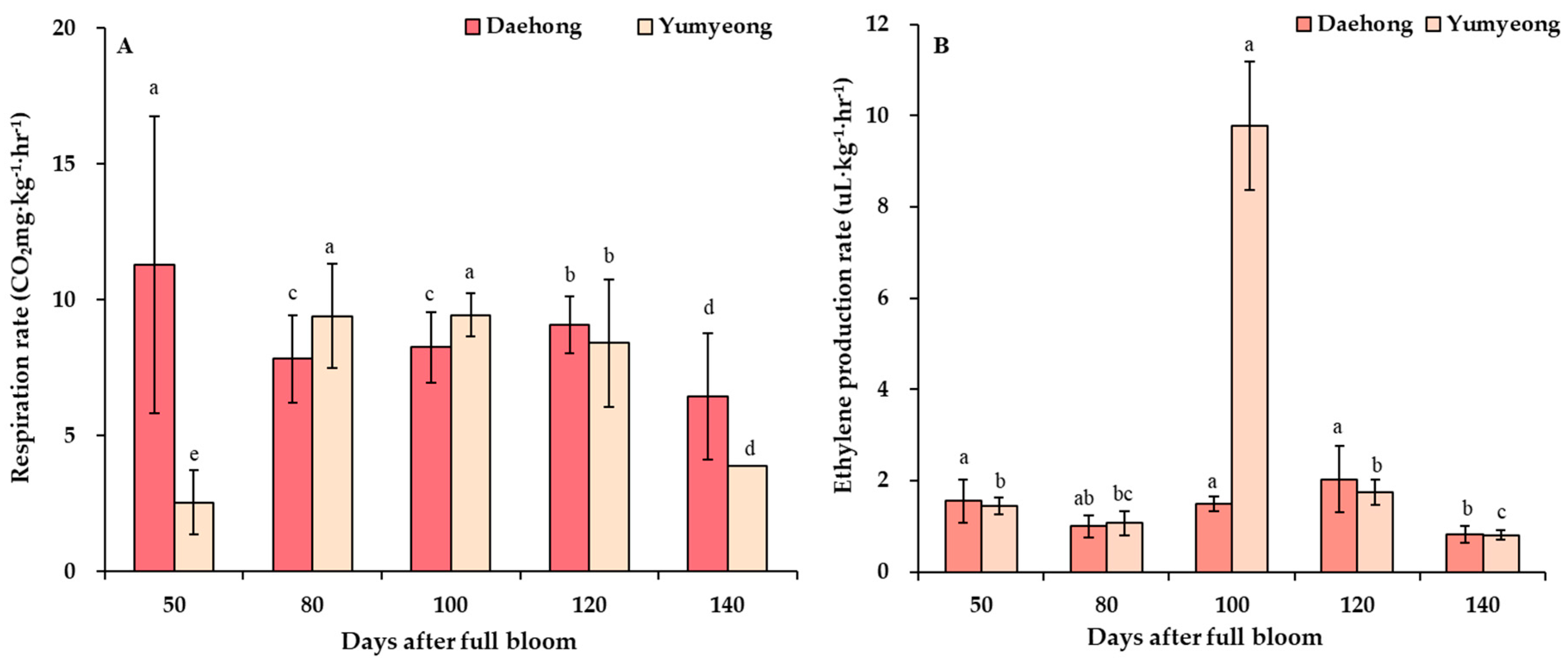
| Cultivars | Days After Full Bloom z | Fresh Weight (g) | Fruit Length (mm) | Fruit Width (mm) | Fruit Shape (Height/Width) |
|---|---|---|---|---|---|
| Daehong | 50 days | 10.3 ± 0.81 d y | 34.5 ± 2.29 d | 25.7 ± 0.89 d | 1.26 ± 1.27 a |
| 80 days | 55.4 ± 7.10 c | 52.6 ± 1.53 c | 43.1 ± 2.57 c | 1.05 ± 1.45 b | |
| 100 days | 134.1 ± 6.25 b | 64.4 ± 2.58 b | 51.4 ± 1.88 b | 1.02 ± 2.11 b | |
| 120 days | 343.1 ± 7.11 a | 73.5 ± 2.84 a | 76.2 ± 2.79 a | 0.96 ± 2.17 c | |
| 140 days | 352.7 ± 5.90 a | 74.0 ± 4.61 a | 80.1 ± 2.94 a | 0.75 ± 1.19 c | |
| Yumyeong | 50 days | 11.7 ± 0.90 d | 34.9 ± 1.29 d | 27.9 ± 1.25 d | 1.25 ± 0.03 a |
| 80 days | 56.1 ± 6.24 c | 50.5 ± 1.15 c | 48.7 ± 1.94 c | 1.05 ± 0.02 b | |
| 100 days | 101.6 ± 5.41 b | 58.2 ± 0.65 b | 58.1 ± 1.43 b | 1.00 ± 0.02 b | |
| 120 days | 342.4 ± 4.41 a | 75.2 ± 0.78 a | 86.6 ± 4.86 a | 0.82 ± 0.04 c | |
| 140 days | 348.2 ± 5.13 a | 78.1 ± 1.79 a | 91.3 ± 1.99 a | 0.79 ± 0.01 c |
| Cultivars | Days After Full Bloom z | Soluble Solid (°Brix) | Total Acidity (%) | Sugar–Acid Ratio (BAR) | Firmness (N) |
|---|---|---|---|---|---|
| Daehong | 120 days | 10.4 ± 0.07 b y | 2.37 ± 0.08 a | 3.96 ± 0.23 b | 36.4 ± 0.56 a |
| 140 days | 10.8 ± 0.03 a | 1.56 ± 0.06 b | 6.32 ± 0.92 a | 35.6 ± 0.24 a | |
| Yumyeong | 120 days | 9.2 ± 0.12 b | 3.21 ± 1.21 a | 2.98 ± 0.22 b | 41.8 ± 1.38 a |
| 140 days | 9.6 ± 0.14 a | 2.68 ± 0.09 b | 3.59 ± 0.18 a | 34.5 ± 1.62 b |
| Days After Full Bloom z | Accumulated Temperature (°C) | ||||
|---|---|---|---|---|---|
| 2012 | 2017 | 2019 | 2021 | 2022 | |
| 50 days | 438.6 | 474.3 | 489.7 | 487.8 | 491.2 |
| 80 days | 908.4 | 969.1 | 998.8 | 1017.5 | 1027.3 |
| 100 days | 1098.9 | 1144.6 | 1179.2 | 1204.6 | 1218.0 |
| 120 days | 1651.7 | 1728.5 | 1788.3 | 1814.8 | 1826.1 |
| 140 days | 1949.8 | 2044.2 | 2098.7 | 2129.3 | 2146.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, Y.H.; Choi, I.-L.; Lee, J.H.; Kwon, Y.B.; Yoon, H.S.; Jeong, H.N.; Kang, H.-M. Physiological Responses and Determination of Harvest Maturity in ‘Daehong’ Peach According to Days After Full Bloom. Horticulturae 2025, 11, 1013. https://doi.org/10.3390/horticulturae11091013
Roh YH, Choi I-L, Lee JH, Kwon YB, Yoon HS, Jeong HN, Kang H-M. Physiological Responses and Determination of Harvest Maturity in ‘Daehong’ Peach According to Days After Full Bloom. Horticulturae. 2025; 11(9):1013. https://doi.org/10.3390/horticulturae11091013
Chicago/Turabian StyleRoh, Yoo Han, In-Lee Choi, Joo Hwan Lee, Yong Beom Kwon, Hyuk Sung Yoon, Haet Nim Jeong, and Ho-Min Kang. 2025. "Physiological Responses and Determination of Harvest Maturity in ‘Daehong’ Peach According to Days After Full Bloom" Horticulturae 11, no. 9: 1013. https://doi.org/10.3390/horticulturae11091013
APA StyleRoh, Y. H., Choi, I.-L., Lee, J. H., Kwon, Y. B., Yoon, H. S., Jeong, H. N., & Kang, H.-M. (2025). Physiological Responses and Determination of Harvest Maturity in ‘Daehong’ Peach According to Days After Full Bloom. Horticulturae, 11(9), 1013. https://doi.org/10.3390/horticulturae11091013






